Abstract
To better understand the role of each of the laryngeal muscles in producing vocal fold movement, activation of these muscles was correlated with laryngeal movement during different tasks such as sniff, cough or throat clear, and speech syllable production. Four muscles (the posterior cricoarytenoid-PCA, lateral cricoarytenoid-LCA, cricothyroid-CT and thyroarytenoid-TA) were recorded with bipolar hooked wire electrodes placed bilaterally in four normal subjects. A nasoendoscope was used to record vocal fold movement while simultaneously recording muscle activity. Muscle activation level was correlated with ipsilateral vocal fold angle for vocal fold opening and closing. Pearson correlation coefficients and their statistical significance were computed for each trial. Significant effects of muscle (p≤0.0005) and task (p=0.034) were found on the r (transformed to Fisher’s Z’) values. All the PCA recordings related significantly with vocal opening while CT activity was significantly correlated with opening only during sniff. The TA and LCA activities were significantly correlated with vocal fold closing during cough. During speech, the CT and TA activity correlated with both opening and closing. Laryngeal muscle patterning to produce vocal fold movement differed across tasks; reciprocal muscle activity only occurred on cough while speech and sniff often involved simultaneous contraction of muscle antagonists. In conclusion, different combinations of muscle activation are used for biomechanical control of vocal fold opening and closing movements during respiratory, airway protection and speech tasks.
Keywords: Correlation, Electromyography, larynx, nasolaryngoscopy, muscle activation, intrinsic laryngeal muscles
I. INTRODUCTION
The human larynx represents a complex arrangement of cartilages and small, compartmentalized intrinsic muscles. Understanding the role of each of these muscles in producing vocal fold movement is needed both for improved understanding of laryngeal biomechanics and for developing new treatments for neuromotor laryngeal disorders. Currently the intrinsic laryngeal muscles can be grouped as adductors for vocal fold closing, abductors for vocal fold opening or lengtheners for increasing vocal fold length or tension when co-contracted with muscles that shorten the vocal folds. Evidence regarding the actions of these muscles comes from several sources: electromyographic (EMG) recordings (16, 18), string models using excised larynges (27); and biomechanical modeling (55). EMG studies of muscle activity have concentrated on muscle activity during a single task such as respiration (35), cough (52), swallowing (50) and speech (43). The posterior cricoarytenoid (PCA) is usually most active during inspiration and is considered a vocal fold opening muscle (41) while the thyroarytenoid (TA) is active for vocal fold closing during both cough and speech (20, 52). The cricothyroid (CT) muscle is active for vocal fold opening during inspiration (49) but also is active during vocal fold lengthening with increases in voice pitch (21). Studies during different speech gestures have found that the PCA is inactive during closing for voice production while the TA, lateral cricoarytenoid (LCA) and interarytenoid (IA) are active during voicing (15, 17, 20, 23, 25). Studies examining the relationship of the CT and the TA muscles have found that these tend to co-contract during pitch elevation, most likely stiffening the folds due to their opposing actions of shortening and lengthening the two folds (54). None of these studies, however, have addressed the relationship between muscle activation and the resulting vocal fold movement. Few studies have examined the role of the laryngeal muscles in producing movement between the different laryngeal functions such as respiration, cough and speech. It is not known, therefore if these different systems pattern the laryngeal muscles differently to produce vocal fold movement.
One approach to determining the role of each of the laryngeal muscles in vocal fold movement is to use strings or force application to emulate the action of muscle contraction in excised larynges (3, 27). Considerable strides have been made in understanding the biomechanics of vocal fold vibration using excised larynges (53). Such studies, however, may not accurately predict the biomechanical effects of intact normal human intrinsic laryngeal muscle contraction because simultaneous forces generated by contractions of multiple muscles could alter the resultant movement. Further, no information on the central nervous system control is available from these studies.
In vivo studies, therefore, relating muscle activation to movement or biomechanical force may be more representative of the use of the laryngeal muscles in humans (10-12). Several studies have focused on the roles of the intrinsic musculature in respiration (6, 28-30, 34, 36-40, 42, 56-58). Little attention, however, has focused on understanding the dynamic control of laryngeal opening and closing for different tasks such as phonation onset and offset, swallowing, cough, etc.
Different central nervous system patterning of laryngeal muscle activation may be used for respiration, deglutition and speech because the motor control demands on movement speed, extent and precision may differ between tasks. Speech production requires the most rapid and precise control of vocal fold movement in the larynx; the changes in muscle activation during speech in contrast with cough for example, are small and rapid (46). As speakers produce syllables with voiceless consonants and vowels, voice onset and offset is controlled by several different gestures (43). These include: hyperadduction to offset voice, a gesture known as a glottal stop /?/ which is often used to mark word boundaries; vocal fold partial opening for voice offset to produce voiceless consonants such as /h/ and /s/; full adduction or closing to onset phonation following inspiration, and partial adduction to produce voice onset following a voiceless consonant as in the syllable /si/.
Observations of the relationship between EMG patterns and speech sound production have assumed that similar movements occurred during each utterance (17). Speakers may approach the production of some gestures differently from other speakers and movements may vary from one production to another within a speaker. EMG recordings also show a great deal of variation during repetition of the same speech sounds in normal speakers (48).
One interpretation of the variation in EMG patterns during repetition of speech gestures has been that individuals learn to use alterations in subglottal air pressure to contribute to vocal fold opening and closing making voice changes less dependent upon laryngeal muscle activation (48). The first step in examining this hypothesis is to determine if vocal fold movement is closely related to intrinsic laryngeal muscle activation. If this is the case, then subglottal pressure may not be sufficient for vocal fold opening and closing gestures for some speech sounds.
Data on the dynamic inter-relationships between intrinsic muscle activations and different laryngeal configurations would provide valuable insight into laryngeal biomechanics and would aid greatly in our understanding of laryngeal control. Intramuscular EMG recordings can provide practical estimates of muscle activation. The relationship between EMG and muscle force, however, is only well understood for static contractions; no generalized model has been developed to relate EMG to muscle fiber force production during movement. Measured EMG also varies with electrode placement in a muscle, and the EMG patterns may vary between individuals, even when producing the same movement or gestures. Additionally, all the intrinsic muscles may interact to control vocal fold adduction and abduction during speech (48).
There is an approximately linear relationship between EMG and active isometric muscle force production (2, 5, 7, 26, 31), at least when the correct electromechanical delay is considered (8). Good correlations have been shown between EMG, intramuscular force and isometric force, for arm (33) and shoulder (31) muscles, but the EMG/force relationship is only linear for isometric contractions and depends on velocity, position, and on whether the contraction is eccentric or concentric (26). Previous studies have related laryngeal EMG and either photoglottography, which reflects the overall degree of opening of the glottis during speech (44, 47), or the anterior glottal angle measured through nasolaryngoscopy (42). These techniques, however, do not allow matching the movement with muscle activation information for the same vocal fold. This study examined the relationship between the EMG and ipsilateral vocal fold movements.
It is well recognized that there is a delay between muscle activation and the resultant movement (8). The duration of the delay will depend upon several factors including: i) the contraction time of the muscle; ii) the relationship of force generated by the muscle to the ongoing level of activation; iii) the number and size of the motor units recruited; iv) the timing of co-contraction of muscle antagonists; and v) tissue compliance. The first three factors depend upon the characteristics of the particular muscle; therefore, we measured the delay between muscle contraction onset and the movement onset, for each muscle in each subject for each task. Then, using the median delays computed for each muscle across al subjects and tasks, we measured the correspondence of muscle EMG and vocal fold movement, by computing a point-by-point Pearson cross-correlation coefficient for each trial.
Another way to determine the delay between muscle contraction and he resulting movement is to calculate the correlation between muscle activation and movement signals at different delays between the onset of the two signals to identify at what delay the correlation is greatest. We designated this the optimum delay and examined the correspondence between these two approaches.
Given the previous findings regarding the role of the intrinsic laryngeal muscles in speech and other gestures, we proposed the following hypotheses regarding the role of the intrinsic laryngeal muscles in sniffing (maximum vocal fold opening), cough (forceful vocal fold closing) and rapid alternation between partial opening and partial closing for repetition of syllables with voiceless consonants and vowels (e.g. /hihihihi/ or /sisisisi/).
The PCA activation is positively correlated with vocal fold abduction, with maximum correlations occurring for rapid and unopposed opening gestures such as sniff;
The TA and LCA muscle activations are negatively correlated with vocal fold abduction, with maximum negative correlations occurring for unopposed, ballistic closing gestures such as cough;
Activations of both abductors and adductors are less well correlated with vocal fold abduction during speech due to antagonist co-contraction and the possible role of air pressure;
The direction of the correlation for CT depends upon the gesture being produced, with maximal positive correlation for gestures that require vocal fold lengthening, such as sniff.
II. METHODS
A. Subjects
The subjects (3 men, 1 woman, aged 23-36 years) participated after giving informed consent. All procedures were approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke. Subjects provided a medical history and received a physical examination. An otolaryngologist confirmed normal laryngeal structure and function via a nasolaryngoscopy exam. All subjects were without speech or neurological abnormalities.
B. Task Training and Tasks
Prior to the study, each subject was trained in performing the muscle verification and laryngeal movement tasks to be performed. The muscle verification tasks included: valsalva produced with the mouth open with a glottal release to demonstrate that closure was only at the glottis; ascending and descending pitch glides on the vowel /i/, prolongation of the vowel /i/ for 3 to 5 s, deep respiration, and swallow.
The experimental tasks were selected to provide rapid vocal fold movements that could be easily visualized using nasolaryngoscopy. Visualization during phonatory tasks was optimized by using the vowel /i/ to move the tongue and epiglottis forward. Tasks were selected that required rapid dynamic vocal fold movement. A sniff produces rapid maximum vocal fold abduction (opening). Rapid repetitions of /hi/ required alternating between a glottal fricative with the vocal folds open for /h/ then vocal fold closure for the vowel /i/, repeated 5 to 7 times on one exhalation. Rapid repetition of /si/ required alternating between partial opening of the vocal folds for the /s/ accompanied by a fricative in the oral cavity followed by rapid closing for the vowel /i/, repeated 5 to 7 times on one exhalation. Cough required a rapid vocal fold closure, build up of subglottal pressure, followed by a rapid vocal fold opening with expulsion of air. Finally “sniff-i” included a rapid vocal fold opening for the sniff followed by a rapid closure for the vowel /i/. The initial design of the study included repetition of the vowel /i/ with phonation offset by a glottal stop. However, it was quickly observed that this voice offset was not accompanied by a change in vocal fold position. The vocal folds remained constantly adducted during rapid repetition of /?i/ while the ventricular folds produced further medialization, squeezing the vocal folds further together to offset voice. Because no change in vocal fold position occurred on this task, the correlation between movement and EMG could not be measured and it was eliminated from the study.
Subjects were trained to produce the speech syllable repetitions at a relatively fast rate, between 5 and 7 repetitions in 1 to 2 seconds while the non-speech gestures such as cough and sniff were produced at ≈1 per second. Subjects were also trained not to shift their voice pitch during performance of any of the tasks to avoid active changes in fundamental frequency or vocal fold tension.
C. Electrode placements and recording procedures
Hooked wire electrodes were placed in four intrinsic laryngeal muscles: two vocal fold adductors (TA and the LCA), one abductor (the PCA), and the CT. Small subcutaneous injections of 1% Xylocaine with epinephrine (1:100,000) were used to reduce discomfort during electrode insertion. Electrodes were placed with the subject supine in a dental exam chair. We first used a 27-gauge bipolar needle electrode with a 0.015 mm2 recording surface (DISA 13K80, Medtronic, Minneapolis) to locate a muscle prior to inserting bipolar, hooked-wire electrodes. Our aim was to record from each of the muscles on the two sides to relate to movement of the fold on the same side. Placement of electrodes into the PCA muscle requires twisting the larynx to allow access to the posterior larynx (4). This laryngeal movement usually dislocates wires already placed in other muscles, therefore placement in only one PCA muscle was attempted in each subject prior to insertion of the other electrodes. The same direction of insertion was used for the TA and the LCA; through the cricothyroid membrane and directed superiorly and laterally into the muscle. The LCA placement was more posterior and lateral to position the wires in the portion of the muscle that inserts on the edge of the cricoid cartilage (9). We confirmed proper electrode positioning using the following verification gestures (20). For TA placement, activation increased during a prolonged vowel, throat clear, effort closure (valsalva), and swallow. Verification gestures for the LCA included increased activation during swallow, throat clear and effort closure with onset and offset bursts for phonation onset and offset but no sustained activation during prolonged phonation. CT verifications were increased activation during the high end of a pitch glide but no activation during swallow. Absence of activity with head raise or turning indicated no contamination of the CT signals with strap muscle activity. PCA verification consisted of increased activity during deep inhalation but no activation during swallowing or vowel prolongation.
Only recordings meeting the verification criteria were used in the study, consequently only a subset of muscles was available on each subject. Each of the 4 muscle types (PCA, CT, TA and LCA) was available on at least one side in each subject except the PCA, which met the verification criteria in only 3 of the 4 subjects.
D. Nasolaryngoscopy and Recording
Four percent Lidocaine was sprayed into one nostril to relieve discomfort during insertion of the Pentax PNL-10RP3 fiberoptic laryngoscope (Pentax Precision Instruments, Orangeburg, NY). The laryngoscope was interfaced with a Pentax camera and a Kay Elemetrics Digital Stroboscope system (Kay Elemetrics Corporation, Lincoln Park, NJ) with a halogen light source. The head-held microphone was placed 2 inches from the subject’s mouth. Marks on the scope where it exited the nares helped the physician maintain the same scope position throughout the recording. The video and audio signals were recorded at 29.97 frames/s on a Panasonic VHS-s recorder. This yielded 2 fields of video image per frame, or 59.94 fields/s. A Horita TRG-50 PC produced SMPTE timestamps that were visible in each field of the video image. Speech was recorded with a Sony EM-2 head-held microphone.
Raw, amplified, bipolar hooked-wire EMG signal recordings were band-pass filtered between 30 and 3000 Hz on a Nicolet Viking V and output to a multiple channel FM instrumentation tape recorder (TEAC XR-70) along with the voice signal and the Horita signal. The binary timestamp signal of the Horita was also streamed simultaneously to both the VHS-s and the FM instrumentation recorder along with the EMG signals to allow time alignment of the video and EMG signals. Interruptions of the timestamp signal before and after the session facilitated the time alignment. The EMG signals were digitized off-line at 6,000 samples/s, after 8th order Butterworth anti-alias filtering at 2 kHz along with the voice signal.
With the fiberoptic nasolaryngoscope in place, recording was started, the time stamp signal interrupted, then the subject was instructed to imitate the examiner on 6 tasks: 1) volitional cough/throat clear; 2) sniff; 3) repeating /hi/; 4) repeating /i?/; 5) repeating /si/; and, 6) repeating sniff followed by /i/. Not all subjects performed all tasks; tasks 5 and 6 were only conducted in one subject.
We used a Peak Performance (Motus) system to digitize each video field. An experimenter identified four points on each digitized video field (Fig. 1): the anterior commissure (AC); the tip of the vocal process of the left arytenoid (LVP); the tip of the vocal process of the right arytenoid (RVP); the midpoint of the posterior glottal wall (PGM). Cases where the labeled structures were not clearly visible were not used, with the exception of the midpoint of the posterior wall, which had to be inferred during tight adduction when the vocal folds were closed. The midline of the glottis was defined as the line connecting the anterior commissure to the midpoint of the posterior glottal wall. Likewise, lines were defined from the vocal process of the arytenoid on each side to the anterior commissure. The angle between the line from the vocal process to the anterior commissure and the midline was the angle reflecting the position of that vocal fold. The angle was zero when the vocal process was exactly at the midline. For each video frame analyzed, the Peak Performance software exported the angle values to a text file. A Matlab program was written to read the angle data files and normalize all the angles to the maximum angle recorded for that subject over the entire session. The angle data were time-aligned with the processed EMG data based on the time stamps. Interruptions in the time stamps at the beginning and end of the sessions were evident in both the data recorded on the FM tape and the video frames, allowing an investigator to time-align the angle data with the EMG data to within one video field sampling interval (±16.68 ms).
Figure 1.
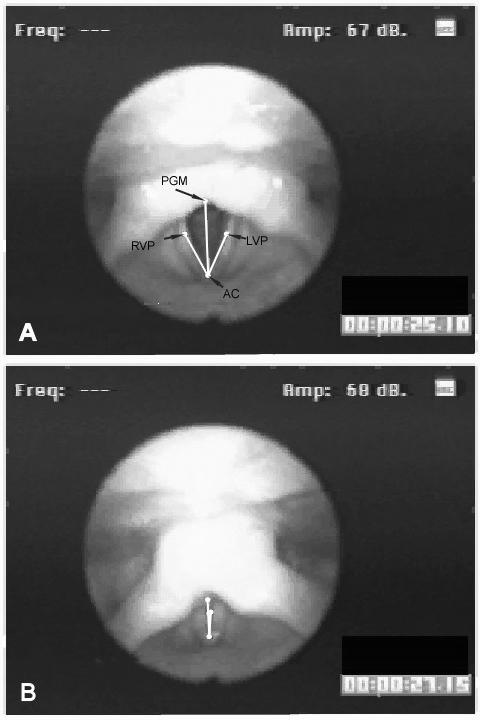
A Example video field showing the marked points and lines used to compute vocal process angles: the anterior commissure (AC), left and right tips of the vocal processes of the arytenoids (LVP and RVP), and the midpoint of the posterior glottal wall (PGM). We defined three angles from these four points: the left vocal process angle, #x2222;(LVP, AC, PGW), the right vocal process angle, #x2222;(RVP, AC, PGW), and the angle of approximation, #x2222;(RVP, AC, LVP). Panel A shows the vocal processes almost fully abducted; Panel B shows the vocal processes fully adducted.
D. Data analysis
The custom Matlab software removed the DC offset from the unrectified EMG data by subtracting the mean offset. The EMG signals were then full-wave rectified and smoothed using a zero-phase-shift, 6th order Butterworth filter with a cutoff frequency of 8 Hz. The smoothed signals were then down-sampled to the same sampling times as the video data points through piecewise cubic Hermite interpolation. The smoothed EMG signals were then normalized to the maximum amplitude across gestures for that muscle and subject.
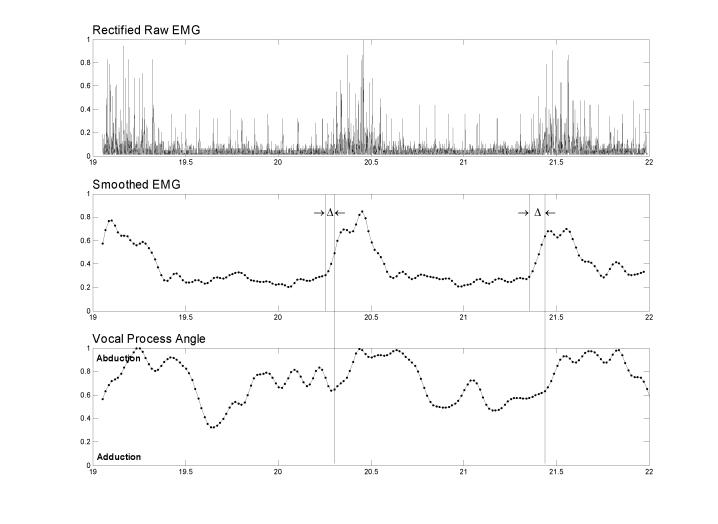
A delay was expected between the onset of muscle activation and the change in vocal fold position because of contraction time, biomechanical damping, and antagonistic muscle co-contraction. Because this information was not known for these gestures where many muscles were active simultaneously, we calculated the delays between muscle activation onset and the onset of change in vocal fold angle on the same side during tasks where the muscle was expected to be the prime mover (Fig. 2.). The EMG burst onset times were chosen manually with visual inspection, using both the raw and the smoothed EMG for guidance, and without the angle data visible. The primary criterion used was the earliest sign of a sustained increase in the slope of the smoothed EMG (i.e. both the velocity and acceleration of the smoothed EMG were positive). The gestures used to calculate the delays were sniff for PCA, cough and phonation onset for the TA and the LCA. Calculating delays for CT was complicated by the fact that CT rarely acts alone to produce either abduction or adduction. The sniff task showed the greatest unopposed CT activation and was therefore used to compute delays for the CT. The median delay across subjects was then computed and used as the delay for all subjects/gestures for that muscle. These median delays were then rounded to the time of the closest video field, making them accurate to ±16.68 ms.
Figure 2.
An example of how the EMG-to-movement delays were calculated from the raw PCA EMG, smoothed PCA EMG, and ipsilateral vocal process angle data for a series of three sniffs. The delays (△) between the onset of muscle activation (short vertical lines in the middle panel) and the onset of the change in vocal fold angle (long vertical lines that span the panels) for the same side during tasks where the muscle was expected to be the prime mover were calculated as shown in this example. The median of all the delays for each muscle was used to calculate the correlations. The delays were rounded to the nearest multiple of the angle data sampling rate (16.68 ms) because they had to be an integer number of samples. The abscissa units are seconds since the beginning of the experimental session and the ordinates are normalized to their maximum values during the session for that subject.
To assess the strength of the relationship between muscle activation and vocal fold movement, we calculated the Pearson’s correlation coefficient, r, between the smoothed EMG and the ipsilateral vocal process angles for each gesture and subject. Because the length of the recordings differed (between 1.03 and 3.55 s) resulting in different sample sizes (between 62 and 213 with a mean N = 142) and because the Pearson’s correlation coefficient is not normally distributed, we used the Fisher’s Z’ transform to calculate confidence intervals and to test for statistical differences between the correlation coefficients. To account for the delay between the onset of muscle activation and the onset of movement, we shifted the EMG signal forward in time by the median onset delay across all subjects for that muscle before calculating the correlations. In this way, the same delay was used to calculate the correlations for every subject and gesture for a given muscle.
We confirmed that the median onset delays described above were appropriate by also calculating the optimum delay that maximized the strength of each correlation between muscle activity and vocal fold angle for each trial. The optimal delay was found by comparing the r values at different delays. These delays were for comparison purposes only; we could not use the optimal delays to compute the correlations we reported because they would have biased the correlation coefficient (by violating the assumption of random sampling). The “optimal” delays were the averages of those that maximized the correlations, whereas the “median” onset delays were the medians of those found by comparing the differences in onset times between the EMG and movement signals.
III. RESULTS
We successfully recorded EMG and synchronized video from 17 muscles in four subjects. The specific muscles for each subject are listed in Table 1. The median movement onset delays relative to EMG onset were about twice as long for the PCA than for the CT, LCA and TA muscles (Table 2). The delay values rounded to the time of the closest video field (a multiple of 16.68 ms) appear in column 3 of Table 2. The optimal delays that maximized the strength the correlations for each muscle are provided in the last column of Table 2. Although the optimum delays were not used for computing the correlations as explained earlier, these optimum delay values were similar to the median delay values rounded to the nearest 16.68 ms, confirming the adequacy of the rounded median delay values.
Table 1.
Verified muscle recordings completed in each subject and the gestures sampled during videotaping that could be analyzed because of an adequate view of both vocal folds
| Subject Number | Left Side Muscles | Right Side Muscles | Gestures |
|---|---|---|---|
| 1 | LCA, TA | CT, PCA | cough, /hi/, sniff, /i/ |
| 2 | LCA, PCA, TA | CT, TA | /hi/,sniff,/i/ |
| 3 | LCA, TA | CT, PCA | cough, /hi/, sniff, /i/, sniff-/i/ |
| 4 | CT, TA | LCA (x2) | cough, /hi/, sniff, /i/, /si/ |
Table 2.
Mean delays (± standard deviations) measured between EMG and movement onsets, values used rounded to time of closest video field time multiple (delays used to compute correlations) and delays producing optimal r values
| Muscle | Delay between EMG and movement onsets | Delay rounded to time of closest video field | Delay producing optimal correlations |
|---|---|---|---|
| PCA | 68 ms | 66.7 ms | 93 ms ± 98 ms |
| TA | 31 ms | 33.4 ms | 38 ms ± 49 ms |
| CT | 42 ms | 33.4 ms | 34 ms ± 79 ms |
| LCA | 31 ms | 33.4 ms | 23 ms ± 42 ms |
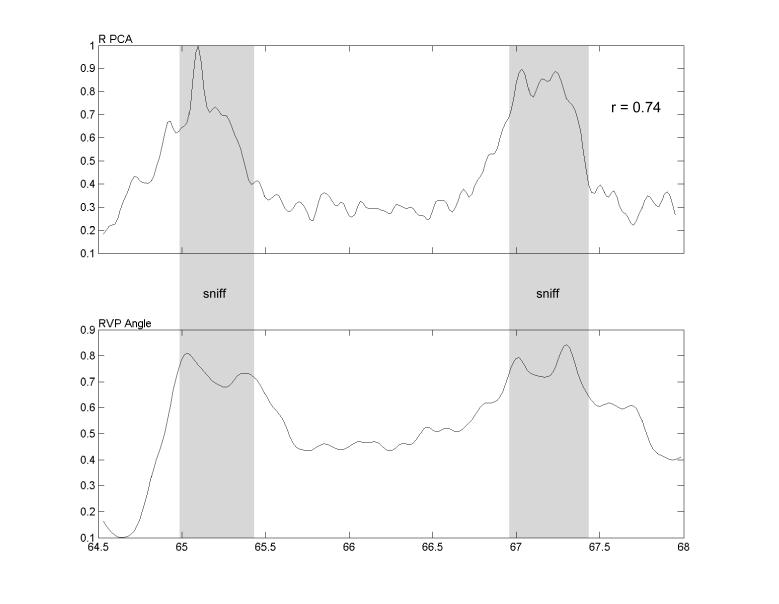
Positive correlations resulted from a muscle being increasingly active while the vocal fold angle increased (abduction). Negative correlations indicated that the muscle was increasingly active during adduction. A typical example of an EMG-movement pair that provided a high correlation (r = 0.74) is shown in Fig. 3. In this example, the subject sniffed twice and the EMG of the PCA, was highly correlated with ipsilateral vocal fold opening. In general, the highest correlations were found for PCA during sniff.
Figure 3.
The subject sniffed twice in this example of a typical trial with high correlation between muscle activation (EMG) and vocal process angle. The top trace displays the smoothed, rectified EMG from the right PCA muscle and the bottom trace shows the resulting ipsilateral vocal process angle. The shaded bands indicate the intervals during which the sniff sounds were audible. The abscissa units are seconds and the ordinates are normalized to their maximum values during the session for that subject.
The tasks (sniff, cough/throat clear, repeated /hi/, repeated /si/, and repeated sniff-i) were sub-grouped as either “cough” that included cough and throat clear, “sniff” that included sniff and sniff followed by the vowel /i/, or “syllable” that included repeated /hi/ and repeated /si/. An ANOVA was computed to examine the main effects of three factors: muscle type (PCA, TA, LCA and CT), side (Right versus Left), and task type (cough, sniff and syllable) on the Z’ values to determine if the correlations between muscle EMG and movement were affected by the three factors or their interactions. Significant differences were found for muscle type (F=10.033, df=3, p≤ 0.0005) and task (F=2.608, df=2, p=0.034) but not for muscle side (F=0.295, df=1, p=0.589). Muscle by task interaction effects approached significance (F=1.841, df=15, p=0.053), reflecting the variable roles of the TA, LCA and CT according to task. Because side did not differ, results from the same muscle on the two sides were combined in further examinations of the results.
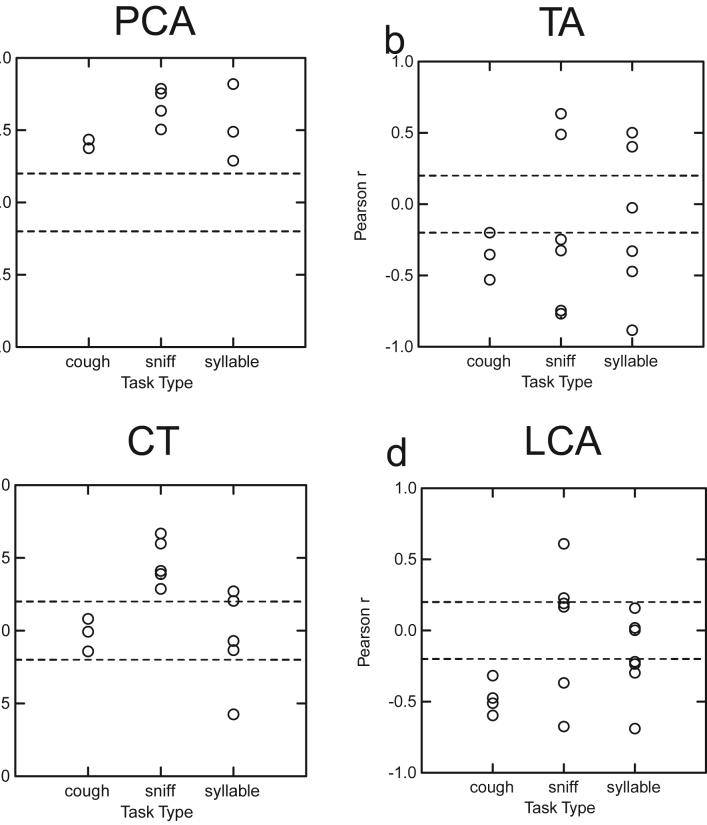
Each of the r values are displayed in Fig. 4 for each muscle according to task type. Because the number of points used to calculate the correlations varied from 62 to 213 (mean = 142), the exact value of r required for statistical significance at p=0.05 varied from trial to trial; however, the approximate correlation values required for significance are indicated by the hatched lines in Fig. 4.
Figure 4.
Plots of Pearson’s correlation coefficients, r, between muscle EMG and vocal process angle are plotted for each task, in separate plots for each muscle. Each circle represents a separate trial. In each case, positive correlations indicate that increases in muscle activity occurred during abduction of the vocal processes, whereas negative correlations indicate activation increased with adduction. Because the number of samples in each correlation varied, the exact value of r required for statistical significance also varied. The dashed lines, however, indicate reasonable approximations of the r values required for significance.
All 9 of the PCA r values were significant and positive demonstrating a significant relationship between muscle EMG amplitude and vocal fold opening regardless of task (Fig. 4a). For the TA muscle, 13 of the 14 r values were statistically significant; 10 of these were in the negative direction indicating that in 71% of the trials TA activation was correlated with vocal fold closing (Fig. 4b). TA EMG was always correlated with closing in cough but correlated significantly with both opening and closing during sniff and syllable repetition. CT r values were significant in 8 (62%) of the 13 trials; 7 of these were positive indicating a correlation with vocal fold opening in almost all cases. In Fig. 4c all of the CT r values during sniff and two of the syllable repetition tasks had significantly positive r values. No significant correlations were found during cough. The LCA r values were significant in 12 (71%) of the trials; 10 of these were negative demonstrating a correlation with vocal fold closing (Fig. 4d). During cough and speech, all of the significant LCA r values during cough and speech were in the negative direction showing a correlation with vocal fold closing. Significant r values during sniff, however, were equally divided in the positive and negative directions.
III. DISCUSSION
The strength and direction of the correlation between the rectified, smoothed EMG of 17 intrinsic laryngeal muscle samples (PCA, TA, CT and LCA) and the abduction angle of the ipsilateral vocal process differed between laryngeal gestures (e.g. cough, repeated-/hi/, sniff). We hypothesized that correlations for the primary adductors and abductors would occur in gestures where they were the prime movers and were largely unopposed by antagonists. These gestures were sniff for the abductor, PCA, cough for the adductors, TA and LCA, and sniff for the CT. Activation in the PCA was significantly and positively correlated with vocal fold abduction in every trial, regardless of task. As expected, correlations for the sniff task were consistently among the highest, however, the trial with the highest correlation for PCA was a speech (syllable repetition) gesture. Likewise, significant negative correlations for the TA and LCA occurred during cough. However, the trials with highest negative correlations for each muscle were for the syllable repetition speech tasks. Although the significant correlations were in the hypothesized direction, the highest correlations did not occur on the expected tasks for the first two hypotheses.
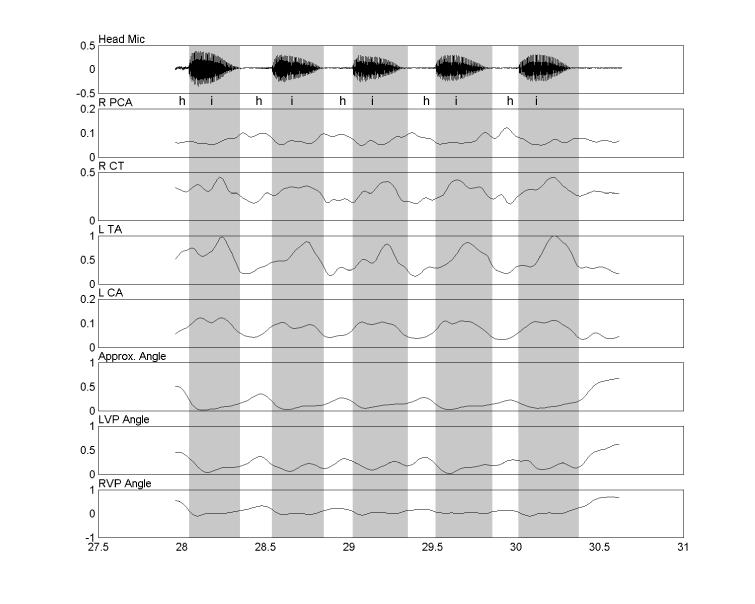
Our third hypothesis, that correlations would be lowest for the speech tasks was neither fully confirmed nor discounted. Some high correlations occurred for both abductors and adductors during the speech tasks. The TA and LCA correlations were not consistent in magnitude or direction and the correlations that were all negative varied in magnitude. During voiceless consonant syllables, the LCA and PCA served more as reciprocal pairs, with the PCA correlated with opening (abduction) and the LCA only being significantly correlated with vocal fold closing (adduction) as has previously been reported (23, 43). This pattern can be seen in Fig. 5, where the subject repeatedly produced the syllable /hi/. During the vowels, the CT and TA tended to co-contract, perhaps to control vocal fold stiffness as has previously been reported during pitch change (21, 54). The variation in direction and degree of correlation during speech suggests that all of the muscles are active and that the resultant movement is the sum of these various forces acting on the cricoarytenoid joint. Thus, many combinations of laryngeal activation levels may be associated with the same movement, and vice versa, as has previously been observed (48).
Figure 5.
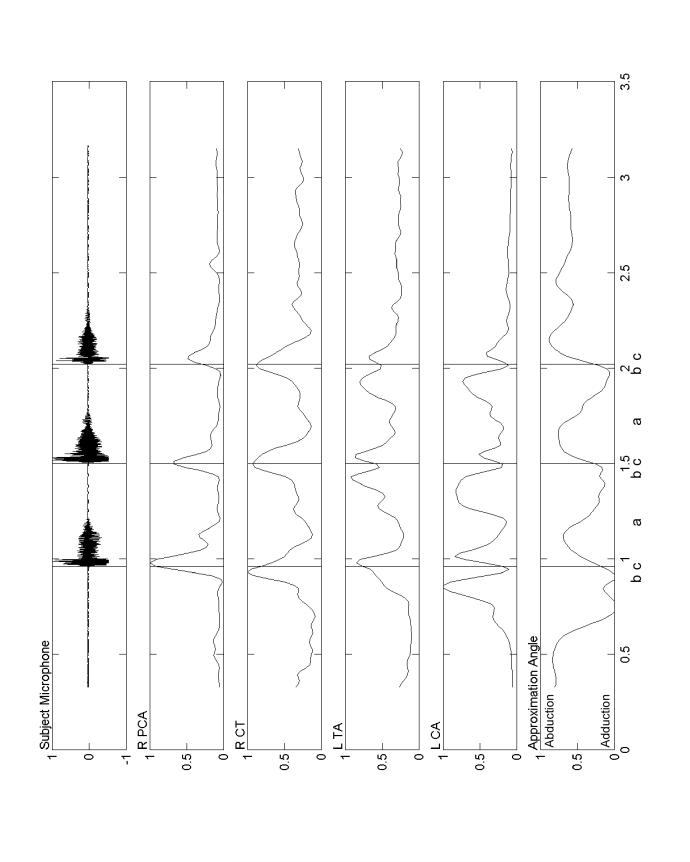
Repeated productions of the syllable /hi/. The acoustic signal, and a phonetic transcription, appears in the top panel. Rectified, smoothed EMG signals are shown in the next four panels. The last three panels display the vocal process angles. Darker shading indicates intervals where the angle of approximation is reduced because of closure for vocalization. The reciprocal relationship between the adductors (LCA and TA) and the abductor (PCA) is clearly evident in each repetition of /hi/. The abscissa units are seconds and the ordinates are normalized to their maximum values during the session for that subject.
Significant correlations also occurred in the CT, although this muscle is thought primarily to have vocal fold lengthening and tensing actions. The significant correlation with vocal fold opening only occurred during sniff when rapid opening is associated with observable lengthening of both vocal folds. The low correlation of CT activation with opening during speech may have occurred because lengthening was not involved. During voiceless consonant syllable repetition, the action of the CT was much less clear; with one negative and two positive significant correlations. Because the speakers were trained not to shift their voice pitch during syllable repetition, CT activity changes were unlikely to be due to active changes in fundamental frequency or vocal fold tension. No significant correlations occurred for the CT during cough. Therefore, CT correlations with movement differed with tasks as was predicted by our fourth hypothesis.
A low correlation does not necessarily imply that the muscle was not active during a given task, only that it was not reliably associated with vocal fold adduction or abduction. For example, a series of three coughs appears in Fig. 6. The airflow timing is indicated by the subject microphone acoustic signal. Although the CT was active during cough, its correlation was nearly zero (rCT = -0.007) while the correlations fo the other muscles were higher (rLCA = -0.511, rTA = -0.200, rPCA = 0.375), possibly because its activity spanned both the closing and opening phase. Others have observed CT activation that was not clearly in phase with either vocal fold abductors or adductors (52), consistent with our results. The PCA correlation, though significant, was also lower during cough than during sniff or speech. This may be because both air pressure and PCA activation contributed to vocal fold opening in these gestures.
Figure 6.
Three successive coughs. Rectified, smoothed EMG from four muscles is shown along with time-aligned vocal fold approximation angle and the acoustic signal. Initially (a), the LCA, then CT and TA were activated before and during glottal closure. Following vocal fold adduction (b), the PCA activated, the LCA became silent, followed by a gradual abduction of the vocal folds. Just before the peak of the PCA activation (c), the glottis was large enough to allow airflow and sound production. This process was repeated for each of the three coughs shown here. The abscissa units are seconds and the ordinates are normalized to their maximum values during the session for that subject.
The delays observed between the onset of EMG and vocal process movement were consistent with the physiology of the intrinsic laryngeal musculature. The TA, CT and LCA had roughly similar, short movement onset latencies while the PCA latencies were roughly twice as long. The TA, CT and LCA muscle are known to be primarily fast-twitch muscles, whereas the PCA is predominately slow-twitch (1, 13, 14) as would be expected based on differences in the ratio of type I and Type II myosin in these muscles (51).
One of the limitations of using the nasoendoscope for image acquisition was demonstrated by the results of our repeated-/i/ gesture. During this gesture, the vocal processes remained adducted with very little change in position, while the EMG signals fluctuated. Likely three dimensional imaging of the tract would have shown that the extent of closure was changing in the superior-inferior dimension within the glottis that could not be seen from the nasoendoscope. Spiral computerized tomography imaging of the vocal tract could provide increased information on changes in vocal tract shaping and the superior-inferior extent of movement associated with adductor muscle activity. The use of computerized tomography, however, would require extensive event-related sampling to visualize changes in shape associated with rapid changes in laryngeal muscle activity (45), causing significant radiation exposure in subjects.
Another limitation was the low sampling rate of the video-recording, with an inter-video field interval of 16.68 ms. High speed video recording techniques are available and could be used in future studies to provide better time resolution (22, 24). Because EMG activity is roughly proportional to active muscle force, the EMG should correlate better with the angular acceleration of the vocal processes than with the vocal process angle. Unfortunately, the coarse temporal resolution of the vocal process angle signal we acquired was not sufficient to allow accurate, stable computations of its derivatives. High speed imaging at 2000 images/s would be adequate and recent technologies have improved image resolution that would now allow identification of the vocal fold processes for tracking. Because of the need for bright illumination, however, most of these systems can only be used with a rigid oral scope, restricting the range of tasks that can be sampled.
Overall, given these limitations, the results from this study are promising. The expected significant correlations between muscle activation and vocal fold position were found. The pattern of correlations differed between tasks, however, demonstrating that vocal fold movement may be achieved somewhat differently for respiratory, airway protection and speech tasks. This will limit the ability to use the same biomechanical models of muscle activity to predict movement across tasks. Different biomechanical models may need to be developed for different tasks. The central movement patterning for respiration, cough and speech may be inter-related but independent in the medulla (19, 32, 59). It is not surprising, therefore, that muscle activation patterning for vocal fold movement may differ between these functions.
Acknowledgements
The authors wish to thank Ms. Mary Zakria and Dr. W. Scott Selbie for their help with the technical setup and data collection.
References
- 1.Alipour-Haghighi F, Titze IR, Durham P. Twitch response in the canine vocalis muscle. J Speech Hear Res. 1987;30:290–294. doi: 10.1044/jshr.3003.290. [DOI] [PubMed] [Google Scholar]
- 2.Aoki F, Nagasaki H, Nakamura R. The relation of integrated EMG of the triceps brachii to force in rapid elbow extension. Tohoku J Exp Med. 1986;149:287–291. doi: 10.1620/tjem.149.287. [DOI] [PubMed] [Google Scholar]
- 3.Berry DA, Montequin DW, Chan RW, Titze IR, Hoffman HT. An investigation of cricoarytenoid joint mechanics using simulated muscle forces. J Voice. 2003;17:47–62. doi: 10.1016/s0892-1997(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 4.Blitzer A, Brin M, Stewart C, Aviv JE, Fahn S. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope. 1992;102:163–167. doi: 10.1288/00005537-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Blumen MB, Perez de La Sota A, Quera-Salva MA, Frachet B, Chabolle F, Lofaso F. Genioglossal electromyogram during maintained contraction in normal humans. Eur J Appl Physiol. 2002;88:170–177. doi: 10.1007/s00421-002-0697-y. [DOI] [PubMed] [Google Scholar]
- 6.Brancatisano A, Dodd DS, Engel LA. Posterior cricoarytenoid activity and glottic size during hyperpnea in humans. J Appl Physiol. 1991;71:977–982. doi: 10.1152/jappl.1991.71.3.977. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TS, Moniz MJ, Dewald JP, Zev Rymer W. Estimation of muscle forces about the wrist joint during isometric tasks using an EMG coefficient method. J Biomech. 1993;26:547–560. doi: 10.1016/0021-9290(93)90016-8. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell GE, Li L. How strongly is muscle activity associated with joint moments? Motor Control. 2000;4:53–59. doi: 10.1123/mcj.4.1.53. discussion 97-116. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos PF, Gates GA, Esselman G, Song F, Vannier MW, Kuo M. Anatomic considerations in botulinum toxin type A therapy for spasmodic dysphonia. Laryngoscope. 1994;104:656–662. doi: 10.1288/00005537-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Choi HS, Berke GS, Ye M, Kreiman J. Function of the posterior cricoarytenoid muscle in phonation - in-vivo laryngeal model. Otolaryngology-Head and Neck Surgery. 1993;109:1043–1051. doi: 10.1177/019459989310900612. [DOI] [PubMed] [Google Scholar]
- 11.Choi HS, Berke GS, Ye M, Kreiman J. Function of the thyroarytenoid muscle in a canine laryngeal model. Annals of Otology,Rhinology and Laryngology. 1993;102:769–776. doi: 10.1177/000348949310201006. [DOI] [PubMed] [Google Scholar]
- 12.Choi HS, Ye M, Berke GS. Function of the interarytenoid(IA) muscle in phonation: in vivo laryngeal model. YonseiMed J. 1995;36:58–67. doi: 10.3349/ymj.1995.36.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DS, Pinczower E, Rice DH. Thyroarytenoid intramuscular pressures. Ann Otol RhinolLaryngol. 1993;102:167–175. doi: 10.1177/000348949310200302. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DS, Shindo M, Hast MH, Sinha U, Rice DH. Dynamic properties of the posterior cricoarytenoid muscle. Ann Otol Rhinol Laryngol. 1994;103:937–944. doi: 10.1177/000348949410301203. [DOI] [PubMed] [Google Scholar]
- 15.Faaborg-Andersen K. Electromyographic investigation of intrinsic laryngeal muscles in humans. Acta Physiologica Scandinavica. 1957;41(140):1–149. [Google Scholar]
- 16.Faaborg-Anderson K. Electromyographic investigation of intrinsic laryngeal muscles in humans. ActaPhysiolScandSuppl. 1957;41:140. [Google Scholar]
- 17.Gay T, Hirose H, Strome M, Sawashima M. Electromyography of the intrinsic laryngeal muscles during phonation. Ann Otol Rhinol Laryngol. 1972;81:401–409. doi: 10.1177/000348947208100311. [DOI] [PubMed] [Google Scholar]
- 18.Gay T, Strome M, Hirose H, Sawashima M. Electromyography of the intrinsic laryngeal muscles during phonation. Annals of Otology,Rhinology and Laryngology. 1972;81(3):401–409. doi: 10.1177/000348947208100311. [DOI] [PubMed] [Google Scholar]
- 19.Gestreau C, Bianchi AL, Grelot L. Differential brainstem fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosc. 1997;17:9340–9352. doi: 10.1523/JNEUROSCI.17-23-09340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano M, Ohala J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res. 1969;12:362–373. doi: 10.1044/jshr.1202.362. [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Ohala J, Vennard W. The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation. J Speech Hear Res. 1969;12:616–628. doi: 10.1044/jshr.1203.616. [DOI] [PubMed] [Google Scholar]
- 22.Hirose H. High-speed digital imaging of vocal fold vibration. Acta Otolaryngol (Stockh) 1988;(458):151–153. doi: 10.3109/00016488809125120. [DOI] [PubMed] [Google Scholar]
- 23.Hirose H, Gay T. The activity of the intrinsic laryngeal muscles in voicing control: an electromyographic study. Phonetica. 1972;25:140–164. doi: 10.1159/000259378. [DOI] [PubMed] [Google Scholar]
- 24.Hirose H, Kiritani S, Imagawa H. High-speed digital image analysis of laryngeal behavior in running speech. In: Fujimura O, editor. Vocal Physiology: Voice Production, Mechanisms and Functions. RavenPress, Ltd.; New York: 1988. pp. 335–345. [Google Scholar]
- 25.Hiroto I, Hirano M, Toyozumi Y, Shin T. Electromyographic investigation of the instrinsic laryngeal muscles related to speech sounds. Ann Otol Rhinol Laryngol. 1967;76:861–872. doi: 10.1177/000348946707600415. [DOI] [PubMed] [Google Scholar]
- 26.Hof AL. The relationship between electromyogram and muscle force. Sportverletz Sportschaden. 1997;11:79–86. doi: 10.1055/s-2007-993372. [DOI] [PubMed] [Google Scholar]
- 27.Inagi K, Connor NP, Suzuki T, Ford CN, Bless DM, Nakajima M. Glottal configuration, acoustic, and aerodynamic changes induced by variation in suture direction in arytenoid adduction procedures. Ann Otol Rhinol Laryngol. 2002;111:861–870. doi: 10.1177/000348940211101001. [DOI] [PubMed] [Google Scholar]
- 28.Insalaco G, Kuna ST, Catania G, Marrone O, Costanza BM, Bellia V, Bonsignore G. Thyroarytenoid muscle activity in sleep apneas. J Appl Physiol. 1993;74:704–709. doi: 10.1152/jappl.1993.74.2.704. [DOI] [PubMed] [Google Scholar]
- 29.Insalaco G, Kuna ST, Cibella F, Villeponteaux RD. Thyroarytenoid muscle activity during hypoxia, hypercapnia, and voluntary hyperventilation in humans. J Appl Physiol. 1990;69:268–273. doi: 10.1152/jappl.1990.69.1.268. [DOI] [PubMed] [Google Scholar]
- 30.Insalaco G, Kuna ST, Costanza BM, Catania G, Cibella F, Bellia V. Thyroarytenoid muscle activity during loaded and nonloaded breathing in adult humans. J Appl Physiol. 1991;70:2410–2416. doi: 10.1152/jappl.1991.70.6.2410. [DOI] [PubMed] [Google Scholar]
- 31.Jarvholm U, Palmerud G, Karlsson D, Herberts P, Kadefors R. Intramuscular pressure and electromyography in four shoulder muscles. J Orthop Res. 1991;9:609–619. doi: 10.1002/jor.1100090418. [DOI] [PubMed] [Google Scholar]
- 32.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 33.Korner L, Parker P, Almstrom C, Andersson GB, Herberts P, Kadefors R, Palmerud G, Zetterberg C. Relation of intramuscular pressure to the force output and myoelectric signal of skeletal muscle. J Orthop Res. 1984;2:289–296. doi: 10.1002/jor.1100020311. [DOI] [PubMed] [Google Scholar]
- 34.Kuna ST, Day RA, Insalaco G, Villeponteaux RD. Posterior cricoarytenoid activity in normal adults during involuntary and voluntary hyperventilation. J Appl Physiol. 1991;70:1377–1385. doi: 10.1152/jappl.1991.70.3.1377. [DOI] [PubMed] [Google Scholar]
- 35.Kuna ST, Insalaco G. Respiratory-related intrinsic laryngeal muscle activity in normal adults. Prog Clin Biol Res. 1990;345:117–123. discussion 123-114. [PubMed] [Google Scholar]
- 36.Kuna ST, Insalaco G, Villeponteaux DR, Vanoye CR, Smickley JS. Effect of hypercapnia and hypoxia on arytenoideus muscle activity in normal adult humans. J Appl Physiol. 1993;75:1781–1789. doi: 10.1152/jappl.1993.75.4.1781. [DOI] [PubMed] [Google Scholar]
- 37.Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol. 1991;70:1655–1664. doi: 10.1152/jappl.1991.70.4.1655. [DOI] [PubMed] [Google Scholar]
- 38.Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1988;65:1332–1339. doi: 10.1152/jappl.1988.65.3.1332. [DOI] [PubMed] [Google Scholar]
- 39.Kuna ST, Smickley JS, Insalaco G. Posterior cricoarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1990;68:1746–1754. doi: 10.1152/jappl.1990.68.4.1746. [DOI] [PubMed] [Google Scholar]
- 40.Kuna ST, Smickley JS, Vanoye CR, McMillan TH. Cricothyroid muscle activity during sleep in normal adult humans. J Appl Physiol. 1994;76:2326–2332. doi: 10.1152/jappl.1994.76.6.2326. [DOI] [PubMed] [Google Scholar]
- 41.Kuna ST, Smickly JS, Insalaco G. Posterior cricoarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1990;68:1746–1754. doi: 10.1152/jappl.1990.68.4.1746. [DOI] [PubMed] [Google Scholar]
- 42.Kuna ST, Vanoye CR. Laryngeal response during forced vital capacity maneuvers in normal adult humans. Am J Respir Crit Care Med. 1994;150:729–734. doi: 10.1164/ajrccm.150.3.8087344. [DOI] [PubMed] [Google Scholar]
- 43.Lofqvist A, McGarr NS, Honda K. Laryngeal muscles and articulatory control. J oust Soc Am. 1984;76:951–954. doi: 10.1121/1.391278. [DOI] [PubMed] [Google Scholar]
- 44.Lofqvist A, Yoshioka H. Interarticulator programming in obstruent production. Phonetica. 1981;38:21–34. doi: 10.1159/000260012. [DOI] [PubMed] [Google Scholar]
- 45.Ludlow CL, Hang C, Bielamowicz S, Choyke P, Hampshire V, Selbie WS. Three-dimensional changes in the upper airway during neuromuscular stimulation of laryngeal muscles. Artifical Organs. 1999;23:463–465. doi: 10.1046/j.1525-1594.1999.06364.x. [DOI] [PubMed] [Google Scholar]
- 46.Ludlow CL, Lou G. Observations on human laryngeal muscle control. In: Fletcher N, Davis P, editors. Controlling complexity and chaos: 9th Vocal Fold Physiology Symposium; Singular Press; Sandiego, CA. 1996.pp. 201–218. [Google Scholar]
- 47.Ludlow CL, Sedory SE, Fujita M. Neurophysiolgical control of vocal fold adduction and abduction for phonation onset and offset during speech. In: Gauffin J, Hammarberg B, editors. Vocal fold physiology. 3 ed. Ravens Press; New York: 1991. pp. 197–205. [Google Scholar]
- 48.Ludlow CL, Yeh J, Cohen LG, Van Pelt F, Rhew k, Hallett M. Limitations of laryngeal electromyography and magnetic stimulation for assessing laryngeal muscle control. Annals of Otology,Rhinology and Laryngology. 1994;103:16–27. doi: 10.1177/000348949410300103. [DOI] [PubMed] [Google Scholar]
- 49.Mathew OP, Sant’Ambrogio FB, Woodson GE, Sant’Ambrogio G. Respiratory activity of the cricothyroid muscle. Annals of Otology,Rhinology and Laryngology. 1988;97:680–687. doi: 10.1177/000348948809700619. [DOI] [PubMed] [Google Scholar]
- 50.Perlman AL, Palmer PM, McCullough TM, VanDaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscle during swallowing. Journal of Applied Physiology. 1999;86:1663–1669. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfield DB, Miller RH, Sessions RB, Pattern BM. Morphologic and histochemical characteristics of laryngeal muscle. ArchOtolaryngol. 1982;108:662–666. doi: 10.1001/archotol.1982.00790580056018. [DOI] [PubMed] [Google Scholar]
- 52.Sant’Ambrogio G, Kuna ST, Vanoye CR, Sant’Ambrogio FB. Activation of intrinsic laryngeal muscles during cough. Am J Respir Crit Care Med. 1997;155:637–641. doi: 10.1164/ajrccm.155.2.9032206. [DOI] [PubMed] [Google Scholar]
- 53.Shiotani A, Fukuda H, Kawaida M, Kanzaki J. Vocal fold vibration in simulated head voice phonation in excised canine larynges. Eur Arch Otorhinolaryngol. 1996;253:356–363. doi: 10.1007/BF00178292. [DOI] [PubMed] [Google Scholar]
- 54.Titze IR, Luschei ES, Hirano M. Role of the thyroarytenoid muscle in regulation of fundamental frequency. Journal of Voice. 1989;3(3):213–224. [Google Scholar]
- 55.Titze IR, Story BH. Rules for controlling low-dimensional vocal fold models with muscle activation. J Acoust Soc Am. 2002;112:1064–1076. doi: 10.1121/1.1496080. [DOI] [PubMed] [Google Scholar]
- 56.Tully A, Brancatisano A, Loring SH, Engel LA. Influence of posterior cricoarytenoid muscle activity on pressure-flow relationship of the larynx. J Appl Physiol. 1991;70:2252–2258. doi: 10.1152/jappl.1991.70.5.2252. [DOI] [PubMed] [Google Scholar]
- 57.Tully A, Brancatisano A, Loring SH, Engel LA. Relationship between thyroarytenoid activity and laryngeal resistance. J Appl Physiol. 1990;68:1988–1996. doi: 10.1152/jappl.1990.68.5.1988. [DOI] [PubMed] [Google Scholar]
- 58.Wheatley JR, Brancatisano A, Engel LA. Respiratory-related activity of cricothyroid muscle in awake normal humans. J Appl Physiol. 1991;70:2226–2232. doi: 10.1152/jappl.1991.70.5.2226. [DOI] [PubMed] [Google Scholar]
- 59.Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. JNeurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]