Abstract
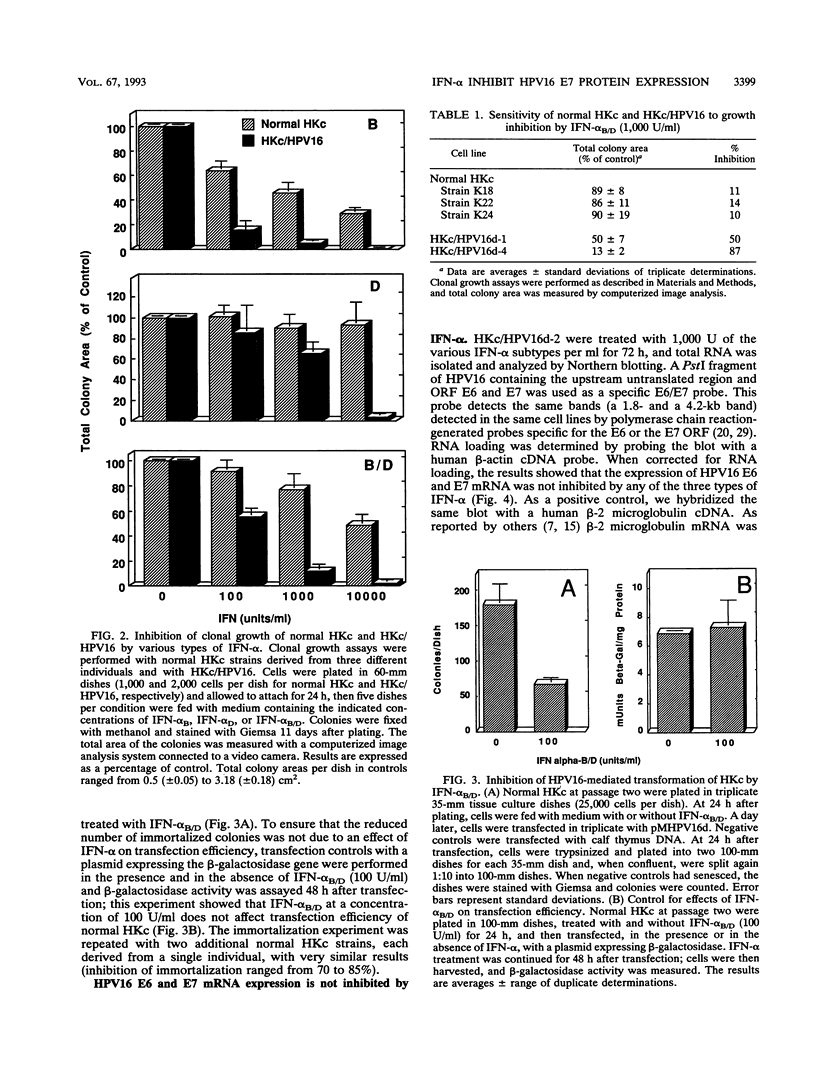
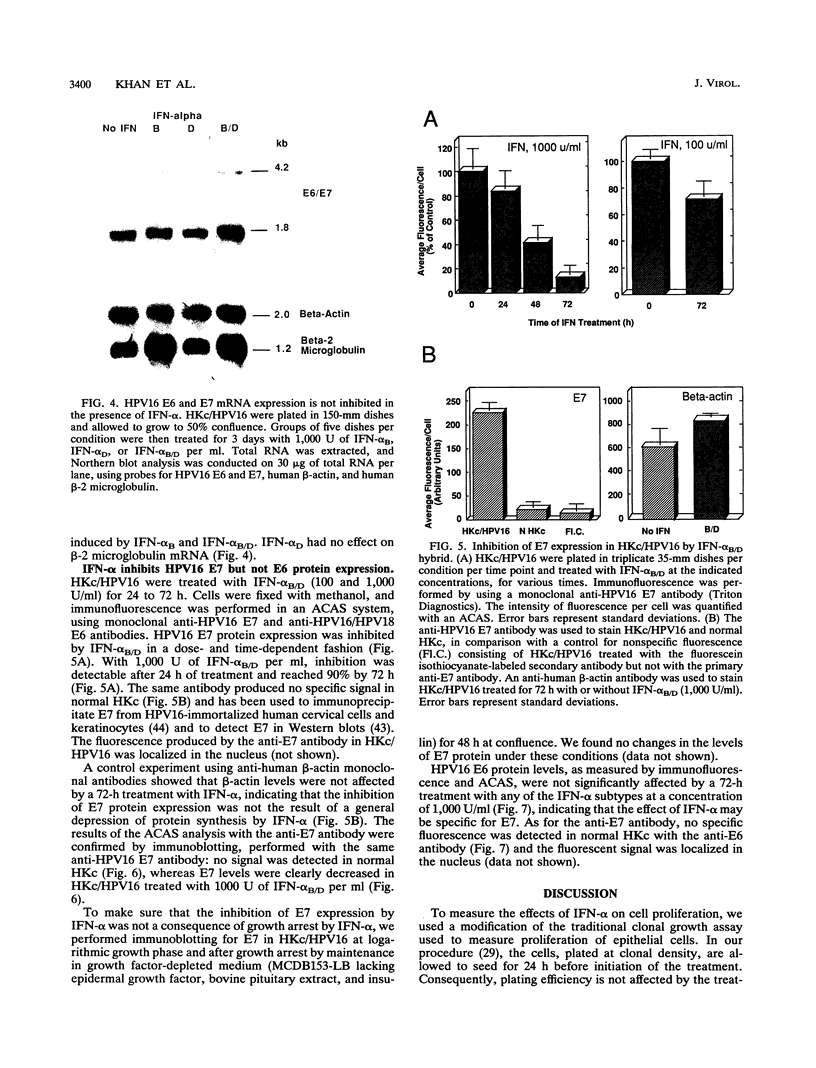
We used a model system of normal human keratinocytes (HKc) and HKc immortalized with human papillomavirus type 16 DNA (HKc/HPV16) to investigate the effects of alpha interferons (IFN-alpha) on the growth of HPV16-immortalized human epithelial cells, on HPV16-mediated immortalization of normal HKc, and on HPV16 gene expression. Normal HKc and HKc/HPV16 were treated with several recombinant human IFN-alpha subtypes (IFN-alpha B, IFN-alpha D, and IFN-alpha B/D). These IFN-alpha subtypes inhibited proliferation of both normal HKc and HKc/HPV16 in a dose-dependent fashion; however, although 1,000 to 10,000 U of IFN-alpha per ml were required to inhibit growth of normal HKc, HKc/HPV16 were substantially growth inhibited by 100 U/ml. In addition, 100 U of IFN-alpha B/D per ml inhibited transformation of normal HKc by HPV16 DNA. Northern (RNA) blot analysis showed no effect of IFN-alpha on the mRNA levels of the HPV16 E6 and E7 open reading frames. However, immunofluorescence studies of the HPV16 E6 and E7 proteins with anti-E6 and anti-E7 monoclonal antibodies showed significant inhibition of E7 protein expression in cells treated with IFN-alpha, whereas E6 protein expression was not altered. The inhibition of E7 protein expression in cells treated with IFN-alpha was further confirmed by Western immunoblot analysis. These results suggest that IFN-alpha may inhibit HPV16-mediated transformation of HKc and proliferation of HKc/HPV16 through an inhibition of HPV16 E7 protein expression.
Full text
PDF


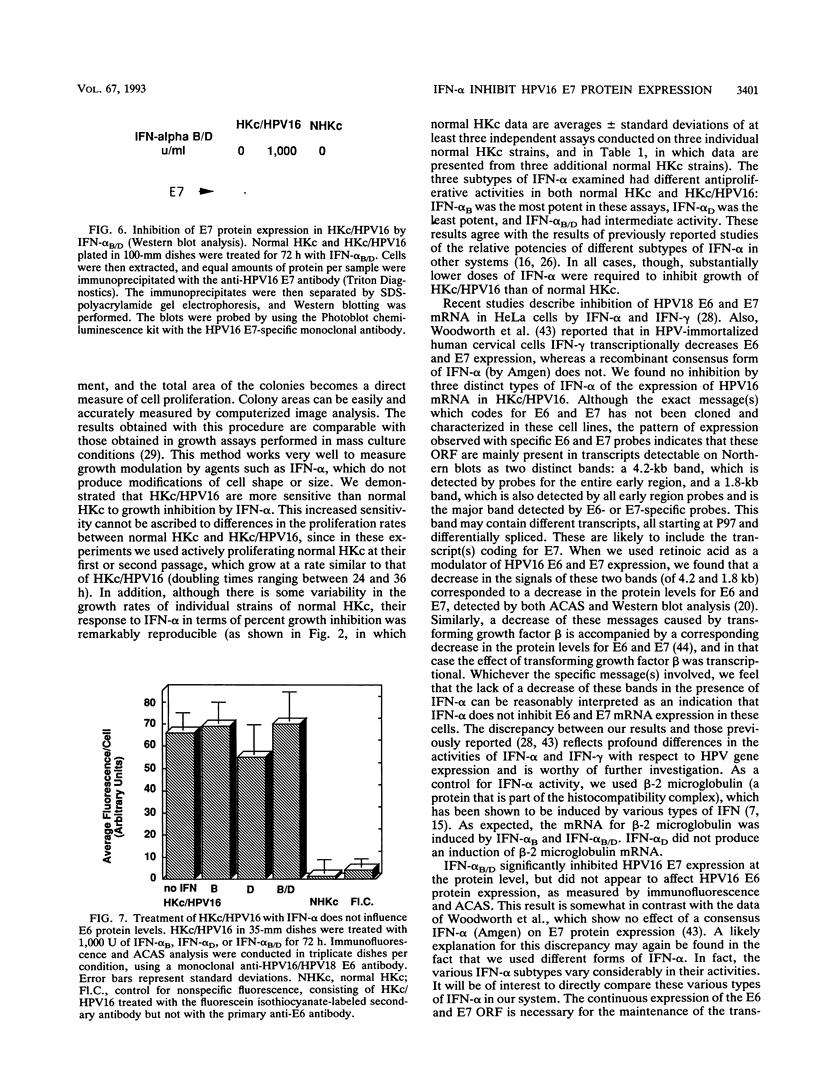


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron E., Narula S. From cloning to a commercial realization: human alpha interferon. Crit Rev Biotechnol. 1990;10(3):179–190. doi: 10.3109/07388559009038206. [DOI] [PubMed] [Google Scholar]
- Crook T., Morgenstern J. P., Crawford L., Banks L. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 1989 Feb;8(2):513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Fellous M., Kamoun M., Gresser I., Bono R. Enhanced expression of HLA antigens and beta 2-microglobulin on interferon-treated human lymphoid cells. Eur J Immunol. 1979 Jun;9(6):446–449. doi: 10.1002/eji.1830090606. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Heicappell R., Saiki I., Grutter M. G., Horisberger M. A., Nuesch J. Direct antiproliferative effects of recombinant human interferon-alpha B/D hybrids on human tumor cell lines. Cancer Res. 1987 Apr 15;47(8):2020–2027. [PubMed] [Google Scholar]
- Fuchs P. G., Girardi F., Pfister H. Human papillomavirus 16 DNA in cervical cancers and in lymph nodes of cervical cancer patients: a diagnostic marker for early metastases? Int J Cancer. 1989 Jan 15;43(1):41–44. doi: 10.1002/ijc.2910430110. [DOI] [PubMed] [Google Scholar]
- Gangemi J. D., Lazdins J., Dietrich F. M., Matter A., Poncioni B., Hochkeppel H. K. Antiviral activity of a novel recombinant human interferon-alpha B/D hybrid. J Interferon Res. 1989 Apr;9(2):227–237. doi: 10.1089/jir.1989.9.227. [DOI] [PubMed] [Google Scholar]
- Gangemi J. D., Matter A., Poncioni B., Hochkeppel H. K. Significant differences in therapeutic responses to a human interferon-alpha B/D hybrid in Rauscher or Friend murine leukemia virus infections. J Interferon Res. 1989 Jun;9(3):275–283. doi: 10.1089/jir.1989.9.275. [DOI] [PubMed] [Google Scholar]
- Haglund S., Lundquist P. G., Cantell K., Strander H. Interferon therapy in juvenile laryngeal papillomatosis. Arch Otolaryngol. 1981 Jun;107(6):327–332. doi: 10.1001/archotol.1981.00790420001001. [DOI] [PubMed] [Google Scholar]
- Halbert C. L., Demers G. W., Galloway D. A. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991 Jan;65(1):473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A., de Staritzky K. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J Gen Virol. 1987 Mar;68(Pt 3):945–948. doi: 10.1099/0022-1317-68-3-945. [DOI] [PubMed] [Google Scholar]
- Ikenberg H., Gissmann L., Gross G., Grussendorf-Conen E. I., zur Hausen H. Human papillomavirus type-16-related DNA in genital Bowen's disease and in Bowenoid papulosis. Int J Cancer. 1983 Nov 15;32(5):563–565. doi: 10.1002/ijc.2910320507. [DOI] [PubMed] [Google Scholar]
- Kaur P., McDougall J. K. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988 Jun;62(6):1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., McDougall J. K., Cone R. Immortalization of primary human epithelial cells by cloned cervical carcinoma DNA containing human papillomavirus type 16 E6/E7 open reading frames. J Gen Virol. 1989 May;70(Pt 5):1261–1266. doi: 10.1099/0022-1317-70-5-1261. [DOI] [PubMed] [Google Scholar]
- Khan M. A., Jenkins G. R., Tolleson W. H., Creek K. E., Pirisi L. Retinoic acid inhibition of human papillomavirus type 16-mediated transformation of human keratinocytes. Cancer Res. 1993 Feb 15;53(4):905–909. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Castellano C., Santos C., Delgado G., Kurman R. J., Jenson A. B. Human papillomavirus deoxyribonucleic acid in cervical carcinoma from primary and metastatic sites. Am J Obstet Gynecol. 1986 Jan;154(1):115–119. doi: 10.1016/0002-9378(86)90405-9. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Contard P. Interferon and condylomata acuminata. Int J Dermatol. 1990 Dec;29(10):699–705. doi: 10.1111/j.1365-4362.1990.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Lippman S. M., Kavanagh J. J., Paredes-Espinoza M., Delgadillo-Madrueño F., Paredes-Casillas P., Hong W. K., Holdener E., Krakoff I. H. 13-cis-retinoic acid plus interferon alpha-2a: highly active systemic therapy for squamous cell carcinoma of the cervix. J Natl Cancer Inst. 1992 Feb 19;84(4):241–245. doi: 10.1093/jnci/84.4.241. [DOI] [PubMed] [Google Scholar]
- Meister A., Uzé G., Mogensen K. E., Gresser I., Tovey M. G., Grütter M., Meyer F. Biological activities and receptor binding of two human recombinant interferons and their hybrids. J Gen Virol. 1986 Aug;67(Pt 8):1633–1643. doi: 10.1099/0022-1317-67-8-1633. [DOI] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa A., Nishiyama Y., Yamamoto N., Maeno K., Goto S., Tomoda Y. Selective suppression of human papilloma virus type 18 mRNA level in HeLa cells by interferon. Biochem Biophys Res Commun. 1990 Jul 31;170(2):793–799. doi: 10.1016/0006-291x(90)92161-r. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Batova A., Jenkins G. R., Hodam J. R., Creek K. E. Increased sensitivity of human keratinocytes immortalized by human papillomavirus type 16 DNA to growth control by retinoids. Cancer Res. 1992 Jan 1;52(1):187–193. [PubMed] [Google Scholar]
- Pirisi L., Creek K. E., Doniger J., DiPaolo J. A. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988 Sep;9(9):1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strander H., Cantell K. Studies on antiviral and antitumor effects of human leukocyte interferon in vitro and in vivo. In Vitro Monogr. 1974;(3):49–56. [PubMed] [Google Scholar]
- Streuli M., Hall A., Boll W., Stewart W. E., 2nd, Nagata S., Weissmann C. Target cell specificity of two species of human interferon-alpha produced in Escherichia coli and of hybrid molecules derived from them. Proc Natl Acad Sci U S A. 1981 May;78(5):2848–2852. doi: 10.1073/pnas.78.5.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. M., Olson S., Lawn R. M. Chromosomal localization of human leukocyte, fibroblast, and immune interferon genes by means of in situ hybridization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7809–7813. doi: 10.1073/pnas.79.24.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofatter K. F., Jr Interferon. Obstet Gynecol Clin North Am. 1987 Jun;14(2):569–579. [PubMed] [Google Scholar]
- Turek L. P., Byrne J. C., Lowy D. R., Dvoretzky I., Friedman R. M., Howley P. M. Interferon induces morphologic reversion with elimination of extrachromosomal viral genomes in bovine papillomavirus-transformed mouse cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7914–7918. doi: 10.1073/pnas.79.24.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., Lichti U., Simpson S., Evans C. H., DiPaolo J. A. Leukoregulin and gamma-interferon inhibit human papillomavirus type 16 gene transcription in human papillomavirus-immortalized human cervical cells. Cancer Res. 1992 Jan 15;52(2):456–463. [PubMed] [Google Scholar]
- Woodworth C. D., Notario V., DiPaolo J. A. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J Virol. 1990 Oct;64(10):4767–4775. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Doniger J., DiPaolo J. A. Differential early viral gene expression in two stages of human papillomavirus type 16 DNA-induced malignant transformation. Mol Cell Biol. 1987 Jun;7(6):2165–2172. doi: 10.1128/mcb.7.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]