Abstract
Controlled release of granulocyte-macrophage colony-stimulating factor (GM-CSF) protein by albumin-heparin microparticles administered via intramuscular vaccination in conjunction with HIV DNA vaccines stimulated HIV Gag-specific immune responses. In the murine model, Gag-specific cytotoxic T lymphocyte (CTL) and T helper (Th) responses were significantly enhanced by administration of murine GM-CSF microparticles. This effect was comparable to a GM-CSF encoded plasmid. In three of four rhesus monkeys, enhancement of Gag-specific antibody (Ab), Th, and CTL responses was observed 1 month after the first immunization with coadministration of human GM-CSF microparticles and HIV Gag plasmid. The second, third, and fourth booster immunizations, however, did not increase the Gag-specific immune responses. Subsequent application of Gag protein in complete Freund's adjuvant (CFA) significantly enhanced Ab and Th, but not CTL. However, Gag-specific CTL response was triggered by cytokine and Gag p55-encapsulated microparticles in all animals. The strategy of priming immune responses by coadministration of cytokine microparticles and DNA vaccines, followed by boosting with cytokine and antigen protein-encapsulated microparticles, may prove effective in improving an HIV DNA vaccine design.
Introduction
There is growing evidence that the CD8+ cytotoxic T lymphocyte (CTL) response plays a central role in the containment of HIV infection.1,2 CTLs are the major contributors to the anti-HIV T cell response. Recognizing target cells with specific T cell receptors, they can directly lyse virus-infected cells, suppress proviral expression by producing antiviral cytokines, and block local spreading of the virus by releasing chemokines that bind to the viral coreceptors, thus interfering with its entry into the cell. Recent studies have highlighted the key role of virus-specific CTLs in controlling simian immunodeficiency virus (SIV) replication in rhesus monkeys.3–5 Therefore, induction of strong CTLs against conserved HIV antigens, such as Gag, is essential for developing an effective and safe HIV vaccine.
DNA vaccination can elicit both humoral and cellular immunity to viral antigens and induce protection in rodent and rhesus monkey models. This strategy has attracted substantial interest in the development of an HIV vaccine.6–8 DNA vaccines offer the advantage of synthesizing endogenously the encoded antigen in a natural form, mimicking the process of producing the antigen during viral infection. Genetic vaccination is, therefore, an efficient means of eliciting protective CTLs as well as T helper (Th) and humoral response.9–11 The virus-specific immunity evoked by DNA vaccines has conferred protection of nonhuman primates against nonpathogenic SIV.12 However, the efficacy of immune responses induced by these DNA vaccines is still suboptimal against pathogenic viruses.13 A more potent and durable cellular immunity is required.
Cytokines play a key role in the initiation and regulation of immune and inflammatory responses. Cytokines can recruit and activate macrophages and dendritic cells (DC) and promote antigen presentation to T cells. Some cytokines, for example, interleukin-2 (IL-2), IL-4, and interferon-γ (IFN-γ), also provide costimulatory signals for T cell activation and differentiation. Coadministration of cytokines with antigens in varying configurations has been shown to be a promising approach for amplifying vaccine-elicited immune responses.14–16 To fully realize the potential of DNA vaccines, cytokines are prime candidates to enhance or redirect the immune response induced by DNA vaccines.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to enhance DNA vaccine-elicited immune responses in a variety of animal models.17,18 It was first described as a growth factor for stem cells of the macrophage and granulocyte.19 Subsequently, GM-CSF was also found to act on somatic and bone marrow-derived cells. In particular, GM-CSF promotes the differentiation and activation of macrophages and DCs.20,21 Differentiation of DCs from primitive hematopoietic precursors, however, would require at least 6 days of culture in the presence of GM-CSF and tumor necrosis factor-α (TNF-α).20,21 GM-CSF protein also has a short half-life, ranging from 0.9 to 2.5 h in plasma.22 In addition, cytokines act in a paracrine fashion, necessitating a sustained presence at the local vaccination site. Therefore, a bolus injection of GM-CSF would not be efficient in achieving a strong immunoadjuvant effect.
To achieve local and sustained delivery, many studies have used the GM-CSF plasmid DNA instead of recombinant protein because of the instability of the cytokine protein itself.23 The plasmid approach is advantageous in being simple and producing the bioactive cytokine. It also has its disadvantages. It is difficult to control and predict the amount, duration, and concentration profile of the cytokine at the vaccination site. The transgene expression level is not easily manipulated by the naked DNA dose. There is also the uncertain and potential adverse effect of long-term expression of cytokine should a rare event of integration into the host genome occur. The protein approach is, therefore, appealing from the standpoint of practicality and safety, and it is valuable as a mechanistic tool to study the relationship between dose and duration of cytokines as an immunoadjuvant.
In this study, we investigated if the immune response to HIV DNA vaccines could be enhanced by applying controlled-release technology to provide a sustained and local delivery of cytokine proteins using biodegradable microspheres. These adjuvant effects were investigated in both murine and rhesus monkey models.
Materials and methods
Animals
Female 6–8-week-old BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA), and 15-year-old female rhesus monkeys were purchased from the Johns Hopkins University Research Farm Rhesus Monkey Colony. The animals were housed in the animal facilities at the Johns Hopkins University School of Medicine (Baltimore, MD).
Reagents
HIV Gag p7g (amino acid 199 AMQMLKETI 207) peptide is an H-2d-restricted peptide and was synthesized at the Peptide Synthesis and Sequencing Facility of the Johns Hopkins University. Human GM-CSF protein and human TNF-a protein were purchased from R&D Systems (Minneapolis, MN). Heparin (1000 U/mL) was purchased from Elkins-Sinn, Inc. (Cherry Hill, NJ). Human serum albumin (HSA, 2.5%) was diluted from the injectable 25% solution (Albumarc 25%, Baxter Healthcare Co., Irvine, CA) and adjusted to pH 3.0. Monkey serum albumin, mouse serum albumin, and complete Freund's adjuvant (CFA) were purchased from Sigma (St. Louis, MO). HIV-1 IIIB Gag p55 protein, mouse GM-CSF protein, and pGM-CSF were gifts from NIH AIDS reagent program, Immunex (Seattle, WA), and the National Gene Vector Laboratory at the University of Michigan (NGVL-UM, Ann Arbor, MI), respectively. pGAGINS24 encoding the HIV-1 HXB2 Gag p55 was kindly provided by Dr. X.F. Yu (Johns Hopkins University School of Public Health, Baltimore, MD). pTPA/GAGINS was generated by cloning of the signal sequence of human tissue plasminogen activator (tPA) to the 5′ of HIV GagINS gene using restriction sites NheI and XhoI. The signal sequence of tPA was amplified from human kidney 293 cell cDNA with PCR generation. The total RNA was isolated from 293 cells using an RNeasy mini kit (Qiagen, Valencia, CA), and cDNA was generated from the total RNA with a OneStep RT-PCR kit according to the manufacturer's recommendations (Qiagen). The pTPA/GAGINS plasmid was constructed by PCR amplification of the cDNA using the following primers: 5′-AAGCTGGCTAGCCCACCATGGATGCAATGAAGAGA-3′ and 5′-CGGCCGCTCGAGGCTGGGCGAAACGAAGAC-3′. The plasmid pGAGINS1 was modified from pTPA/GAGINS by adding DNA sequence GAATTCTTGATCCCCATTGCTGTGGGCGGTGCCCTGGCAGGGCTGATCCTCATCGTCCTCATTGCCTACCTCATTGGCAGGAAGAGGAGTCACGCCGGCTATCAGACCATCTAGGAATTC with restriction sites EcoRI and KpnI. The plasmid DNA was produced in bacteria and purified with Endo Free Plasmid kit (Qiagen).
Synthesis of microparticles and cytokine release profile study
Albumin-heparin microparticles (Mps) containing GM-CSF protein or tumor TNF-α protein were prepared by adding 2 mL albumin solution (2.5%, pH 3.0) into 3 mL heparin solution (1000 U/mL) containing GM-CSF, TNF-α, or HIV Gag p55, with continuous vortexing for 10 sec. The Mps were further stabilized by a cross-linking reaction with 1-ethyl 3 (3-propylamino) carbodiimide hydrochloride (EDC) at ambient temperature for 15 min. To study the release profile of GM-CSF, albumin-heparin Mps encapsulating 100 μg human GM-CSF (HuGM-CSF) protein were made and incubated with 10 mL minimum essential medium (MEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Invitrogen) at 37°C. The supernatant was refreshed every day, and the bioactive concentration of GM-CSF was measured using a TF-1 cell proliferation assay.25 The cell proliferation reagent WST-1 (Roche Diagnostics, Indianapolis, IN) was used to determine the proliferation rate of TF-1 cells.
Vaccination of mice
The tibialis muscles of BALB/c mice (n = 5) were injected twice with 20 μg pGAGINS1 in 50 μL phosphate-buffered saline (PBS) via a 28-gauge needle at a 2-week interval with or without 10 μg pGM-CSF plasmid DNA, 20 μg pGM-CSF plasmid DNA, 1 μg bolus mouse GM-CSF protein, blank Mps, or Mps encapsulating 1 μg recombinant mouse GM-CSF (rMuGM-CSF). Animals were killed 2–3 weeks after the second vaccination, and the splenocytes were isolated for testing Gag-specific Th and CTL responses.
Vaccination of rhesus monkeys (Macaca mulatta)
The vaccine formulation for each monkey immunization was composed of 1 mg pGAGINS,24 1 mg pGAGINS1, and 1 mg pTPA/GAGINS in the presence of 20 μg Mps-encapsulated HuGM-CSF and 6 μg Mps-encapsulated HuTNF-α. Each of four rhesus monkeys (10Y, 24Y, 25Y, and 31Y) was immunized four times (weeks 0, 4, 8, and 12) with this vaccine formulation intramuscularly (two sites at the quadriceps) and intradermally (four sites on the back) using 100 μL of the vaccine formulation for each site of injection. At week 16, equal volumes of CFA and 200 μg HIV Gag p55 protein in PBS were emulsified and inoculated into rhesus monkeys intradermally and intramuscularly in a similar manner. At week 20, the animals were finally vaccinated with a mixture of 20 mg albumin-heparin Mps encapsulating 200 μg HIV Gag p55 protein and 5 mg Mps containing 20 μg HuGM-CSF and 6 μg HuTNF-α. Blood samples were collected, and peripheral blood mononuclear cells (PBMC) were isolated from each animal 4 weeks after each immunization for measuring HIV Gag-specific antibody (Ab), Th, and CTL responses.
ELISA for Gag-specific Ab response
Fifty microliters of 2 μg/mL Gag p55 in PBS buffer, pH 7.4, was absorbed onto microtiter wells overnight at 4°C. The plates were washed three times with PBS/0.05% Tween-20 (PBST) and blocked with 3% dehydrated milk and 1% normal goat serum in PBST for 2 h at 37°C. Monkey antisera were diluted with PBST and incubated for 1 h at 37°C, with subsequent incubation with goat antimonkey IgG-conjugated horseradish peroxidase (HRP) for 1 h. The plates were washed and developed with 3,3′,5,5′ tetramethyl benzidine (TMB). The optical density (OD) at 450 nm was read on a microplate reader (Bio-Rad, Hercules, CA).
Gag-specific Th response
Splenocytes (2 × 105/well) from immunized mice or PBMCs from rhesus monkeys were incubated in RPMI medium 1640 (Invitrogen) with 10% FBS (RPMI 10), with or without 0.5 μg/well recombinant Gag p55 or 0.5 μg/well concanavalin A (ConA). The cells were incubated for 4 days, after which 1 μCi/well 3H-thymidine was added for an additional incubation of 16–20 h. The cells were harvested, and the amount of incorporated tritiated thymidine was measured in a Topcount reader. The stimulation index (SI), defined as the ratio between the experimental count and the spontaneous count, was determined. Spontaneous count wells (medium only) included 10% FBS as a protein control. To ascertain the health of the cells, ConA was used as a polyclonal stimulator positive control.
CTL assay
T cells from immunized mice were purified with nylon wool columns and incubated with RPMI 10 in the presence of IL-2 for 6 days as effector cells. Target P815 cells (H-2d) were prepared by incubation for 2 h with p7g and 100 μCi 51Cr. For the monkey vaccination studies, PBMCs from rhesus monkeys were incubated for 6 days with RPMI 10 and IL-2 in the presence of irradiated autologous monkey B lymphoblastoid cell lines (B-LCLs) that were previously infected with vaccinia virus (vv) expressing HIV-1 HXB2 Gag p55 at a multiplicity of infection (moi) of 5 per cell. The monkey B-LCLs were generated by transforming PBMCs with Herpesvirus papio,26 a generous gift from Dr. Norman L. Letvin (Beth Israel Deaconess Medical Center, Boston, MA). Target autologous B-LCLs were also infected with vv expressing HIV Gag and incubated for 2 h with 40 μCi 51Cr. A standard 4-h cytolytic assay was carried out, and the percent of specific lysis was calculated as:
Results
In vitro evaluation of Mps
The Mps had an opaque appearance in saline solution, with sizes ranging from submicrometer to 5 μm (Fig. 1A, B). The encapsulation efficiency of the cytokines was close to 100%.27 Encapsulated cytokines showed a negligible release in PBS solution, whereas in 10% FBS-containing MEM, an initial burst release of bioactive GM-CSF was observed in the first week (Fig. 1C). Release continued for up to 1 month, concomitant with the decrease of particle size and concentration evidenced by qualitative observation under microscopy. Bioactive GM-CSF was released for over a month, as determined by the TF-1 cell assay, which measures the proliferation of TF-1 cells in the presence of bioactive GM-CSF in a dose-dependent manner with the cell proliferation reagent WST-1.
FIG. 1.
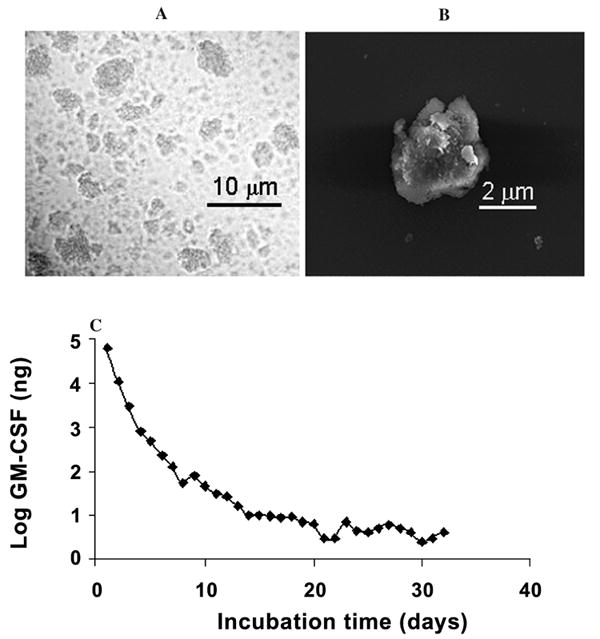
(A) Electron and (B) optical micrographs of albumin-heparin microparticles. (C) Release kinetics of MuGM-CSF from mouse serum albumin-heparin Mps in MEM with 10% FBS (MEM 10). Albumin-heparin Mps containing GM-CSF protein were prepared and incubated in MEM 10. The supernatant was refreshed every day, and the bioactive concentration of GM-CSF was measured using a TF-1 cell proliferation assay.
Effects of local sustained delivery of GM-CSF on CTL and Th responses elicited by pGAGINS1 DNA vaccine in mice
pGAGINS1 was coinoculated intramuscularly with bolus GM-CSF protein (1 μg), GM-CSF Mps (1 μg GM-CSF), or blank albumin-heparin Mps into BALB/c mice. Significantly enhanced Gag-specific lymphoproliferative activity (Fig. 2A) was observed in the GM-CSF Mps group, with an SI of 6.4. In contrast, coadministration of bolus GM-CSF or blank Mps did not affect the Gag-specific Th responses. Analogous to the Th activity, the highest cytotoxic activity (Fig. 2B) was achieved with T cells from mice coadministered with both Gag expression plasmid and GM-CSF Mps. This caused a 48% specific lysis of Gag p7g peptide-pulsed target cells at an effector/target (E:T) ratio of 40. Only a modest increase in CTL was observed in animals immunized with Gag encoding plasmid and bolus GM-CSF protein, in comparison to the CTL in naive animals and animals vaccinated with Gag plasmid and blank albumin-heparin Mps.
FIG. 2.
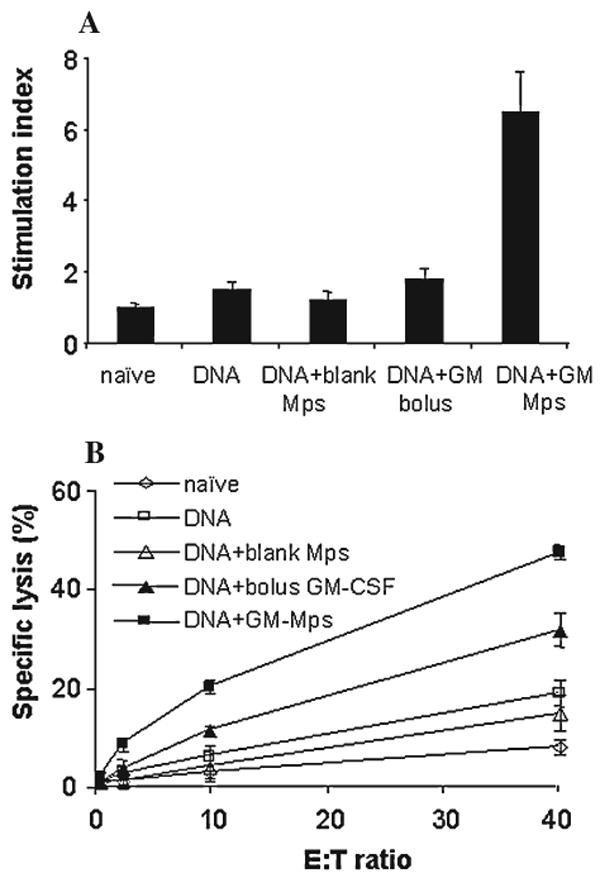
HIV Gag-specific Th response (A) and CTL-specific lysis (B) to pGAGINS1 DNA vaccine. BALB/c mice (n = 5) were immunized intramuscularly with 20 μg DNA vaccine with bolus GM-CSF, GM-CSF Mps, or blank Mps at weeks 0 and 2. DNA vaccine alone was used as control. Animals were studied 2 weeks after the second immunization.
Effects of GM-CSF protein in controlled-release formulation and GM-CSF encoding plasmid on Gag-specific T cell response
The co-inoculation of either albumin-heparin Mps encapsulating 1 μg GM-CSF protein or 10 or 20 μg unencapsulated pGM-CSF plasmid with 20 μg pGAGINS1 enhanced the Gag-specific Th response (Fig. 3A) and CTL response (Fig. 3B) compared with inoculation of 20 μg pGAGINS1 alone. Gag-specific SI was increased to the range of 3–3.8 in Th responses, and specific lysis of target cells was achieved to 25%–38% at an E:T ratio of 40 in the CTL response.
FIG. 3.
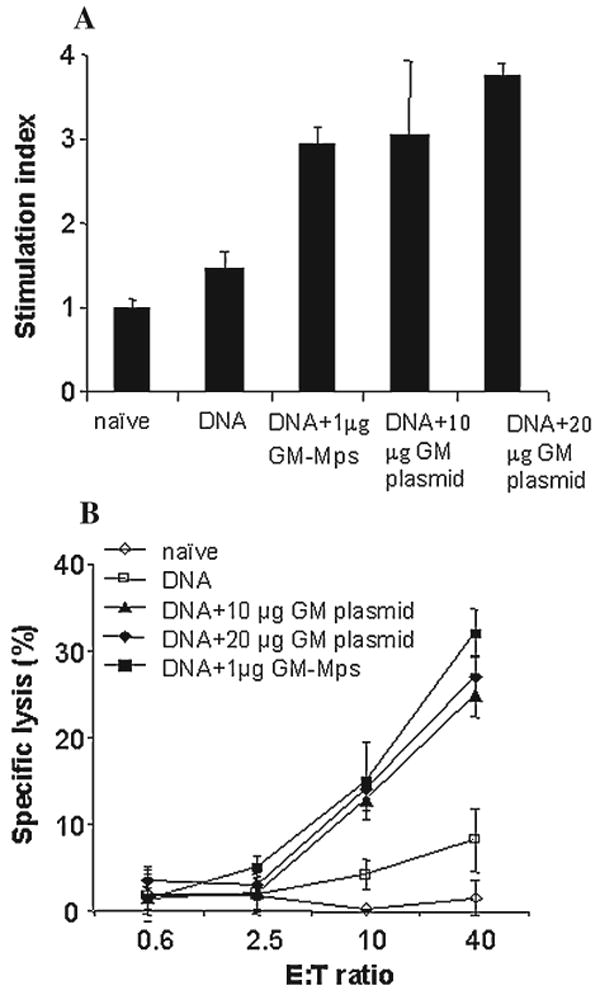
Gag-specific Th response (A) and CTL-specific lysis (B) induced by 20 μg Gag DNA vaccine with or without Mps encapsulating 1 μg GM-CSF or 10 μg or 20 μg of GM-CSF plasmids. BALB/c mice (n = 5) were inoculated intramuscularly at weeks 0 and 2.
CTL responses in individual vaccinated animals
To ascertain the efficacy of the coadministration of DNA vaccine and GM-CSF Mps, the cytotoxic activity of T cells from each individual immunized mouse was measured after two inoculations. A high CTL response was detected in all 8 experimental animals, with Gag-specific lysis of target cells ranging from 33% to 77% at an E:T ratio of 40 (Fig. 4), in comparison to a specific lysis of 4% in naive animals.
FIG. 4.
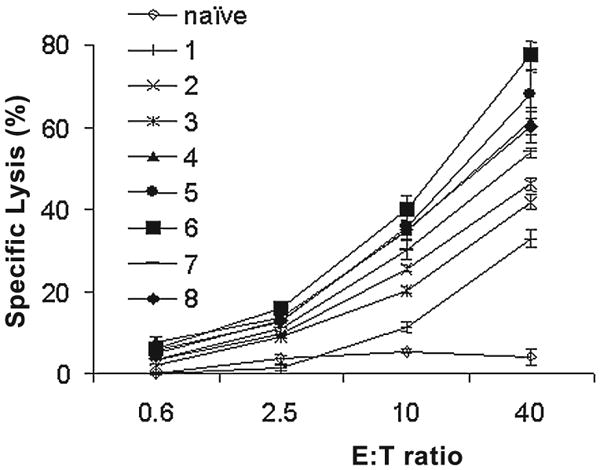
Gag-specific CTL response in individual BALB/c mice immunized with HIV plasmid and GM-CSF Mps. Animals were immunized twice with 20 μg Gag DNA vaccine with Mps encapsulating 1 μg GM-CSF twice at a 2-week interval.
Gag-specific immunity induced in rhesus monkeys after first immunization with cytokine Mps and HIV Gag plasmids
To investigate the effect of cytokine Mps on a macaque model, 4 rhesus monkeys (10Y, 24Y, 25Y, and 31Y) were immunized at a 4-week interval (Scheme 1). This was achieved by intradermal and intramuscular injections with DNA vaccines in the presence of 20 μg Mps-encapsulated HuGM-CSF protein in conjunction with 6 μg Mps-encapsulated HuTNF-a protein. The basal levels of PBMC and sera, obtained from each of the animals before immunization, were used as controls. Negligible Gag-specific CTL, Th, and Ab responses (Fig. 5, top) were observed in the animals before immunization except for animal 24Y, which has a slightly positive Ab response to Gag. A significant Gag-specific Th response was observed with PBMCs from 2 of the 4 immunized animals, 10Y and 24Y (Fig. 5, bottom). In 3 of the 4 animals, 10Y, 24Y, and 25Y, elevated levels of Gag-specific CTL were detected ranging from 30% to 42% specific lysis of target cells at an E:T ratio of 40. The Gag-specific Ab response was potently enhanced in all rhesus monkeys, with anti-Gag IgG titers ranging from 180 to 20,000.
SCHEME 1.
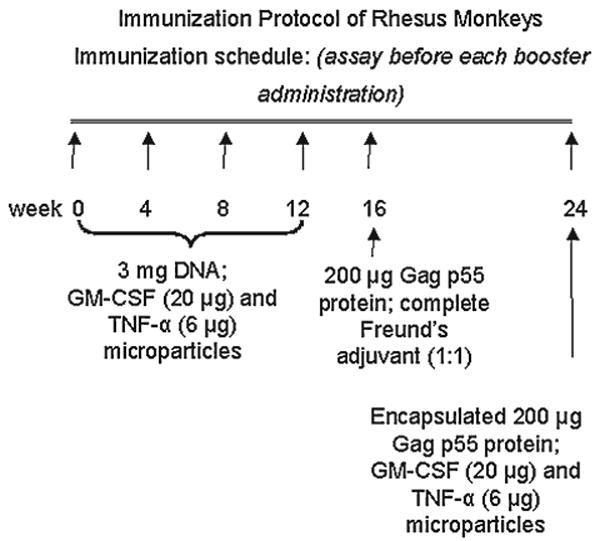
Immunization protocol of rhesus monkeys.
FIG. 5.
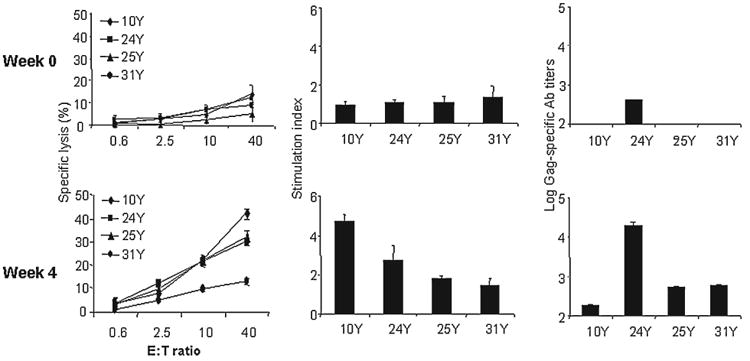
Effect of 3 mg Gag encoding plasmid immunization in the presence of Mps encapsulating 20 μg HuGM-CSF and 6 μg HuTNF-a on immune response in individual rhesus monkeys. (Top) Basal responses (week 0) of Gag-specific CTL, Th, and Ab. (Bottom) Immune responses 4 weeks postimmunization.
Gag-specific immunity induced by DNA priming and DNA boosting, with coadministration of cytokine Mps
The Gag-specific CTL, Th, and Ab responses (Fig. 6) in rhesus monkeys after the three booster shots at 1-month intervals continued to decrease after the initial immunization. At the end of the fourth month, the Gag-specific immune response declined to the prevaccination level, with the SI the Th response <1.5 and specific lysis of target cells <13% at an E:T ratio of 40.
FIG. 6.
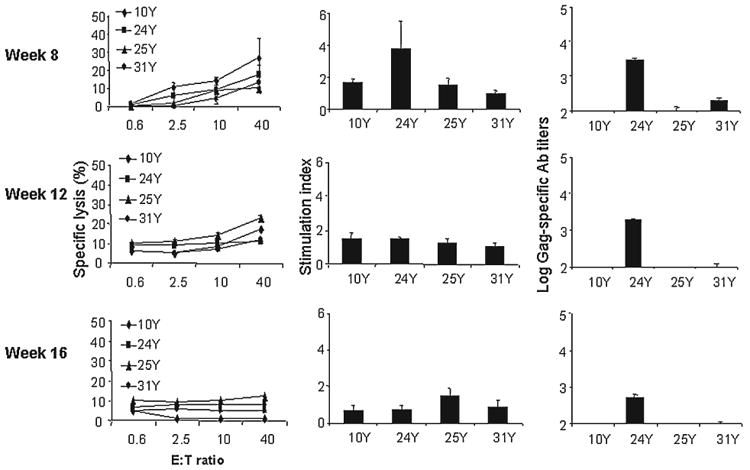
Gag-specific immune response induced by DNA priming and DNA boosting with 3 mg Gag encoding plasmids in the presence of Mps encapsulating 20 μg HuGM-CSF and 6 μg HuTNF-α in individual rhesus monkeys at weeks 8, 12, and 16. After the first immunization, the 4 rhesus monkeys were vaccinated three more times at monthly intervals using the strategy described in Materials and Methods. PBMCs and sera were collected 1 month after each inoculation and assayed.
Boosting with Gag p55 protein and CFA or Gag p55 protein Mps and cytokine Mps
One month after immunization with 200 μg HIV Gag p55 mixed with CFA (Fig. 7, top), the Th response was enhanced in 2 animals with an SI of 4.7 and 3 in animals 25Y and 10Y, respectively. Boosters with Gag p55 protein and CFA also significantly enhanced the anti-Gag IgG titers in all the experimental animals. However, the CTLs remained close to the background level, with the highest specific lysis of 20% in monkey 25Y. Coadministration of 200 μg Gag p55 protein Mps with Mps-encapsulated 20 μg GM-CSF/6 μg TNF-α to the monkeys 2 months after the previous vaccination (Fig. 7, bottom) yielded significantly enhanced CTLs in all 4 animals, ranging from 22% to 37% specific lysis at an E:T ratio of 40. Th Gag-specific Th response in 31Y was also substantially enhanced. The SI level in this monkey, which did not respond to the former two vaccination strategies, was 7.8 1 month postimmunization. The Gag-specific SI in monkey 25Y was 2.3. The Gag-specific Ab response was increased in all 4 animals.
FIG. 7.
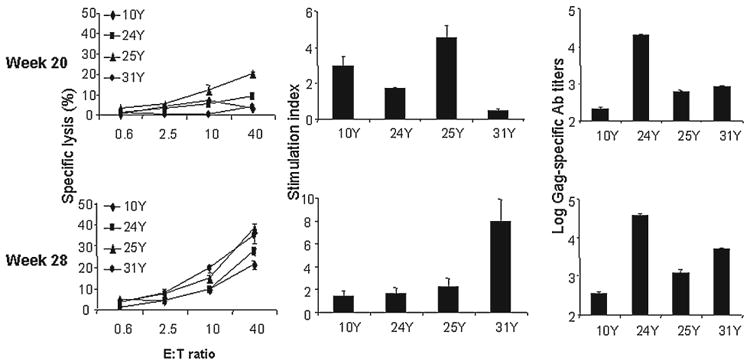
Boosting with Gag p55 protein and CFA (top) or Gag p55 protein and cytokine Mps (bottom). Gag-specific CTL, Th, and Ab responses 4 weeks after rhesus monkeys were immunized with 200 μg HIV Gag p55 protein, mixed with CFA (1:1) or 200 μg Gag p55 protein-encapsulated Mps and 20 μg GM-CSF/6 μg TNF-α Mps.
Discussion
The HIV Gag-specific T cell responses induced in BALB/c mice and rhesus monkeys described in this study highlighted the potential of local and sustained delivery of cytokines in recruiting antigen-presenting cells (APCs) and promoting their activation and differentiation. This enhances the immune response to vaccination strategies, such as DNA vaccines, which offer the advantage of reduced toxicity in comparison with traditional vaccines but have the disadvantage of lower immunogenicity.
The murine experiments were designed to test if GM-CSF Mps could enhance Gag-specific T cell responses in comparison to bolus GM-CSF protein administration. Clearly, a sustained presence of GM-CSF at the vaccine site is required for it to exert its immunoadjuvant effect. Co-injection of a GM-CSF protein bolus could not improve the immune responses. This is consistent with previous results using poly(glycolide-co-d,l-lactide) (PLGA) and poly(d,l-lactide) (PLA) microspheres for controlled release of GM-CSF.28 In this study, mice injected subcutaneously with HuGM-CSF Mps maintained serum levels of HuGM-CSF between 10 and 100 ng/mL for at least 9 days after administration, whereas injection of soluble HuGM-CSF protein was rapidly cleared, with serum elimination half-lives of approximately 1.5 h.
The GM-CSF expression plasmid has been studied extensively for enhancement of immune responses to DNA or protein vaccines. Therefore, to compare the effect of GM-CSF Mps with GM-CSF expression plasmid, BALB/c mice were immunized with GM-CSF Mps encapsulating 1 μg MuGM-CSF protein or two different doses of GM-CSF plasmid as adjuvant. The immunoadjuvant effects of these two modes of GM-CSF delivery are similar in this study. Quantification of GM-CSF transgene expression in the tissue was difficult because of the likely low expression level and the instability of the cytokine. Judging from the in vitro release profile of the GM-CSF Mps, it appears that a low dose of GM-CSF in the nanogram range within the tissue would already be efficacious in stimulating an enhanced immune response. Previous reports have shown that GM-CSF plasmid, when administered at a 3 × 50 μg dose intramuscularly, can induce both antibody and CTL responses.24 In these studies, pGAGINS1 was used at a dose of only 2 × 20 μg intramuscularly, and the CTL response observed was weaker than reported. In the murine studies, improved Gag-specific Th and CTL responses were observed in the vaccination group injected with the combination of GM-CSF Mps and pGAGINS1 compared with animals immunized with pGAGINS1 alone. In the efficacy study, the combined cytokine Mps and DNA vaccines were able to induce positive Gag-specific CTLs in all 8 experimental animals, highlighting the significant potential of this vaccination strategy.
Despite the promising development of a number of strategies to induce potent immune responses in a murine model, HIV-specific or SIV-specific T cell responses induced in monkey models have, by and large, been disappointing, particularly in protection studies.26 Based on our initial observation that HIV Gag-specific immune responses were stimulated by the coadministration of DNA vaccine and cytokine Mps in mice, we applied a similar vaccination strategy to the macaque model. To maximize our chance of success, we also included microencapsulated TNF-α in the monkey studies because previous studies have hypothesized that differentiation of DCs from primitive hematopoietic precursors would be optimal with the presence of both GM-CSF and TNF-α.20,21
In this study, HIV Gag plasmids, Mps encapsulating HuGM-CSF and HuTNF-α, were coadministered to 4 rhesus monkeys. One month postimmunization, potent HIV Gag-specific Th responses and CTL responses were detected in 3 of the 4 immunized monkeys, and the Gag-specific Ab response was observed in all 4 experimental animals. These observations suggest that coadministration of DNA vaccine and cytokine Mps is effective in priming antigen-specific immune responses in rhesus monkeys. However, the fact that the Gag-specific Ab response, Th response, and CTL levels all declined after additional similar immunizations suggests that this approach may be inefficient in boosting an existing immune response. The decreased virus-specific immune responses may be related to the short 1-month interval between the vaccinations. Sustained expression of a reporter gene product encoded by DNA plasmid is observed for at least 2 months after intramuscular injection,29 Therefore, clonal exhaustion may occur when the antigen encoded by the plasmid is continuously expressed in animals with vaccination intervals shorter than the duration of antigen expression. The optimal relationship between the duration of antigen expression and the intervals between DNA vaccine immunizations, however, has yet to be elucidated in any DNA vaccination studies.
The role of CFA in T cell responses, particularly antigen-specific CTL responses, remains controversial. This is despite well-established vigorous enhancement effect of CFA on antigen-specific antibody responses.30–33 To investigate the effect of CFA on HIV Gag-specific immune responses, we inoculated the same animals with HIV Gag p55 protein mixed with CFA. Enhanced antibody response was observed in all the animals, and enhanced Th response was detected in 2 of the 4 animals, although Gag-specific CTL levels remained low. Our results suggest that immunization with protein antigen mixed with CFA is effective in inducing antigen-specific Ab response and Th response but is not as potent in the induction of Gag-specific CTLs, presumably because insufficient access of the antigen to the MHC class I pathway.
Exogenous soluble antigens are generally presented by MHC class II molecules. Because of the segregation of the MHC class I and the exogenous processing pathways, soluble proteins in general cannot induce antigen-specific CTLs. Protein antigen entrapped in biodegradable Mps, however, can elicit MHC class I presentation and CD8+ CTLs.34–37 We, therefore, explored the role of heparin-albumin Mps encapsulating HIV Gag p55 in the induction of Gag-specific immune responses. Enhanced Gag-specific CTLs and antibody responses were observed in all the animals. The Gag-specific Th response was also increased in 1 animal. The heparin-albumin Mps encapsulating the HIV Gag antigen probably mediate their effects through uptake into APCs by priming the MHC class I and II pathway owing to their small sizes, which range from submicrometer to 5 μm.
A salient feature of the heparin-albumin Mps is their ability to simultaneously or singly entrap and provide controlled-release cytokines and antigens.38 This opens up the possibility of single-dose vaccines with improved compliance, which would be particularly valuable in the developing world.
In summary, the studies demonstrate the value of controlled-release technology in realizing the immunoadjuvant potential of cytokines in DNA vaccination. The murine studies show that a localized controlled release of bioactive GM-CSF at the injection site significantly enhances the HIV Gag-specific CTL as well as Th responses. Although the results of the rhesus monkey experiment should be interpreted with caution because of the limited sample size, this pilot study shows that coadministration of DNA vaccines and heparin-albumin Mps-encapsulating cytokines is a potent approach to prime an HIV Gag-specific immune response, although inefficient in providing a boosting effect. Finally, the co-inoculation of Mps encapsulating HIV Gag protein antigen and Mps entrapping cytokines is an effective strategy for inducing antigen-specific antibody, Th, and CTL responses.
Acknowledgments
This research was supported by NIH-CA68011 and NIH-AI42718. The pGAGINS1 construct used in this study was built on pGAGINS kindly provided by Drs. X.F. Yu and G.N. Pavlakis from the National Institutes of Health, Bethesda, MD. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NI-AID, NIH: HIV-1 IIIB p55 Gag. We thank Drs. Eytan A. Klausner and Alisager K. Salem from the Department of Biomedical Engineering of The Johns Hopkins University School of Medicine for their technical discussion.
References
- 1.Sewell AK, Price DA, Oxenius A, Kelleher AD, Phillips RE. Cytotoxic T lymphocyte responses to human immunodeficiency virus: control and escape. Stem Cells. 2000;18:230–244. doi: 10.1634/stemcells.18-4-230. [DOI] [PubMed] [Google Scholar]
- 2.Wick WD, Yang OO, Corey L, Self SG. How many human immunodeficiency virus type 1-infected target cells can a cytotoxic T-lymphocyte kill? J Virol. 2005;79:13579–13586. doi: 10.1128/JVI.79.21.13579-13586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, Perelson AS, Marx PA, Ho DD, Kostrikis LG, Connor RI. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Exp Med. 2000;191:1921–1931. doi: 10.1084/jem.191.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 5.Barouch DH, Powers J, Truitt DM, Kishko MG, Arthur JC, Peyerl FW, Kuroda MJ, Gorgone DA, Lifton MA, Lord CI, Hirsch VM, Montefiori DC, Carville A, Mansfield KG, Kunstman KJ, Wolinsky SM, Letvin NL. Dynamic immune responses maintain cytotoxic T lymphocyte epitope mutations in transmitted simian immunodeficiency virus variants. Nat Immunol. 2005;6:247–252. doi: 10.1038/ni1167. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JD, Chattergoon M, Muthumani K, Kudchodkar S, Kim J, Bagarazzi M, Pavlakis G, Sekaly R, Weiner DB. Next generation DNA vaccines for HIV-1. J Liposome Res. 2002;12:137–142. doi: 10.1081/lpr-120004786. [DOI] [PubMed] [Google Scholar]
- 7.Nabel GJ. HIV vaccine strategies. Vaccine. 2002;20:1945–1947. doi: 10.1016/s0264-410x(02)00074-9. [DOI] [PubMed] [Google Scholar]
- 8.Lian Y, Srivastava I, Gomez-Roman VR, Zur Megede J, Sun Y, Kan E, Hilt S, Engelbrecht S, Himathongkham S, Luciw PA, Otten G, Ulmer JB, Donnelly JJ, Rabussay D, Montefiori D, van Rensburg EJ, Barnett SW. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J Virol. 2005;79:13338–13349. doi: 10.1128/JVI.79.21.13338-13349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis PJ, Babiuk LA. DNA vaccines: a review. Adv Virus Res. 1999;54:129–188. doi: 10.1016/s0065-3527(08)60367-x. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly JJ, Ulmer JB. DNA vaccines for viral diseases. Braz J Med Biol Res. 1999;32:215–222. doi: 10.1590/s0100-879x1999000200010. [DOI] [PubMed] [Google Scholar]
- 11.Fong CL, Mok CL, Hui KM. Intramuscular immunization with plasmid coexpressing tumour antigen and Flt-3L results in potent tumour regression. Gene Ther. 2006;13:245–256. doi: 10.1038/sj.gt.3302639. [DOI] [PubMed] [Google Scholar]
- 12.Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu S, Arthos J, Montefiori DC, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro JC, Wissink J, Mullins JI, Haynes JR, Letvin NL, Wyand M, Robinson HL. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 15.Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, Boyer JD, Weiner DB. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 16.Hess PR, Boczkowski D, Nair SK, Snyder D, Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2005;55:672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas EK, Berzofsky JA. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L. Proc Natl Acad Sci USA. 2002;99:13020–13025. doi: 10.1073/pnas.192251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss WR, Ishii KJ, Hedstrom RC, Sedegah M, Ichino M, Barnhart K, Klinman DM, Hoffman SL. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol. 1998;161:2325–2332. [PubMed] [Google Scholar]
- 19.Metcalf D, Begley CG, Nicola NA. The proliferative effects of human GM-CSF alpha and beta and murine G-CSF in microwell cultures of fractionated human marrow cells. Leuk Res. 1985;9:521–527. doi: 10.1016/0145-2126(85)90131-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- 21.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 22.Stute N, Santana VM, Rodman JH, Schell MJ, Ihle JN, Evans WE. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood. 1992;79:2849–2854. [PubMed] [Google Scholar]
- 23.Sedegah M, Charoenvit Y, Aguiar J, Sacci J, Hedstrom R, Kumar S, Belmonte A, Lanar DE, Jones TR, Abot E, Druilhe P, Corradin G, Epstein JE, Richie TL, Carucci DJ, Hoffman SL. Effect on antibody and T-cell responses of mixing five GMP-produced DNA plasmids and administration with plasmid expressing GM-CSF. Genes Immun. 2004;5:553–561. doi: 10.1038/sj.gene.6364125. [DOI] [PubMed] [Google Scholar]
- 24.Qiu JT, Song R, Dettenhofer M, Tian C, August T, Felber BK, Pavlakis GN, Yu XF. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, Piao YF, Miyazono K, Urabe A, Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 26.Yasutomi Y, Robinson HL, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins JI, Voss G, Manson K, Wyand M, Letvin NL. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SQ, Aoki H, Ohno T, Leong K. Generation of tumor-specific cytotoxic T lymphocytes from naive mouse splenocytes in vitro. 89th Annual Meeting of American Association for Cancer Research; 1998. p. 612. [Google Scholar]
- 28.Pettit DK, Lawter JR, Huang WJ, Pankey SC, Nightlinger NS, Lynch DH, Schuh JA, Morrissey PJ, Gombotz WR. Characterization of poly(glycolide-co-d,l-lactide)/poly(d,l-lactide) microspheres for controlled release of GM-CSF. Pharm Res. 1997;14:1422–1430. doi: 10.1023/a:1012176823155. [DOI] [PubMed] [Google Scholar]
- 29.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 30.Karanikas V, Rowley MJ, MacKay IR, Loveland BE. Autoreactive cytotoxic T cells in mice are induced by immunization with a conserved mitochondrial enzyme in Freund's complete adjuvant. Immunology. 1999;97:264–271. doi: 10.1046/j.1365-2567.1999.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernando GJ, Stewart TJ, Tindle RW, Frazer IH. Vaccine-induced Th1-type responses are dominant over Th2-type responses in the short term, whereas preexisting Th2 responses are dominant in the longer term. Scand J Immunol. 1998;47:459–465. doi: 10.1046/j.1365-3083.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh NA, Rajananthanan P, Attard GS, Morrow WJ. Generation of antigen-specific CD8+ cytotoxic T cells following immunization with soluble protein formulated with novel glycoside adjuvants. Vaccine. 1999;17:2974–2982. doi: 10.1016/s0264-410x(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 33.Skinner MA, Prestidge R, Yuan S, Strabala TJ, Tan PL. The ability of heat-killed Mycobacterium vaccae to stimulate a cytotoxic T-cell response to an unrelated protein is associated with a 65 kilodalton heat-shock protein. Immunology. 2001;102:225–233. doi: 10.1046/j.1365-2567.2001.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Men Y, Tamber H, Audran R, Gander B, Corradin G. Induction of a cytotoxic T lymphocyte response by immunization with a malaria specific CTL peptide entrapped in biodegradable polymer microspheres. Vaccine. 1997;15:1405–1412. doi: 10.1016/s0264-410x(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 35.Maloy KJ, Donachie AM, O'Hagan DT, Mowat AM. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology. 1994;81:661–667. [PMC free article] [PubMed] [Google Scholar]
- 36.Nixon DF, Hioe C, Chen PD, Bian Z, Kuebler P, Li ML, Qiu H, Li XM, Singh M, Richardson J, McGee P, Zamb T, Koff W, Wang CY, O'Hagan D. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine. 1996;14:1523–1530. doi: 10.1016/s0264-410x(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 37.Kuang M, Liu SQ, Saijo K, Uchimura E, Huang L, Leong KW, Lu MD, Huang JF, Ohno T. Microwave tumour coagulation plus in situ treatment with cytokine microparticles: induction of potent anti-residual tumour immunity. Int J Hyperthermia. 2005;21:247–257. doi: 10.1080/02656730500052027. [DOI] [PubMed] [Google Scholar]
- 38.Peng BG, Liu SQ, Kuang M, He Q, Totsuka S, Huang L, Huang J, Lu MD, Liang LJ, Leong KW, Ohno T. Autologous fixed tumor vaccine: a formulation with cytokine-microparticles for protective immunity against recurrence of human hepatocellular carcinoma. Jpn J Cancer Res. 2002;93:363–368. doi: 10.1111/j.1349-7006.2002.tb01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


