Abstract
Recognition of Staphylococcus aureus and its cell-wall component peptidoglycan (PGN) by microglia is mediated, in part, by Toll-like receptor 2 (TLR2). However, the pattern recognition receptor (PRR) CD14 can also bind PGN and enhance TLR2-mediated signaling in macrophages, suggesting a similar phenomenon might occur in microglia. To assess the functional significance of CD14 on microglial activation, we evaluated the responses of primary microglia isolated from CD14 knockout (KO) and wild type (WT) mice. PGN-dependent microglial activation was partially CD14-dependent as demonstrated by the attenuated expression of TNF-α, macrophage inflammatory protein-2 (MIP-2/CXCL2), and the soluble PRR pentraxin-3 in CD14 KO microglia compared to WT cells. In contrast, microglial responses to intact S. aureus occurred primarily via a CD14-independent manner. Collectively, these findings reveal the complex nature of gram-positive bacterial recognition by microglia, which occurs, in part, via CD14.
Keywords: CD14, Microglia, S. aureus, Peptidoglycan, Lipopolysaccharide, Central nervous system, Pentraxin-3
1. Introduction
Microglia represent one effector arm of innate immunity in the central nervous system (CNS) parenchyma as evident by their roles in pathogen recognition and antigen presentation (Aloisi, 2001; Hanisch, 2002). As such, microglia are uniquely poised to provide an initial line of protection against invading microorganisms into CNS prior to leukocyte infiltration. The defense function of microglia is mainly provided by the expression of pattern recognition receptors (PRR) capable of recognizing pathogen-associated molecular patterns (PAMPs) (Akira and Hemmi, 2003; Husemann et al., 2002; Marzolo et al., 1999). PAMPs are defined as invariant molecular motifs of bacteria, fungi, and viruses that are essential for pathogen survival and are conserved across broad subclasses of pathogens (Akira and Hemmi, 2003; Takeda et al., 2003). Studies have demonstrated that Toll-like receptors (TLR) are important for PAMP recognition, and microglia express the majority of TLRs identified to date (TLR1–9)(Bsibsi et al., 2002; Kielian et al., 2002, 2005; Laflamme et al., 2003; Olson and Miller, 2004), which, in theory, enables these cells to recognize a wide spectrum of PAMPs (Akira and Hemmi, 2003; Sieling and Modlin, 2002; Takeda et al., 2003). Recently, we reported the functional importance of TLR2 in mediating microglial activation in response to Staphylococcus aureus (S. aureus) and its cell wall component peptidoglycan (PGN) using primary microglia isolated from TLR2 knockout (KO) and wild-type (WT) mice (Kielian et al., 2005). Our results revealed that TLR2 is pivotal for PGN recognition and subsequent activation in microglia, as demonstrated by the attenuated expression of proinflammatory mediators in PGN-treated TLR2 KO microglia compared with WT cells. In contrast, the importance of this receptor in microglial responses to S. aureus was not as dramatic, indicating that in addition to TLR2, alternative receptors are responsible for recognizing intact bacteria (Kielian et al., 2005).
CD14 is another PRR involved in PAMP recognition, and is found in two forms, namely membrane-bound (mCD14) and soluble CD14 (sCD14). Membrane-bound CD14 is expressed on cells of the myeloid lineage including microglia and macrophages (Becher et al., 1996; Kielian et al., 2002, 2005; Nadeau and Rivest, 2000; Saito et al., 2000; Tsan et al., 2001). It has been well established that mCD14 plays a pivotal role in enabling cells to recognize the gram-negative bacterial cell wall component lipopolysaccharide (LPS) (Dobrovolskaia and Vogel, 2002; Palsson-McDermott and O’Neill, 2004). Since mCD14 is a glycosylphosphatidyl inositol (GPI)-anchored receptor, it requires interaction with TLR4 to transduce activation signals in response to LPS (Dobrovolskaia and Vogel, 2002; Fitzgerald et al., 2004; Haziot et al., 1988; Palsson-McDermott and O’Neill, 2004). However, recent evidence also supports a role for mCD14 in the recognition of gram-positive PAMPs such as PGN and lipoteichoic acid (Cleveland et al., 1996; Dziarski et al., 2000a; Gupta et al., 1996). With respect to gram-positive bacterial antigens, mCD14 is thought to interact with either TLR2/TLR1 or TLR2/TLR6 heterodimers to induce cell activation and subsequent effector functions (Henneke et al., 2001; Kielian et al., 2002; Manukyan et al., 2005; Schroder et al., 2003; Weber et al., 2003). Soluble CD14 can also bind both PGN and LPS (Dziarski, 2003; Gupta et al., 1996; Hailman et al., 1994); however, these complexes have disparate effects on cell activation in mCD14-negative cells. For example, sCD14-LPS complexes are capable of activating mCD14-negative cells (Cohen et al., 1995; Haziot et al., 1993), whereas sCD14-PGN aggregates are not active (Dziarski et al., 2000b; Jin et al., 1998). The reason(s) responsible for the differential effects of sCD14-LPS versus -PGN complexes on mCD14-negative cells remain unknown. With regard to mCD14-positive cells, sCD14-antigen aggregates act in an additive manner to enhance cell activation compared with each PAMP alone (Dziarski et al., 2000b; Hailman et al., 1996).
S. aureus is one of the main pathogens of CNS parenchymal infection leading to the establishment of brain abscess (Kielian, 2004). However, the mechanisms by which S. aureus influences immune activation in the CNS remain to be fully defined. Previous studies from our laboratory have shown that both S. aureus and PGN enhance microglial PRR expression, which may serve to potentiate the antibacterial immune response in infected brain parenchyma prior to leukocyte infiltration (Kielian et al., 2002, 2005). In addition, upon exposure to S. aureus and PGN, microglia respond with the robust synthesis and release of numerous proinflammatory mediators as well as internalizing and killing S. aureus in vitro (Kielian et al., 2002, 2005). Although previous studies have demonstrated that PGN binds to CD14 and enhances TLR2-mediated signaling in macrophages (Iwaki et al., 2002; Koedel et al., 2003; Schwandner et al., 1999), the fundamental role of CD14 in S. aureus- and PGN-dependent microglial activation is not known. In the current study, we evaluated the relative importance of CD14 on S. aureus and PGN recognition using primary microglia isolated from CD14 KO and WT mice. Since fetal bovine serum (FBS) serves as a source of sCD14 and LPS binding protein (LBP), the latter of which facilitates the transfer of PGN to mCD14 (Cohen et al., 1995; Dziarski, 2003; Wright et al., 1990; Yang et al., 1994), we examined microglial activation in response to gram-positive stimuli under serum-free conditions to prevent any confounding effects of serum on the results obtained. The findings presented demonstrate that CD14 participates in PGN-dependent microglial activation, whereas responses to intact S. aureus are primarily CD14-independent. Collectively, these findings reveal the complex nature of gram-positive pathogen recognition by microglia which occurs, in part, via CD14.
2. Materials and methods
2.1. Primary microglia cell culture and reagents
CD14 KO mice backcrossed for six generations (C57BL/ 6 background) were generously provided by Dr. Mason Freeman (Harvard Medical School, USA). Primary microglia were isolated from CD14 KO or WT C57BL/6 pups (Harlan Labs, Indianapolis, IN; 1–8 days of age) as previously described (Esen et al., 2004; Kielian et al., 2005). The purity of microglial cultures was evaluated by immunohistochemical staining using antibodies against CD11b (BD PharMingen, San Diego, CA) and glial fibrillary acidic protein (GFAP; DAKO Corp., Carpenteria, CA) to identify microglia and astrocytes, respectively. The purity of primary microglial cultures was routinely greater than 95%. Microglia were maintained in culture medium supplemented with 10% FBS (Kielian et al., 2004); however, for all of the experiments described herein, cells were plated and stimulated under serum-free conditions to rule out any potential confounding effects of sCD14 and LBP that are present in FBS on cell activation.
Heat-inactivated S. aureus (strain RN6390, kindly provided by Dr. Ambrose Cheung, Dartmouth Medical School) was prepared as previously described (Kielian et al., 2002). PGN derived from S. aureus was obtained from Fluka (St. Louis, MO) and Escherichia coli O11:B1 LPS was from List Biological Laboratories (Campbell, CA). All non-LPS reagents and culture media were verified to have endotoxin levels <0.03 EU/ml as determined by Limulus amebocyte lysate assay (LAL; Associates of Cape Cod, Falmouth, MA). In addition, a phosphate assay (Pierce, Rockford, IL) was performed on the PGN stock to ensure reagent purity since commercial PGN preparations have been reported to be contaminated with LTA and bacterial DNA (Travassos et al., 2004), whereas PGN does not contain phosphate residues. The phosphate content of the PGN preparation used in this study was below the limit of detection, indicating that it was not contaminated with alternative microbial products. Unless otherwise indicated, heat-inactivated S. aureus (107 cfu/ml) PGN (10 μg/ml), and LPS (100 ng/ml) were added to microglial cultures at the final concentrations indicated, representing doses that were previously determined to be optimal for inducing maximal proinflammatory cytokine expression in microglia without any evidence of cytotoxicity (Kielian et al., 2004, 2005).
2.2. ELISA
Comparisons in cytokine and chemokine expression between CD14 WT and KO primary microglia were performed using standard sandwich ELISA kits according to the manufacturer’s instructions (OptEIA mouse TNF-α, IL-12 p40, and IL-1β, BD PharMingen; DuoSet mouse MIP-2/CXCL2, R&D Systems, Minneapolis, MN). The level of sensitivity for all ELISAs was 15.6 pg/ml.
2.3. Cell viability assays
To confirm that the observed changes in inflammatory mediator expression between CD14 WT and KO microglia were not due to differences in cell seeding densities or potential confounding proliferative effects, a standard MTT assay based upon the mitochondrial conversion of (3-[4,5-dimethylthiazol–2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) into formazan crystals was performed as previously described (Kielian et al., 2004).
2.4. Quantitative real-time RT-PCR (qRT-PCR)
Total RNA from CD14 WT and KO primary microglia was isolated using the TriZol reagent and treated with DNAse1 (both from Invitrogen, Carlsbad, CA) prior to use in qRT-PCR studies. The experimental procedure was performed as previously described (Kielian et al., 2005). Briefly, TLR2, CD14, and GAPDH primers and TAMRA Taqman probes were designed as previously described (Esen et al., 2004; Kielian et al., 2005) and synthesized by Applied Biosystems (ABI, Foster City, CA). ABI Assays-on-Demand™ Taqman kits were utilized to examine microglial TLR9, pentraxin-3 (PTX3), and lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) expression. Comparisons in gene expression between CD14 WT and KO primary microglia were calculated after normalizing cycle thresholds against the ‘‘housekeeping’’ gene GAPDH and are presented as the fold-induction (2−ΔΔCt) value relative to unstimulated microglia.
2.5. Protein extraction and Western blotting
Protein extracts were prepared and quantitated from both CD14 WT and KO primary microglia as previously described (Kielian et al., 2004). The effects of heat-inactivated S. aureus, PGN, and LPS on microglial CD14 and MyD88 protein expression were evaluated by Western blot analysis. Blots were probed using rabbit anti-mouse MyD88 (E-Biosciences, San Diego, CA) or rat anti-mouse CD14 antibodies (clone RMC5-3, BD Pharmingen) followed by a donkey anti-rabbit IgG-HRP conjugate (Jackson Immunoresearch, West Grove, PA). Duplicative blots were probed with a rabbit anti-actin polyclonal antibody (Sigma, St. Louis, MO) for normalization. Blots were developed using the ChemiGlow West substrate (Alpha Innotech, San Leandro, CA) and visualized by exposure to X-ray film.
2.6. Statistics
Significant differences between experimental groups were determined using the unpaired Student’s t-test at the 95% confidence interval with Sigma Stat (SPSS Science, Chicago, IL).
3. Results
3.1. Effects of S. aureus and PGN on CD14 expression in primary microglia
It is well established that CD14 is expressed on macrophages and plays an important role in the generation of immune responses to gram-negative bacteria (Dobrovolskaia and Vogel, 2002; Triantafilou and Triantafilou, 2002; Wright et al., 1990). However, recent studies have revealed that CD14 can also bind gram-positive bacterial components such as PGN and lipoteichoic acid (Cleveland et al., 1996; Dziarski, 2003; Dziarski et al., 2000a; Gupta et al., 1996). Since microglia represent the first line of defense against bacterial infections in the CNS parenchyma and modulating PRR expression may have implications on the extent of microglial antibacterial responses, the effects of S. aureus and PGN on CD14 protein expression in primary microglia was evaluated by Western blotting. Since serum contains sCD14 and LBP that are capable of augmenting cell responses to microbial stimuli (Cohen et al., 1995; Wright et al., 1990; Yang et al., 1994), the ability of S. aureus and PGN to modulate CD14 expression was evaluated in the absence of serum. Under these conditions, both S. aureus and PGN stimulation led to an increase in CD14 protein expression in primary WT microglia (Fig. 1). In addition, LPS was also found to augment CD14 protein levels. Taken together, these results indicate that gram-positive bacterial stimuli augment CD14 expression, which may serve to enhance microglial activation in situ during gram-positive CNS infections.
Fig. 1.
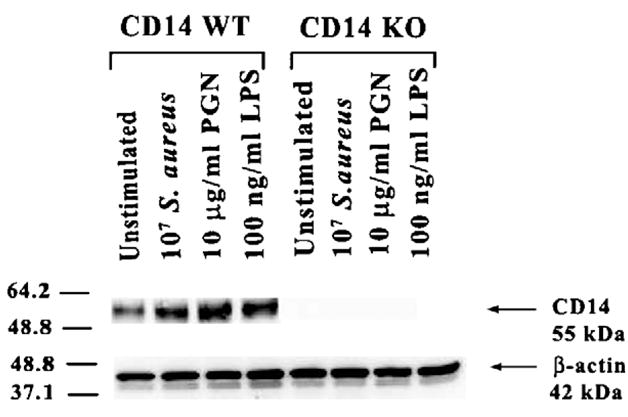
Both S. aureus and PGN augment CD14 protein expression in primary microglia. CD14 KO and WT microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h, whereupon whole cell extracts (40 μg of protein per sample) were prepared and analyzed for CD14 expression by Western blotting as described in the Materials and methods section. Duplicative blots were probed with an antibody specific for β-actin to verify uniformity in gel loading. Results are representative of three independent experiments.
3.2. CD14 differentially regulates PGN and S. aureus recognition by microglia
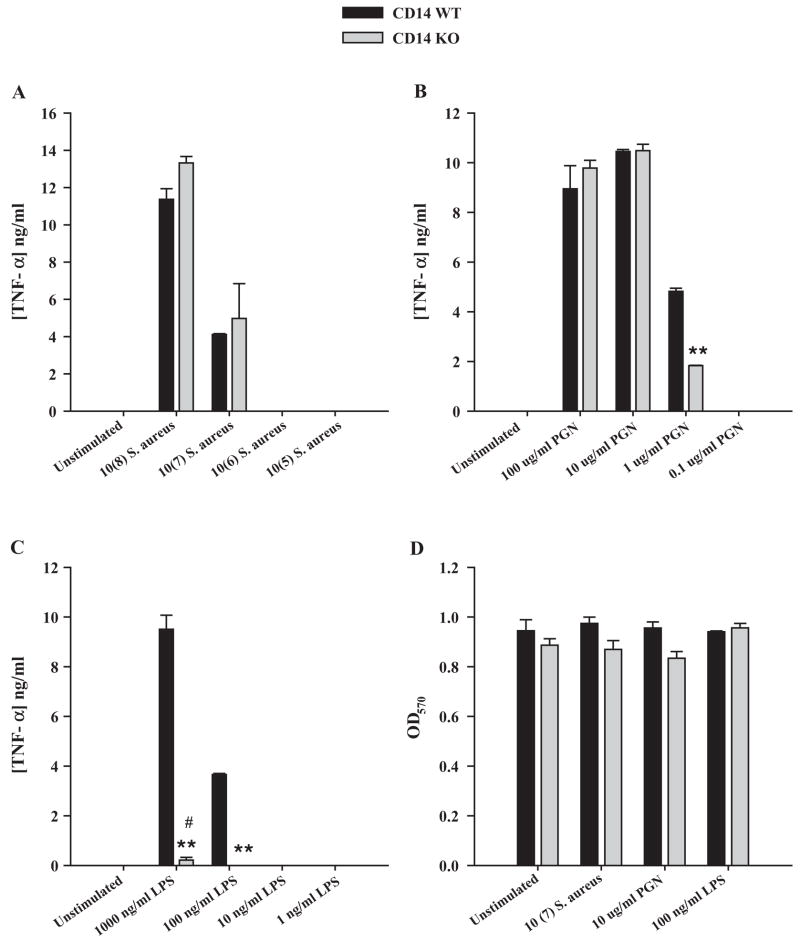
The functional role of mCD14 in LPS-related macrophage responses has been studied extensively and the signal transduction mechanisms in cooperation with TLR4 are well-defined (Dobrovolskaia and Vogel, 2002; Hailman et al., 1994; Palsson-McDermott and O’Neill, 2004; Takeda et al., 2003). However, the direct functional importance of CD14 in mediating microglial responses to gram-positive stimuli has not yet been examined. Therefore, we compared the responses of primary microglia isolated from CD14 KO and WT mice to intact S. aureus and PGN. The absence of CD14 expression in KO microglia was confirmed by both qRT-PCR (data not shown) and Western blot analysis (Fig. 1). As mentioned earlier, in all of the reported studies microglia were evaluated under serum-free conditions to eliminate any confounding effects of sCD14 in serum, which would effectively restore CD14-dependent responses to KO cells. In addition, a dose–response study was performed with each bacterial stimulus since previous studies with LPS have revealed that macrophage activation in response to high-dose LPS can occur via a CD14-independent mechanism (Haziot et al., 1999; Lynn et al., 1993; Perera et al., 1997; Tsan et al., 2001). Interestingly, proinflammatory mediator production by microglia stimulated under serum-free conditions was consistently lower compared to our previous studies where cells were activated in the presence of serum (Kielian et al., 2002, 2004, 2005), revealing that FBS potentiates microglial activation by an, as of yet, unidentified mechanism. S. aureus-dependent microglial activation did not require CD14 at any of the concentrations examined (Fig. 2A). In contrast, CD14 was found to participate in PGN recognition and subsequent TNF-α production (Fig. 2B). Interestingly, higher concentrations of PGN (i.e. >10 μg/ml) stimulated microglial TNF-α release in a CD14-independent manner, suggesting the engagement of alternative, lower affinity PRRs (Fig. 2B). Similar to what has been described in macrophages, CD14 was essential for mediating cell activation in response to LPS; however, higher doses began to act independently of the receptor as evident by residual TNF-α release in CD14 KO microglia when exposed to 1 μg/ml LPS, although the differences in cytokine production remained significantly different between CD14 KO and WT cells (Fig. 2C). Importantly, cell viability assays revealed that the differential responses between CD14 KO and WT microglia were not the result of differential cell density or toxicity (Fig. 2D). Similar results were obtained for IL-1β (data not shown). Importantly, the commercial PGN preparation used in this study was not found to contain detectable phosphate levels, indicating that any differences observed between CD14 KO and WT cells throughout this study cannot be explained by effects of contaminating LTA or DNA, which have been previously reported to be present in various commercial PGN preparations (Travassos et al., 2004).
Fig. 2.
CD14 is involved in mediating microglial activation in response to PGN and LPS. CD14 KO and WT microglia were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to various concentrations of heat-inactivated S. aureus (A), PGN (B), or LPS (C) for 24 h, whereupon conditioned supernatants were collected and analyzed for TNF-α protein expression by ELISA. Results are presented as the amount of TNF-α (ng) per ml of culture supernatant (mean ± S.D. of three biological replicate wells). Microglial cell viability was assessed using a standard MTT assay and the raw OD570 absorbance values are reported (mean ± S.D.; D). Significant differences between CD14 KO versus WT microglia are denoted with asterisks (**p <0.001), whereas significant differences in TNF-α expression between unstimulated versus LPS-stimulated CD14 KO microglia are indicated by a hatched sign (*p <0.001). Results are presented from one of four independent experiments.
Compared to TNF-α release, examination of chemokine expression in CD14 KO and WT microglia revealed slightly different requisites for CD14 in the responses of cells to various doses of S. aureus and PGN (Fig. 3). Specifically, CD14 was found to participate in S. aureus-dependent MIP-2/CXCL2 production at a dose previously determined by our laboratory to be optimal for proinflammatory mediator induction (i.e. 107 cfu, Fig. 3A) (Kielian et al., 2002, 2004, 2005). In addition, CD14 KO microglia were less responsive to PGN compared to WT cells at the majority of doses examined (Fig. 3B). Microglial responses to LPS were CD14-dependent at all antigen doses examined; however, at high concentrations (i.e. 1 μg/ml), LPS was found to partially activate microglia via a CD14-independent manner as evident by detectable MIP-2 production by CD14 KO cells (Fig. 3C).
Fig. 3.
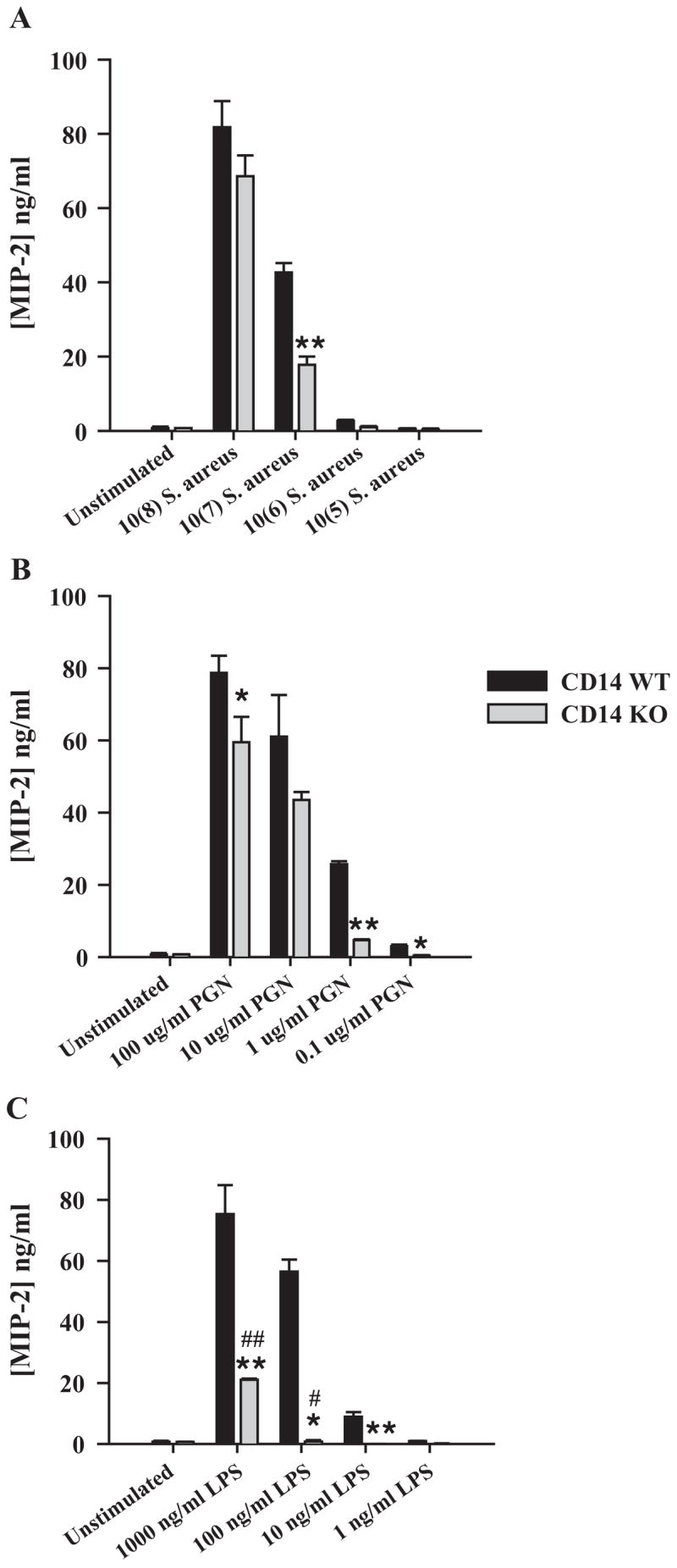
CD14 participates in regulating microglial chemokine expression following stimulation with either PGN or LPS. Primary microglia from CD14 KO and WT mice were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to various concentrations of heat-inactivated S. aureus (A), PGN (B), or LPS (C) for 24 h, whereupon conditioned supernatants were collected and analyzed for MIP-2 protein expression by ELISA. Results are presented as the amount of MIP-2 (ng) per ml of culture supernatant (mean ± S.D. of three biological replicate wells). Significant differences between CD14 KO versus WT microglia are denoted with asterisks (*p <0.05; **p <0.001), whereas significant differences in MIP-2 expression between unstimulated versus LPS-stimulated CD14 KO microglia are indicated by a hatched sign (*p <0.05; **p <0.001). Results are presented from one of four independent experiments.
We have previously demonstrated that, unlike the majority of proinflammatory mediators examined, IL-12 p40 expression was significantly increased in S. aureus-stimulated TLR2 KO microglia compared to WT cells (Kielian et al., 2005). Since TLR2 and CD14 have been reported to cooperate in mediating macrophage activation in response to gram-positive stimuli (Medvedev et al., 1998; Schwandner et al., 1999; Yoshimura et al., 1999), we examined whether a similar phenomenon occurs in CD14 KO microglia. Similar to our findings with TLR2 KO microglia, IL-12 p40 production was consistently elevated in CD14 KO microglia in response to S. aureus compared to WT cells (Fig. 4), suggesting that these receptors may cooperate to regulate IL-12 p40 expression. In contrast, PGN-induced IL-12 p40 expression was significantly decreased in CD14 KO cells (Fig. 4). Collectively, these results demonstrate that CD14 participates, in part, in PGN-dependent microglial activation; however, additional receptors cooperate with CD14 for maximal cellular responses. In contrast, microglia do not utilize CD14 for S. aureus recognition and subsequent microglial proinflammatory mediator production with the exceptions of IL-12 p40 and MIP-2, whereas microglial responses to LPS were CD14-dependent.
Fig. 4.
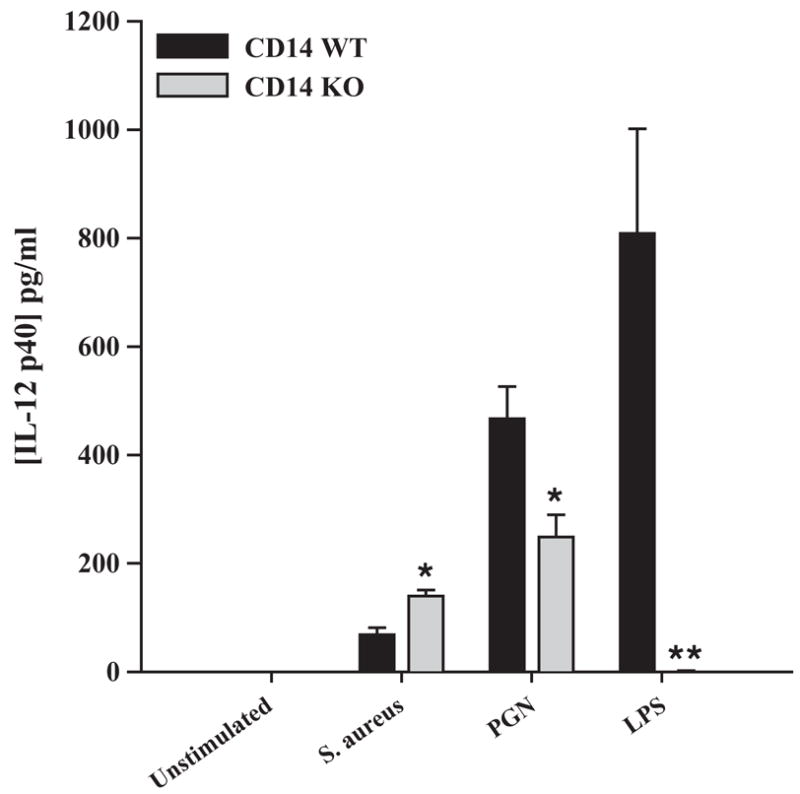
The loss of CD14 results in differential IL-12 p40 production in response to S. aureus or PGN. Primary microglia from CD14 KO and WT mice were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h, whereupon conditioned supernatants were collected and analyzed for IL-12 p40 protein expression by ELISA. Results are presented as the amount of IL-12 p40 (pg) per ml of culture supernatant (mean ± S.D. of three biological replicate wells). Significant differences between CD14 KO versus WT microglia are denoted with asterisks (*p <0.05; **p <0.001). Results are presented from one of four independent experiments.
3.3. Modulation of CD14 KO microglial activation in response to bacterial stimuli is not due to defective MyD88 expression
Upon LPS binding, CD14 physically interacts with TLR4 to initiate intracellular signaling since the former is GPI-anchored and lacks a cytoplasmic signaling domain (Dobrovolskaia and Vogel, 2002; Palsson-McDermott and O’Neill, 2004; Triantafilou and Triantafilou, 2002). In the case of gram-positive bacterial components, evidence suggests that CD14 can enhance TLR2-dependent signaling in macrophages (Harokopakis and Hajishengallis, 2005; Manukyan et al., 2005; Schroder et al., 2003). The majority of Toll-like receptors identified to date utilize a common signal transduction pathway with minor differences that appear to confer specificity in response to distinct ligands (Akira and Hemmi, 2003; Gantner et al., 2003; Takeda et al., 2003). MyD88 is a central player in the signal transduction pathways of the IL-1R, IL-18R, and TLRs (with the exception of TLR3), providing a bridge between the cytoplasmic domains of these receptors and IL-1R-associated kinase (IRAK) (Akira and Hemmi, 2003; Kawai and Akira, 2005; Takeda et al., 2003; Yamamoto et al., 2004). To determine the role of CD14 in regulating MyD88 expression in response to bacterial stimuli, we evaluated the levels of these proteins in both CD14 WT and KO microglia. Interestingly, constitutive levels of MyD88 protein were low in unstimulated CD14 KO microglia; however, following bacterial exposure MyD88 levels were enhanced in CD14 KO microglia (Fig. 5). The extent of MyD88 protein expression in activated CD14 KO and WT microglia was comparable, suggesting that the observed alterations in proinflammatory mediator expression in the former did not result from alterations in this key signaling intermediate (Fig. 5).
Fig. 5.
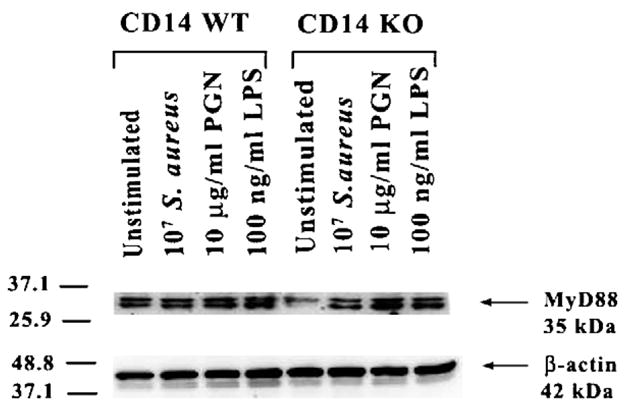
Modulation of cellular activation in response to bacterial stimuli in CD14 KO microglia is not due to defective MyD88 expression. CD14 KO and WT primary microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h, whereupon protein extracts from whole cell lysates (40 μg per sample) were evaluated for MyD88 expression by Western blotting as described in the Materials and methods section. Duplicative blots were probed with an antibody specific for β-actin to verify uniformity in gel loading. Results are representative of three independent experiments.
3.4. Role of CD14 on the expression of alternative PRRs in microglia
Recently we have demonstrated that responses to S. aureus were not abolished in TLR2 KO microglia, implying a role for alternative receptors in S. aureus recognition in these cells (Kielian et al., 2005). Although CD14, which has been shown to mediate macrophage activation in response to PGN and other gram-positive bacterial components (Cleveland et al., 1996; Dziarski et al., 1998; Kusunoki et al., 1995), was another logical candidate, in the present study we have shown that S. aureus activates microglia primarily via a CD14-independent manner. Therefore, to determine the effects of S. aureus on additional PRRs that could be responsible for bacterial recognition, we evaluated differences in pentraxin-3 (PTX3), lectin-like oxidized low density lipoprotein receptor-1 (LOX-1), and TLR9 expression in both CD14 KO and WT microglia using qRT-PCR. Both PTX3 and LOX-1 represent phagocytic PRRs that may be responsible for S. aureus uptake by microglia, whereas TLR9 may be important for inducing proinflammatory mediator production in response to non-methylated CpG DNA motifs contained within heat-inactivated bacteria. Alterations in microglial receptor expression following PGN stimulation were also evaluated to assess potential differential effects of the two bacterial stimuli.
PTX3 is a secreted PRR that is produced by activated macrophages (Alles et al., 1994; Introna et al., 1996) and physically binds to several bacteria and fungi, implying an important role for PTX3 in the innate immune response (Garlanda et al., 2002; Mantovani et al., 2003). The expression of this PRR in microglia and the role of CD14-dependent signals in regulating PTX3 levels have not yet been reported, although a recent study has revealed that TLR2-dependent activation is important for modulating PTX3 levels in macrophages (Jeannin et al., 2005). The present study is the first report demonstrating PTX3 expression in primary microglia (Fig. 6A). Compared to PGN, S. aureus was not capable of stimulating PTX3 gene expression in WT microglia (Fig. 6A). When evaluating PTX3 expression in CD14 KO and WT microglia, the former did not display detectable PTX3 constitutively, whereas basal levels were readily apparent in WT microglia (data not shown). Interestingly, although PTX3 expression was induced in both CD14 WT and KO microglia in response to PGN, the degree of PTX3 upregulation was significantly lower in KO cells, suggesting that CD14 regulates PTX3 mRNA induction (Fig. 6B).
Fig. 6.
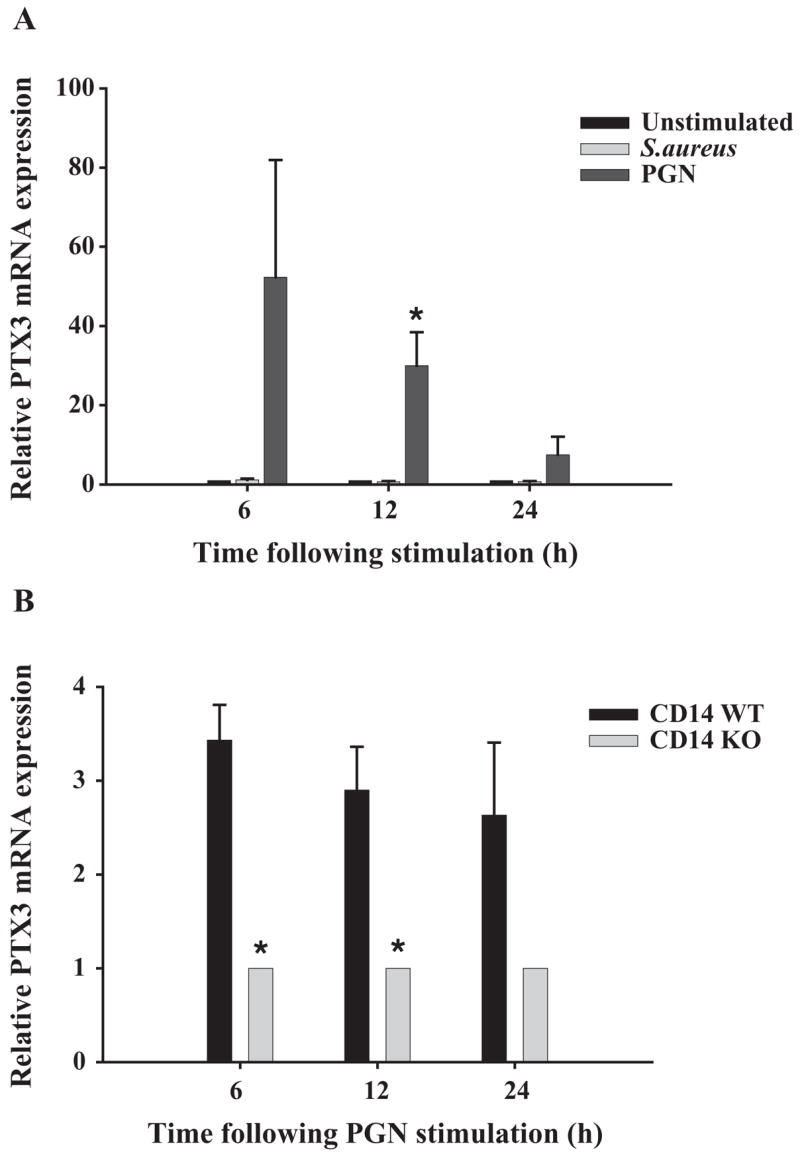
Expression of the secreted PRR pentraxin-3 is regulated by CD14-dependent signals in PGN-stimulated microglia. CD14 WT (A) or CD14 KO and WT (B) microglia were seeded at 2 × 106 cells per well in 6-well plates and incubated overnight. The following day, cells were stimulated with either 107 heat-inactivated S. aureus or 10 μg/ml PGN (A) or PGN only (B), whereupon total RNA was isolated and examined for PTX3 expression by qRT-PCR as described in the Materials and methods section. Each real-time PCR reaction was performed in duplicate for PTX3 and the ‘‘housekeeping’’ gene GAPDH. The level of gene expression was calculated after normalizing PTX3 signals against GAPDH and is presented in relative mRNA expression units (mean ± S.D. of fold-changes pooled from three independent experiments). Significant differences between CD14 WT untreated versus S. aureus- or PGN-stimulated microglia (A) and CD14 WT versus CD14 KO PGN-stimulated microglia (B) are denoted with asterisks (*p <0.05).
Additional PRR candidates that could be involved in mediating microglial responses to S. aureus include scavenger receptors which represent a large class of cell surface molecules that recognize a wide array of both exogenous and endogenous ligands including PAMPs and modified low-density lipoproteins (Husemann et al., 2002; Peiser et al., 2002). Within this family we chose to investigate LOX-1 since this receptor has been reported to bind S. aureus directly (Shimaoka et al., 2001). LOX-1 mRNA expression was significantly elevated in response to both S. aureus and PGN (Fig. 7 and data not shown); however, its levels were similar between CD14 WT and KO microglia (data not shown), suggesting that CD14 is not pivotal for regulating microglial LOX-1 expression in response to these gram-positive stimuli.
Fig. 7.
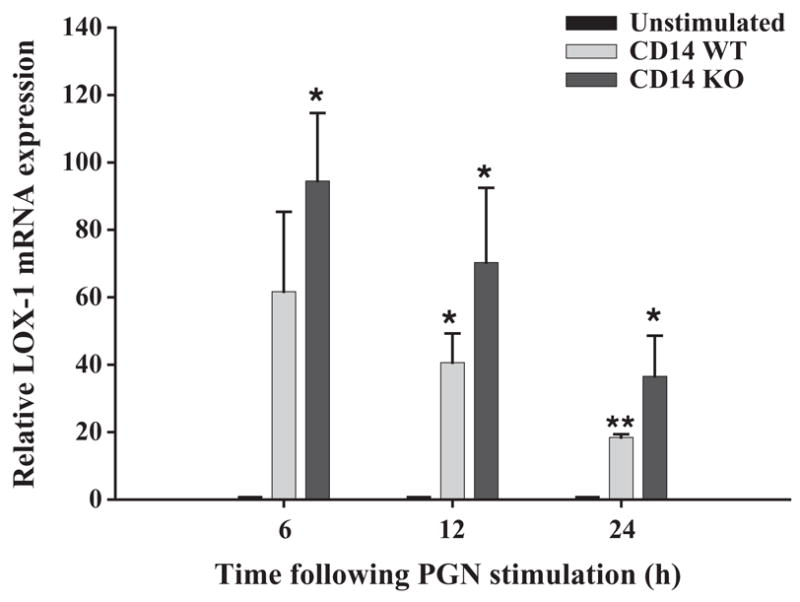
Regulation of microglial LOX-1 expression is CD14-independent. The time course profile of LOX-1 mRNA expression following PGN stimulation (10 μg/ml) in both CD14 KO and WT microglia was measured by qRT-PCR as described in the Materials and methods section. Each real-time PCR reaction was performed in duplicate for LOX-1 and the ‘‘housekeeping’’ gene GAPDH. The level of gene expression was calculated after normalizing LOX-1 signals against GAPDH and is presented in relative mRNA expression units (mean ± S.D. of fold-changes pooled from three independent experiments). Significant differences between unstimu-lated versus PGN-stimulated CD14 WT or CD14 KO microglia are denoted with asterisks (*p <0.05; **p <0.001).
Although studies have established that TLR2 is required for cellular responses to S. aureus (Akira and Hemmi, 2003; Kaisho and Akira, 2002), recent evidence has revealed that DNA can influence cellular activation by engaging the intracellular PRR TLR9 (Athman and Philpott, 2004; Dziarski, 2003; Hemmi et al., 2000). Intact S. aureus presents microglia with a complex milieu of antigens including DNA containing non-methylated CpG motifs, the only known bacterial ligand of TLR9, suggesting that S. aureus may induce immunological responses through this receptor. In the present study we have determined that TLR9 expression was differentially modulated by S. aureus and PGN. Specifically, the extent of TLR9 inhibition following microglial activation was significantly more pronounced in PGN-treated compared to S. aureus-treated cells, with the latter displaying a delayed increase in TLR9 mRNA levels at 24 h post-stimulation (Fig. 8). The effects of S. aureus and PGN on TLR9 expression occurred independently of CD14 since similar responses were observed in both CD14 KO and WT microglia (data not shown).
Fig. 8.
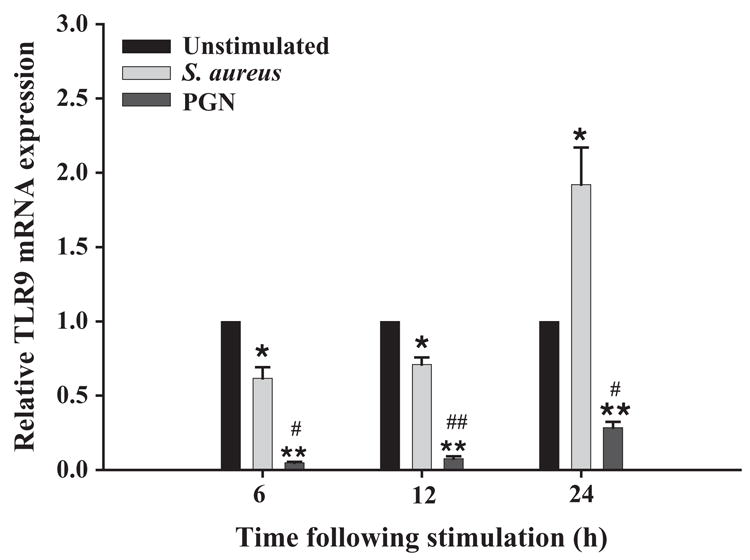
S. aureus and PGN differentially modulate TLR9 expression. The time course profile of TLR9 mRNA expression following exposure to either 107 heat-inactivated S. aureus or 10 μg/ml PGN in CD14 WT microglia was measured by qRT-PCR as described in the Materials and methods section. Each real-time PCR reaction was performed in duplicate for TLR9 and the ‘‘housekeeping’’ gene GAPDH. The level of gene expression was calculated after normalizing TLR9 signals against GAPDH and is presented in relative mRNA expression units (mean ± S.D. S.D. of fold-changes pooled from three independent experiments). Significant differences between unstimulated versus bacterially stimulated microglia are denoted with asterisks (*p <0.05; **p <0.001, whereas significant differences between S. aureus- and PGN-treated microglia are also indicated (*p <0.05; **p <0.001).
Collectively, the evaluation of additional PRRs indicates that CD14-dependent activation signals influence microglial PTX3 expression in response to PGN; however, the effects of S. aureus and PGN on LOX-1 and TLR9 expression are CD14-independent. The S. aureus-induced effects on LOX-1 and TLR9 expression may compensate for the loss of CD14 in KO microglia, thus explaining, in part, the inability to detect widespread differences in activation status between CD14 KO and WT cells in response to intact bacteria.
4. Discussion
Pathogen recognition by cells of the myeloid lineage represents a first step towards initiating complex inflammatory reactions characteristic of innate immune responses. Although gram-positive pathogens are common etiologic agents of CNS bacterial infections (Mathisen and Johnson, 1997; Townsend and Scheld, 1998), the innate immune mechanisms elicited by these organisms in microglia still remain to be completely defined. In the CNS, microglia likely play an essential role in initiating innate immunity through their extensive expression of PRRs including TLRs and CD14 (Bsibsi et al., 2002; Dalpke et al., 2002, Kielian et al., 2002; Laflamme et al., 2001; Olson and Miller, 2004).
Using primary microglia from CD14 KO and WT mice, we demonstrated that CD14 participates in PGN recognition by microglia. However, the residual levels of proinflammatory mediator expression detected in PGN-stimulated CD14 KO microglia indicate that CD14 cooperates with additional PRRs for maximal cellular activation. Indeed, the findings reported here are in close agreement with our recently published studies investigating the functional importance of TLR2 in PGN-dependent microglial activation, where the loss of TLR2 did not result in the complete ablation of proinflammatory mediator release from PGN-activated microglia (Kielian et al., 2005). Taken together, these findings suggest that TLR2 and CD14 may function cooperatively to mediate maximal microglial activation in response to S. aureus-derived PGN. Indeed, evidence to support the collaborative actions of TLR2 and CD14 in the recognition of gram-positive bacteria is provided by recent studies demonstrating that macrophage activation in response to group B streptococci (GBS) involves the coordinated efforts of both receptors (Medvedev et al., 1998; Yoshimura et al., 1999). Studies are underway to directly assess the cooperation between TLR2 and CD14 in regulating PGN-dependent microglial activation by examining primary microglia from TLR2/CD14 double KO mice.
Although we were able to demonstrate that CD14 participates in LPS-dependent microglial activation presumably via its interactions with TLR4, it was evident that at higher antigen doses cell activation could begin to proceed via a CD14-independent manner. Indeed, the ability of high-dose LPS to activate macrophages through CD14/TLR4-independent pathways has been well established and may be a consequence of engaging lower affinity receptors under conditions of excess antigen (Haziot et al., 1999; Lynn et al., 1993; Perera et al., 1997). The identity of receptor(s) that may be responsible for mediating CD14-, and presumably TLR4-independent microglial activation in response to LPS are currently unknown.
The finding that microglial responses to intact bacteria were not dramatically affected by the loss of CD14 suggests that microglia utilize alternative PRRs for S. aureus recognition. Indeed, recent evidence has revealed the existence of receptor cross-talk between TLRs and phagocytic receptors (Underhill and Gantner, 2004; Underhill and Ozinsky, 2002). For example, recent studies suggest that TLRs may regulate phagosome formation and maturation as well as modulate the transcription of some phagocytic receptors, while signaling via phagocytic receptors can also modulate TLR signaling (Underhill and Gantner, 2004). Relevant to the current study, through its ability to enhance TLR-dependent signaling, CD14 may also influence microglial activation by regulating the expression and/or activity of phagocytic PRRs. Although TLRs and CD14 are pivotal PRRs, they do not function directly as phagocytic receptors. This is supported by our findings where MyD88 KO microglia were still capable of phagocytizing heat-inactivated S. aureus (Kielian, unpublished observations) in addition to studies by others demonstrating that macrophages with a deletion of TLR2 or MyD88 were not capable of producing proinflammatory cytokines but were still able to internalize pathogens (Henneke et al., 2002; Underhill et al., 1999). To begin to investigate the potential involvement of alternative phagocytic PRRs in microglial responses to S. aureus, we examined the expression of the scavenger receptor LOX-1 and PTX3 in primary microglia. Our results represent the first evidence demonstrating that CD14-dependent signals are essential for maximal PTX3 expression in PGN-activated microglia. In addition, we have recently found that the PGN-induced increase in PTX3 expression is also attenuated in TLR2 KO microglia (Kielian, unpublished observations). Taken together, we suggest that CD14, possibly in cooperation with TLR2, induces PTX3 expression in primary microglia, which may result from the autocrine action of proinflammatory cytokines released from activated microglia following bacterial exposure. Interestingly, although our objective in examining PTX3 expression in microglia was to determine whether this soluble PRR may participate in the recognition event of intact bacteria, we found that S. aureus was not capable of augmenting microglial PTX3 expression. However, a definitive role for PTX3 in mediating microglial responses to S. aureus awaits the examination of cells from PTX3 KO animals. With regard to LOX-1, the expression of this phagocytic PRR was significantly increased in microglia following S. aureus exposure, suggesting that it may participate in bacterial recognition. Although we were unable to detect any significant differences in LOX-1 expression between CD14 KO and WT microglia, it is important to acknowledge that CD14-dependent signals may influence the subsequent extent of LOX-1-dependent phagocytosis by modulating constitutive LOX-1 activity. Therefore, definitive information regarding the potential functional cooperativity of LOX-1 with CD14 in mediating S. aureus-dependent microglial activation awaits the generation of LOX-1/CD14 double KO mice.
It is well established that primary microglia respond to S. aureus and PGN with the robust production of proinflam-matory cytokines and chemokines, such as TNF-α, IL-1β, MIP-2, and MCP-1 (Kielian et al., 2002, 2005). In addition, we have recently reported that TLR2 is pivotal for proinflammatory cytokine production in response to PGN (Kielian et al., 2005), with the present study extending these findings to demonstrate a role for CD14 in this recognition event, possibly via receptor cooperation. However, one consistent finding that surfaced during the course of the current study and previous work with TLR2 KO microglia (Kielian et al., 2005) relates to the heightened release of IL-12 p40 by S. aureus activated KO microglia. Specifically, both CD14 and TLR2 KO microglia produced more IL-12 p40 in response to S. aureus compared with WT cells, suggesting that these receptors play an integral role in regulating IL-12 expression. Interestingly, IL-12 p40 levels were not elevated in either CD14 or TLR2 KO microglia in response to PGN, indicating that the influence of these PRRs on cytokine regulation is antigen-specific. These findings suggest that under normal conditions, TLR2 and CD14 may serve to regulate IL-12 p40 release by inducing suppressor cytokines and/or activating a direct suppressor pathway. Candidate molecules may include IL-10 and/or the suppressors of cytokine signaling (SOCS) proteins (Alexander and Hilton, 2004; Elliott and Johnston, 2004; Williams et al., 2004), possibilities that are currently under investigation in our laboratory.
In conclusion, these results demonstrate that CD14 participates in regulating microglial proinflammatory mediator release in response to PGN. We have recently established that CD14 plays a functional role in dictating the ensuing CNS host immune response in a mouse model of S. aureus-induced experimental brain abscess. Specifically, CD14 appears to participate in regulating the release of select proinflammatory mediators as well as modulating blood–brain barrier permeability at the later stages of infection (Kielian, manuscript in preparation). However, despite these differences bacterial titers were not significantly different between CD14 KO and WT mice, indicating that alternative PRRs are responsible for controlling CNS bacterial burdens. Based upon the functional similarities in responses between CD14 and TLR2 KO microglia following PGN exposure, we propose that these receptors function in a multi-receptor complex to coordinate maximal proin-flammatory mediator production and PTX3 expression in microglia to facilitate pathogen recognition and phagocytosis in the infected CNS.
Acknowledgments
The authors would like to thank Dr. Mason Freeman for generously providing the CD14 KO mice, Dr. Paul Drew for critical review of the manuscript, and Patrick Mayes for excellent technical assistance. This work was supported by the NIH National Institute of Mental Health (RO1 MH65297) to T.K. and the National Institute of Neurological Disorders and Stroke supported Core facility at UAMS (P30 NS047546).
References
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Athman R, Philpott D. Innate immunity via Toll-like receptors and Nod proteins. Curr Opin Microbiol. 2004;7:25–32. doi: 10.1016/j.mib.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Becher B, Fedorowicz V, Antel JP. Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res. 1996;45:375–381. doi: 10.1002/(SICI)1097-4547(19960815)45:4<375::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuro-pathol Exp Neurol. 2002;61(11):1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Haziot A, Shen DR, Lin XY, Sia C, Harper R, Silver J, Goyert SM. CD14-independent responses to LPS require a serum factor that is absent from neonates. J Immunol. 1995;155:5337–5342. [PubMed] [Google Scholar]
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci. 2003;60:1793–1804. doi: 10.1007/s00018-003-3019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R, Tapping RI, Tobias PS. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Ulmer AJ, Gupta D. Interactions of CD14 with components of gram-positive bacteria. Chem Immunol. 2000a;74:83–107. doi: 10.1159/000058761. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Viriyakosol S, Kirkland TN, Gupta D. Soluble CD14 enhances membrane CD14-mediated responses to peptidoglycan: structural requirements differ from those for responses to lipopolysac-charide. Infect Immun. 2000b;68:5254–5260. doi: 10.1128/iai.68.9.5254-5260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J, Johnston JA. SOCS: role in inflammation, allergy and homeostasis. Trends Immunol. 2004;25:434–440. doi: 10.1016/j.it.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- Gupta D, Kirkland TN, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, Busse LA, Zukowski MM, Wright SD. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, Detmers PA, Wright SD. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- Haziot A, Rong GW, Silver J, Goyert SM. Recombinant soluble CD14 mediates the activation of endothelial cells by lip-opolysaccharide. J Immunol. 1993;151:1500–1507. [PubMed] [Google Scholar]
- Haziot A, Hijiya N, Schultz K, Zhang F, Gangloff SC, Goyert SM. CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-alpha production. J Immunol. 1999;162:4801–4805. [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT. Novel engagement of CD14 and multiple Toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–7076. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- Henneke P, Takeuchi O, Malley R, Lien E, Ingalls RR, Freeman MW, Mayadas T, Nizet V, Akira S, Kasper DL, Golenbock DT. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J Immunol. 2002;169:3970–3977. doi: 10.4049/jimmunol.169.7.3970. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, Srinivasan N, Blundell TL, Hamilton TA, Mantovani A. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872. [PubMed] [Google Scholar]
- Iwaki D, Mitsuzawa H, Murakami S, Sano H, Konishi M, Akino T, Kuroki Y. The extracellular Toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem. 2002;277:24315–24320. doi: 10.1074/jbc.M107057200. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, Thieblemont N, Corvaia N, Garlanda C, Delneste Y, Mantovani A. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jin Y, Gupta D, Dziarski R. Endothelial and epithelial cells do not respond to complexes of peptidoglycan with soluble CD14 but are activated indirectly by peptidoglycan-induced tumor necrosis factor-alpha and interleukin-1 from monocytes. J Infect Dis. 1998;177:1629–1638. doi: 10.1086/515318. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol. 2002;130(1–2):86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- Kielian T, McMahon M, Bearden ED, Baldwin AC, Drew PD, Esen N. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) J Neurochem. 2004;90:1163–1172. doi: 10.1111/j.1471-4159.2004.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- Kusunoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Echchannaoui H, Landmann R, Rivest S. Cooperation between Toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur J Immunol. 2003;33:1127–1138. doi: 10.1002/eji.200323821. [DOI] [PubMed] [Google Scholar]
- Lynn WA, Liu Y, Golenbock DT. Neither CD14 nor serum is absolutely necessary for activation of mononuclear phagocytes by bacterial lipopolysaccharide. Infect Immun. 1993;61:4452–4461. doi: 10.1128/iai.61.10.4452-4461.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21(Suppl 2):S43–S47. doi: 10.1016/s0264-410x(03)00199-3. [DOI] [PubMed] [Google Scholar]
- Manukyan M, Triantafilou K, Triantafilou M, Mackie A, Nilsen N, Espevik T, Wiesmuller KH, Ulmer AJ, Heine H. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur J Immunol. 2005;35:911–921. doi: 10.1002/eji.200425336. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Inestrosa NC. Mannose receptor is present in a functional state in rat microglial cells. J Neurosci Res. 1999;58:387–395. [PubMed] [Google Scholar]
- Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. quiz 780–761. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Flo T, Ingalls RR, Golenbock DT, Teti G, Vogel SN, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, De Winther MP, Makepeace K, Hollinshead M, Coull P, Plested J, Kodama T, Moxon ER, Gordon S. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lip-opolysaccharide and not required for secretory responses. Infect Immun. 2002;70:5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- Saito S, Matsuura M, Tominaga K, Kirikae T, Nakano M. Important role of membrane-associated CD14 in the induction of IFN-beta and subsequent nitric oxide production by murine macrophages in response to bacterial lipopolysaccharide. Eur J Biochem. 2000;267:37–45. doi: 10.1046/j.1432-1327.2000.00956.x. [DOI] [PubMed] [Google Scholar]
- Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Modlin RL. Toll-like receptors: mammalian ‘‘taste receptors’’ for a smorgasbord of microbial invaders. Curr Opin Microbiol. 2002;5:70–75. doi: 10.1016/s1369-5274(02)00288-6. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004;5:1000–1006. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Clark RN, Goyert SM, White JE. Induction of TNF-alpha and MnSOD by endotoxin: role of membrane CD14 and Toll-like receptor-4. Am J Physiol, Cell Physiol. 2001;280:C1422–C1430. doi: 10.1152/ajpcell.2001.280.6.C1422. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Weber JR, Freyer D, Alexander C, Schroder NW, Reiss A, Kuster C, Pfeil D, Tuomanen EI, Schumann RR. Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity. 2003;19:269–279. doi: 10.1016/s1074-7613(03)00205-x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation—a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yang Z, Khemlani LS, Dean DF, Carter CD, Slauson DO, Bochsler PN. Serum components enhance bacterial lipopoly-saccharide-induced tissue factor expression and tumor necrosis factor-alpha secretion by bovine alveolar macrophages in vitro. J Leukoc Biol. 1994;55:483–488. doi: 10.1002/jlb.55.4.483. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]