Abstract
Recent work suggested that the energy intake and weight gain of rats maintained on chow and 32% sucrose solution could be increased by simply offering more sources of sucrose (Tordoff, 2002). In Experiment 1 this procedure was replicated but the effect was not: rats given one bottle of sucrose and five bottles of water consumed as much sucrose as those given five bottles of sucrose and one of water. Adding different flavors to the sucrose did not increase intakes further in Experiment 2. The relative potency of sucrose and other optional foods was studied in Experiment 3. Sucrose solution stimulated more overeating and weight gain than fat (vegetable shortening), and offering both sucrose and shortening did not generate further increases in energy intake. Finally, foods commonly used to produce overeating and weight gain were compared. Sucrose was less effective than a high-fat milk diet, and offering cookies in addition to the milk did not increase energy intake further. The nature of optional foods (nutrient composition and physical form) was markedly more important than the number of food sources available to the animals, and is a better contender as the reason for “obesity by choice”.
Keywords: Food choice, Variety, Fat, Sugar
The widespread availability of tasty, inexpensive, energy dense foods, which are typically rich in sugar and fat, is thought to contribute to the increasing prevalence of human obesity [e.g., 26,32]. A useful animal model for this phenomenon exists: laboratory rats overeat and gain excessive weight when offered a cafeteria of palatable high-fat and high-sugar foods in addition to their nutritionally complete chow diet [e.g., 34,36,37]. Another potentially important variable in diet-induced obesity studies is the availability of food, e.g., the number of sources of food offered [43]. In confirmation of prior studies [e.g, 16,29,40], rats offered a 32% sucrose solution in addition to chow and water consumed more total energy (sucrose + chow) and gained more weight than rats given only chow and water. The surprising finding of Tordoff’s study was that rats given access to five bottles of sucrose and one of water consumed significantly more sucrose and total energy than did rats given only one bottle of sucrose and five bottles of water. This is seemingly a situation that mimics the human case of abundant palatable food, with a larger number of sources leading to greater overeating. Tordoff [43] suggested that this was related to the overeating observed in cafeteria studies.
Beyond the contributions of sugar and fat per se, variety in the available foods and their flavors has been linked to overeating and obesity in humans and animals [e.g., 27,28,36]. Laboratory rats are normally fed an unvarying chow maintenance diet, and even the 32% sucrose, while palatable, has an invariant flavor. We were interested in the possibility that intakes could be increased still further by combining Tordoff’s availability effect with that of variety of flavors added to the sucrose. We began by reproducing Tordoff’s procedure but did not replicate his results. In a second experiment we asked whether variety in the flavors added to sucrose solutions would lead to greater intake than that of unflavored sucrose. Sensory similarity can be reduced further by providing a choice of palatable foods with different nutrient contents. A third experiment compared the overeating and weight gain with a sucrose option to that with other foods previously used to generate overeating: vegetableshortening [10,11,22], high-fat milk [2,24,49,51], and chocolate chip cookies [37,38].
Experiment 1A
Our initial study was designed as a replication of Tordoff’s [43] sucrose experiment, to obtain the basic data using equipment available in our laboratory. There were two differences that could influence the results: one was the use of somewhat smaller cages than those of the original study, and the other was the use of larger bottles. Tordoff used 50-ml bottles, so that an animal given only one bottle of sucrose was constrained to a maximum intake of about 45 ml due to the space occupied by the stopper. We used bottles that held 80 ml, which exceeds typical daily intakes of 32% sucrose in rats. We used animals that closely resembled those in the Tordoff experiment in strain, sex, and initial body weights.
Method
Subjects
Female Sprague-Dawley rats (n = 36) were bred in the lab from CD stock (Charles-River Laboratories, Wilmington, MA). The animals were housed in a colony room maintained at 70 degrees F with a 12:12 h light:dark cycle (lights on 0800). Because of the limited number of cages of the requisite size, the experiment was run in two cohorts of 18 rats each with six rats from each group. At the start of sucrose access, the rats were 76-90 days old with an average body weight of 259 g.
Apparatus
Stainless steel cages (40 × 24 × 18 cm W × L × H) with wire mesh front wall and floor were used to house the animals. These medium-sized cages have 2.2 times the floor area of standard (18 cm wide) hanging cages for rats (960 vs. 432 cm2). Powdered chow (PMI Nutrition International, Brentwood, MO) was offered in a small glass jar inside a larger glass jar to contain spillage. These jars were secured in the left rear corner of the cage by two stainless steel bolts fastened to the floor. The cages for the control group had one standard glass water bottle with curved stainless steel spout; the bottle was secured to the front of the cage with a spring. The cages for the other two groups had six 80-ml plastic bottles with straight stainless steel drinking spouts inserted ∼5 cm above the floor of the cage. They were secured in three plexiglas mounts evenly spaced along the front wall of the cage. Each mount held a pair of bottles with the spouts 5 cm apart; adjacent spouts on different mounts were 7 cm apart. The spouts extended ∼1 cm into the cage; this distance was fixed by stainless steel collars on the spouts. Rubber bands were used to stabilize the bottles in order to minimize movement caused by the animals.
Procedure
The rats were accustomed to the experimental cages for 4 days. They were then divided into three groups equated for chow and water intake and body weight on days 3 and 4. The control group continued to receive chow and one bottle of water. The S-1 group had five bottles of water and one bottle of 32% sucrose (w/w). The S-5 group had five bottles of 32% sucrose and one bottle of water. For both groups the single-bottle fluid was placed in the fourth slot from the left. There was also an empty spillage cage to correct for the small fluid losses from removal and placement of the bottles. The spillage measurements were averaged and the corresponding fluid values were adjusted. All sucrose bottles were cleaned and refilled daily. For 20 days, chow and fluid intakes were recorded daily, and body weights were recorded every fourth day. Intakes were averaged in 4-day blocks.
Statistical analysis
Intake data were entered in repeated-measures analyses of variance. To remove any influence of differences in initial body weights, they were converted to weight gains relative to the baseline period for analysis. However, they are plotted in the graphs as absolute body weights.
Results and Discussion
The S-1 and S-5 groups maintained similar sucrose intakes during the 20-day access period (Fig. 1A). Sucrose intake and percent of total energy from sucrose increased across the first three 4-day blocks and then stabilized (Block F(4,88) = 22.13 and 81.73, p < 0.001); the final sucrose proportion was 68% of total energy. Chow and water intakes (not shown) of the sucrose groups decreased when sucrose was available (Group × Block F(10,165) = 50.27 and 98.80, p < 0.001); the S-1 and S-5 groups consumed significantly less chow and water than did the controls (Group F(2,33) = 97.68 and 215.77, p < 0.001), and chow intake in the last four sucrose blocks was lower than in the first (Block F(5,165) = 140.11, p < 0.001). The S-1 water intake was greater than that of the S-5 group (5.5 vs. 1.5 g/day, p < 0.001). The total energy intake of the two sucrose groups did not differ and exceeded that of the controls throughout the sucrose period (Group × Block F(10,165) = 9.74, p < 0.001; Fig. 1B). Body weight gains of the two sucrose groups did not differ and exceeded that of the controls in all but the first block of the sucrose period (Group × Block F(8,132) = 18.56, p < 0.001; Fig. 1C).
Figure 1.
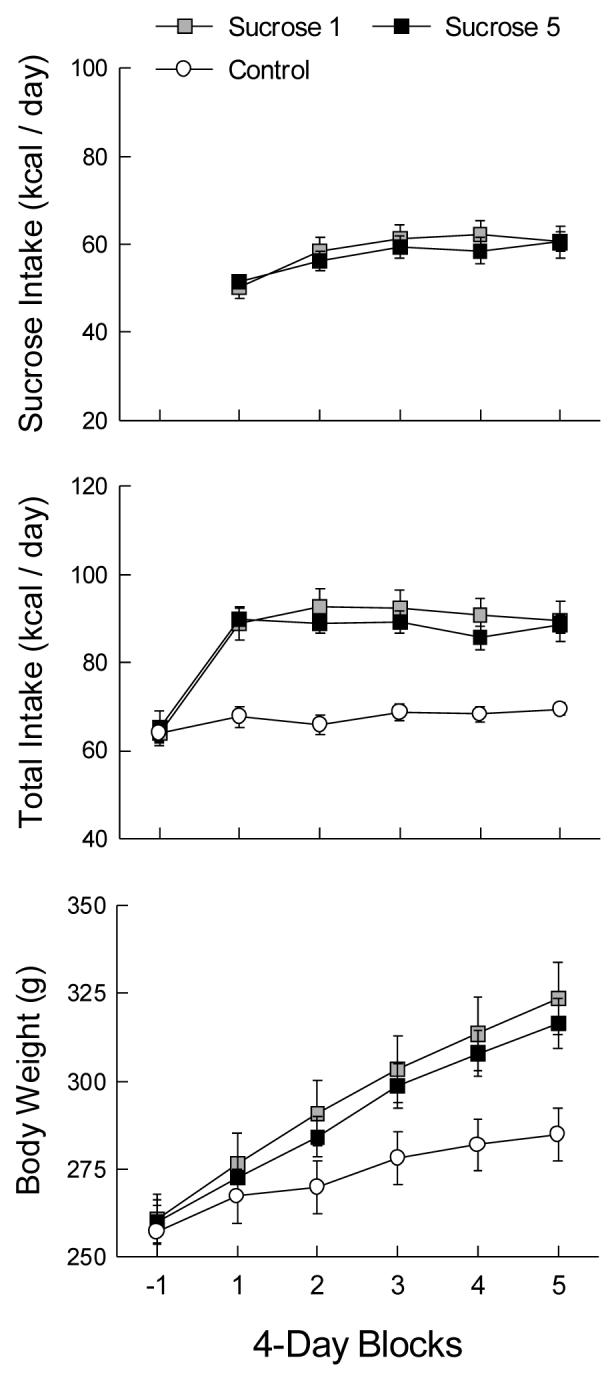
Mean (± sem) sucrose and total energy intakes and body weights in 4-day blocks of Experiment 1A. Block -1 is the baseline measure prior to the introduction of sucrose access. The Control group did not receive sucrose. The Sucrose 1 group received one bottle of 32% sucrose solution and five bottles of water during blocks 1-5; the Sucrose 5 group received five bottles of 32% sucrose and one of water. The animals were housed in medium-sized cages (see text).
In contrast to Tordoff’s rats with one or five bottles of sucrose, our animals did not differ in sucrose or total energy intake as a function of the number of bottles available. His five-bottle group was heavier than the one-bottle group by day 16 of sucrose access, whereas our rats did not differ and showed no sign of diverging at day 20. Tordoff’s one-bottle group took 20 days to reach an asymptotic sucrose intake of about 40 ml (∼51 kcal) per day, whereas his five-bottle group began at about 55 ml and stabilized by the second 4-day block at about 48 ml (∼61 kcal). In contrast, our animals attained that intake in the first block and exceeded it thereafter. Average intakes of sucrose exceeded 45 ml in six of 12 one-bottle rats and seven of 12 five-bottle rats, so using the smaller bottles would have limited their intakes and may have limited Tordoff’s animals as well. The difference between the one- and five-bottle groups in water intake, though small, is one result consistent with an availability notion, and could reflect more frequent encounters with water spouts in the group with multiple water bottles.
Experiment 1B
Experiment 1A did not replicate Tordoff’s finding of greater sucrose intake when more bottles were offered. His one-bottle group behaved atypically for females; previous studies have recorded intakes more akin to those of our rats [e.g., 1,39] though there are exceptions [15]. One possible reason for the discrepancy is cage size: Tordoff housed his rats in cages that were larger than those used in Experiment 1A (2296 vs. 960 cm2 floor area). Cage size has been observed to affect rats’ feeding and drinking behaviors [e.g., 8,53], reflecting different allocation of time to these and other activities and perhaps greater physical separation from food and fluid sources. Experiment 1B tested the possibility that Tordoff’s availability finding depends on cage size by using still larger cages (2650 cm2 floor area).
Method
Twenty adult female rats (69-95 days old) were divided into two groups of 10 equated for chow and water intake and body weight (mean 261 g). They were housed under similar conditions except that the cages were larger (53 × 50 × 18 cm; 2.8 times the floor area of the Experiment 1 cage, and 6.1 times that of a standard cage). The same bottle mounts were used; two were attached so that the second and third spouts were 8 cm apart, and due to an obstruction on the cage front the third was attached so that the fourth and fifth bottles were 22 cm apart. The chow jar was attached in the right front corner of the cage. Because of the limited number of cages available, the experiment was run in two cohorts, each with five rats from each group. One group of rats (S-5L) were given five bottles of sucrose and one bottle of water, and the other group (S-1L) received one bottle of sucrose and five bottles of water. As in Experiment 1A, intakes and body weights were collected for 20 days.
Results and Discussion
Sucrose intake of the rats with five sucrose bottles did not differ from that of rats with only one sucrose bottle (Fig. 2A). Both groups increased sucrose intake from the first to the second 4-day block, and then maintained intake for the remainder of the access period (Block F(4,72) = 29.54, p < 0.001). Percentage energy consumed as sucrose was similar in the two groups but did not stabilize (at 72%) until the third block (F(4,72) = 58.03, p < 0.001). The groups were also similar in chow intake (not shown), and reduced intake from baseline to the sucrose access period (Block F(5,90) = 120.47, p < 0.001). The chow reduction was not compensatory, however, so that total energy intake increased from baseline to the sucrose period, Block F(5,90) = 88.02, p < 0.001 (Fig. 2B). Total energy intake peaked in the second and third blocks of the sucrose period; blocks 1, 4 and 5 were similar in total energy. The groups did not differ in weight gain (Fig. 2C). Water intake decreased markedly from baseline levels and remained low during sucrose access (F(5,90) = 259.60, p < 0.001). When sucrose was available, the S-1L rats drank more water than the S-5L rats (7.2 vs 1.3 g/day, p < 0.001).
Figure 2.
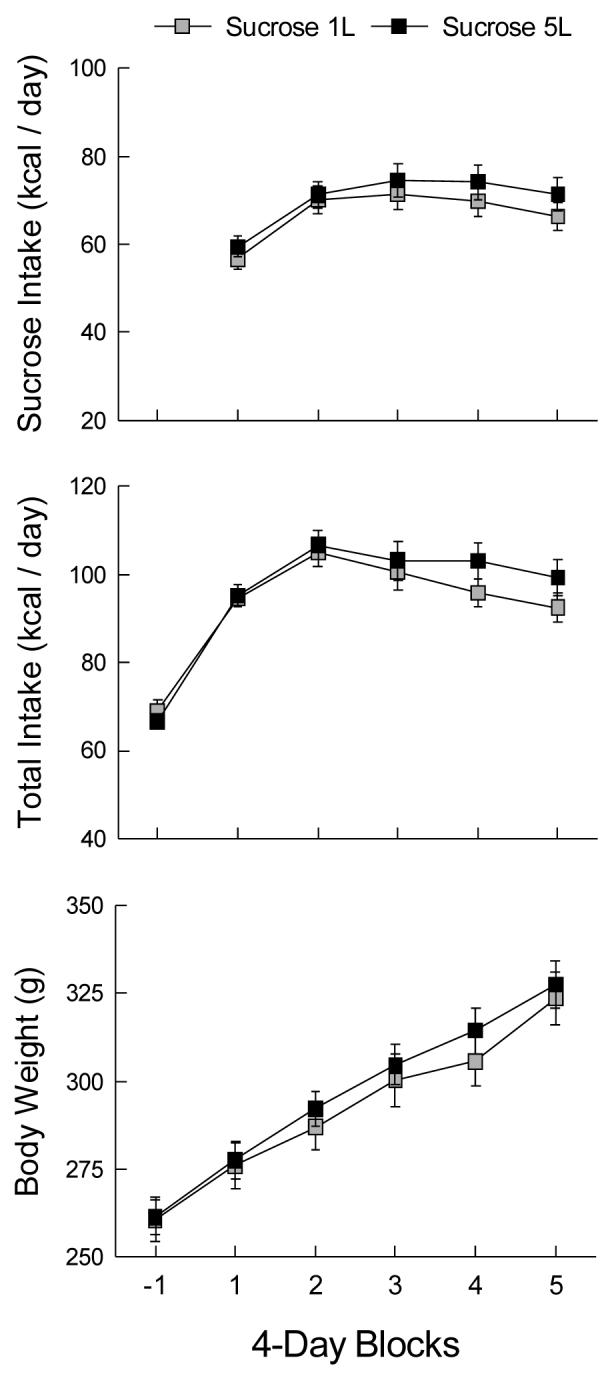
Mean (± sem) sucrose and total energy intakes and body weights in 4-day blocks of Experiment 1B. Block -1 is the baseline measure prior to the introduction of sucrose access. The Sucrose 1L group received one bottle of 32% sucrose solution and five bottles of water during blocks 1-5; the Sucrose 5L group received five bottles of 32% sucrose and one of water. The animals were housed in large cages (see text).
Combined analyses of average intakes during the 20-d sucrose period were used to compare the five groups of Experiment 1. The groups did not differ in baseline measures of water and chow intake. Sucrose intake of the large-cage groups (52-55 kcal) exceeded that of the medium-cage groups (45-46 kcal), F(3,40) = 5.02, p < 0.01. However, the proportion of energy consumed as sucrose did not differ among the groups, averaging 66.5% of total energy. When the comparison included the control group, water intake of the sucrose groups did not differ and all drank markedly less than the control average of 38 g/day, F(4,51) = 94.38, p < 0.001. A comparison including only the sucrose groups found that water intakes of the one-bottle groups were similar and exceeded the intakes of the five-bottle groups, F(3,40) = 13.83, p < 0.001. Cage size had no effect on chow intake in the sucrose groups, which all ate about half as much as the control group (31 vs. 68 kcal, F(4,51) = 153.54, p < 0.001). Total energy intake of the S-5L group (101 kcal) did not differ from that of the S-1L group (98 kcal), but exceeded that of both medium-cage groups (S-5 88 kcal, S-1 91 kcal, F(4,51) = 21.12, p < 0.001. Body weight gains of the sucrose groups did not differ at day 8, when sucrose intakes were stabilizing, or at the end of sucrose access (F(4,51) = 10.67 and 15.26, ps < 0.001). A possible explanation for the similar weight gains despite dissimilar intakes is that energy expenditure may have been greater in the larger cages.
Rather than reducing intake, as in some other large-cage studies, the large cages increased sucrose intake, and slightly slowed the stabilization of that intake. However, there was no effect of the number of sucrose bottles available, and chow intakes were unaffected. This suggests that the discrepancy between our findings and Tordoff’s result is unlikely to reflect caging differences. We used cages that were smaller (Experiment 1A) and larger (Experiment 1B) than Tordoff’s cages, and in both cases found no difference based on a the number of sucrose bottles available. The surprising result in his experiment was the time it took the group with one sucrose source to increase intake to asymptotic levels; our one-source groups did not lag in this fashion. His bottles were spaced only 3 cm apart, which should have reduced the effort of locating the single sucrose bottle in the array. Although we did not replicate his basic effect in either part of Experiment 1, we continued to look for an availability effect in Experiment 2 by testing our original idea that increasing flavor variety might increase the daily intake from multiple sucrose sources.
Experiment 2A
The flavor-based variety effect is thought to involve stimulation by assorted sensory characteristics of foods. In Experiment 1 the same unflavored 32% sucrose was offered in all bottles. Would intake increase if the sucrose was accompanied by various flavors? To assess the effect of flavor we obtained a baseline measure of sucrose intake so that groups could be equated prior to flavor introduction.
Procedure
Female rats (110 days old, mean body weight 325 g) were individually housed in the same cages used in Experiment 1A. They were given chow and water only for 2 days, and then a bottle of 32% sucrose was added for 2 more days. The rats were then divided into two groups (n = 10 each) equated for body weight and intakes in the 2-day sucrose access period. Then all rats were given 1 bottle of water and 5 bottles of 32% sucrose solution. The Flavor group was given one bottle of unflavored sucrose and four bottles with added flavors: 0.05% cherry and 0.05% strawberry Kool-Aids, and 2% vanilla and 2% maple extracts. The positions of the flavors were constant for each rat; unflavored sucrose and water were always presented in the third and fourth positions from the left, and the remaining flavors were arranged in a different order for each cage. The No Flavor group was given only unflavored sucrose, with water in the fourth position. Because intakes were stable after 8 days, corresponding to the first two 4-day blocks of Experiment 1, the study was terminated at this point.
Results and Discussion
Intakes were analyzed in 2-day blocks (Fig. 3). Sucrose intake and total energy intake increased from the baseline period to the six-bottle period, F(4,72) = 15.30 and 8.53, ps < 0.001; there were no differences within the six-bottle period. The Flavor group showed no preferences among the different flavors of sucrose (F < 1). Chow and water intakes did not change across blocks. The only difference between the groups was that the Flavor group drank less water than the No Flavor group (1.6 vs 3.4 g/day, F(1,18) = 5.22, p < 0.05).
Figure 3.
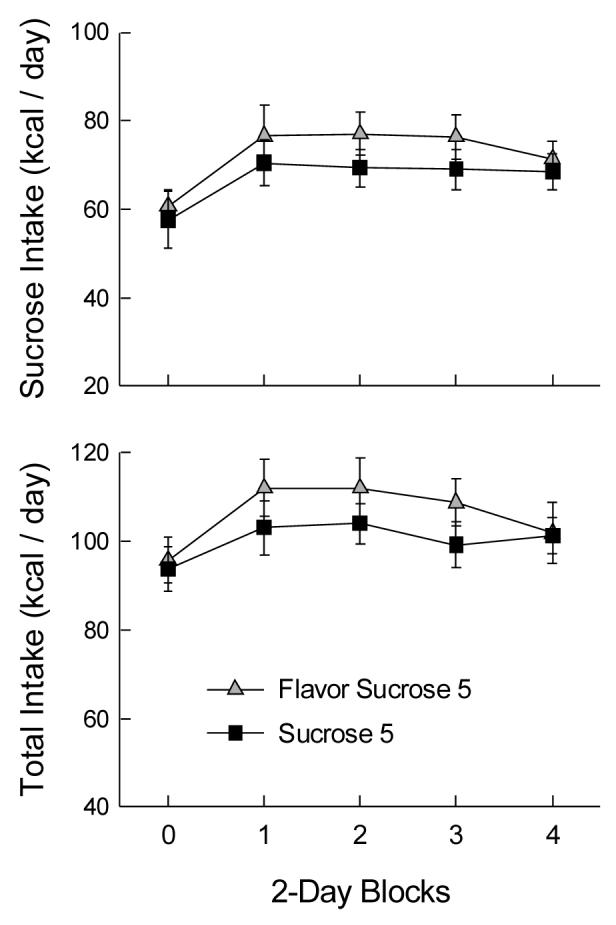
Mean (± sem) sucrose and total energy intakes in 2-day blocks of Experiment 2A. Block 0 is the baseline measure with a single bottle of sucrose. The Flavor Sucrose 5 group received five bottles of 32% sucrose solution (four with added flavors) and one bottle of water during blocks 1-4; the Sucrose 5 group received five bottles of 32% sucrose without added flavors and one of water.
Unlike the within-meal enhancement of intake by variety in the flavor of otherwise identical food [17,46], prolonged access to multiple flavors did not alter the daily intake of 32% sucrose. Previous studies of solid foods with added flavors have yielded mixed results, as noted in the general discussion. However, a within-group effect of the number of sucrose sources occurred: the pretest with a single bottle of sucrose, followed by the shift to five bottles, did appear to produce a sustained increase in sucrose intake. The elevated intake could have been produced by the stimulus change of the switch to a larger number of bottles. Alternately, it may have simply represented the animals’ adaptation to continued sucrose access. The next experiment examined these two alternative explanations.
Experiment 2B
In Experiment 1, the rats were offered one or five bottles of sucrose throughout the access period, whereas the animals in Experiment 2A, because of our concern with matching the groups on sucrose intake, had initial experience with one sucrose bottle before the shift to five bottles. The more rapid adjustment in sucrose intake with access to multiple bottles in Experiment 2A than in Experiment 1 suggested that the initial exposure to a single bottle of sucrose might have been responsible. Experiment 2B repeated the procedure of Experiment 2A with two changes: both groups were given only unflavored sucrose, and while one group was shifted from one to five sucrose bottles as before, the other group continued with one sucrose bottle and one water bottle.
Procedure
Female rats (82 days old, mean body weight 275 g) were individually housed in the same cages used in Experiment 1A. They were given chow and water only for 4 days, and then a bottle of 32% sucrose was added for 2 more days (baseline period). The rats were then divided into two groups (n = 10 each) equated for body weight and intakes in the 2-day sucrose access period. For the next 8 days, the S5W1 group was given one bottle of water and five bottles of 32% sucrose solution, and the S1W1 group continued on one bottle of 32% sucrose solution and one bottle of water. Sucrose, chow and total energy intakes were averaged over 2-day blocks.
Results and Discussion
Sucrose intakes changed across blocks: the first 2-day block of the test period did not differ from the baseline period, but then intake increased and remained constant (Fig. 4A; F(4,72) = 8.22, p < 0.001). Water and chow intakes decreased from baseline, and then were constant across the access period, F(4,72) = 19.19, 10.47, ps < 0.001. Total energy intake remained constant across baseline and test periods (Fig. 4B), and there were no differences in any measures between the S1W1 and S5W1 groups.
Figure 4.
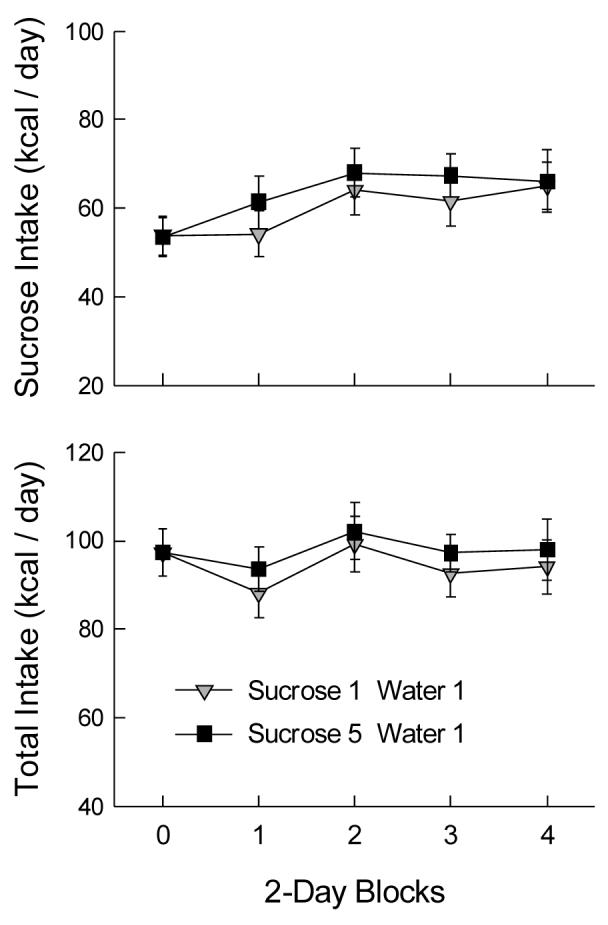
Mean (± sem) sucrose and total energy intakes in 2-day blocks of Experiment 2B. Block 0 is the baseline measure with a single bottle of sucrose. The Sucrose 1 Water 1 group received one bottle of 32% sucrose solution and one bottle of water during blocks 1-4; the Sucrose 5 Water 1 group received five bottles of 32% sucrose and one of water.
The increase from the initial two-bottle test to greater intake levels appeared slightly later than in Experiment 2A, but occurred equally in the two groups. Thus the increased intake observed in Experiment 2A cannot be attributed to a shift from one to five bottles of sucrose. The increase occurred over the first few days of sucrose access, regardless of how many bottles were offered, and may reflect an improved ability to metabolize sucrose [5,33] or a conditioned increase in sucrose acceptability based on flavor-nutrient conditioning [42].
Experiment 3
The absence of differences in three experiments (1A, 1B, 2B) between groups that received one or five bottles of sucrose in a six-bottle choice suggests that Tordoff’s study is the unusual case. Even adding flavors to increase the sucrose variety had no effect compared to plain sucrose. Flavored and plain versions of otherwise identical nutritionally balanced diets also did not lead to differential energy intake [28], though presenting various nutritionally similar diets that varied on multiple dimensions [21] or offering flavored high-fat and high-sugar diets [28] did increase total intake. It is possible that our rats’ intakes were limited by a ceiling effect: either the rats were overeating as much as possible, or they were consuming as much sucrose as possible. Offering other foods in addition to sucrose could distinguish between these potential ceilings. An established procedure for obtaining marked increases in energy intake is to provide a cafeteria diet high in fat and sugar [35,37]. In Experiment 3A the effects of sucrose solution and fat (hydrogenated shortening), offered singly or together to different groups of animals, were compared to controls.
Experiment 3A
Subjects
Female rats born in the laboratory were divided into groups equated for chow and water intake and body weight. The animals were 87-103 days old (263 g) at the start of the 20-day food option period. They were housed in cages with a somewhat larger floor area (18 × 34 × 18 cm; 10 cm longer than a standard cage), to accommodate the extra food jar given to some groups.
The control group (n=9) continued to receive chow and water. In addition to chow and water, the Fat group also received vegetable shortening (Crisco, J.M. Smucker Co., Orrville, OH), the Sucrose group was given a bottle of 32% sucrose, and the Sucrose+Fat group received both 32% sucrose and shortening. The chow was in a double jar (as described in Experiment 1) attached to the center of the left wall, and the fat, whipped briefly to produce a smooth texture, was in a jar attached in the right rear corner. Sucrose was offered in a 100-ml bottle, fastened to the right front of the cage with a drinking spout protruding through the front wall. The three experimental groups each had 10 rats. Intakes were measured daily for 20 days. Then the optional foods were removed and animals were maintained on chow and water for 20 days.
Results and Discussion
The primary data for Experiment 3A are shown in Figure 5. Option intake of the Fat group was less than that of the Sucrose and Sucrose+Fat groups, F(2,27) = 23.57, p < 0.001. Option intake was greatest in the first block, (Block F(4,108) = 3.72, p < 0.01); thereafter, the Fat and Sucrose+Fat groups reduced their option intake, while the Sucrose group consumed more in blocks 2 and 5 than in block 1 (Group × Block F(4,108) = 9.05, p < 0.001). Average intakes of chow and the option foods are shown in Table 2, along with proportional nutrient intake. Note that the Sucrose and Sucrose+Fat groups, which were very similar in total intake, consumed disparate proportions of their energy from fat and from carbohydrate. The Sucrose+Fat group selected 40% of option energy from the shortening, composing a diet that was 32% fat, whereas the sucrose group consumed only 3.5% as fat (from chow intake).
Figure 5.
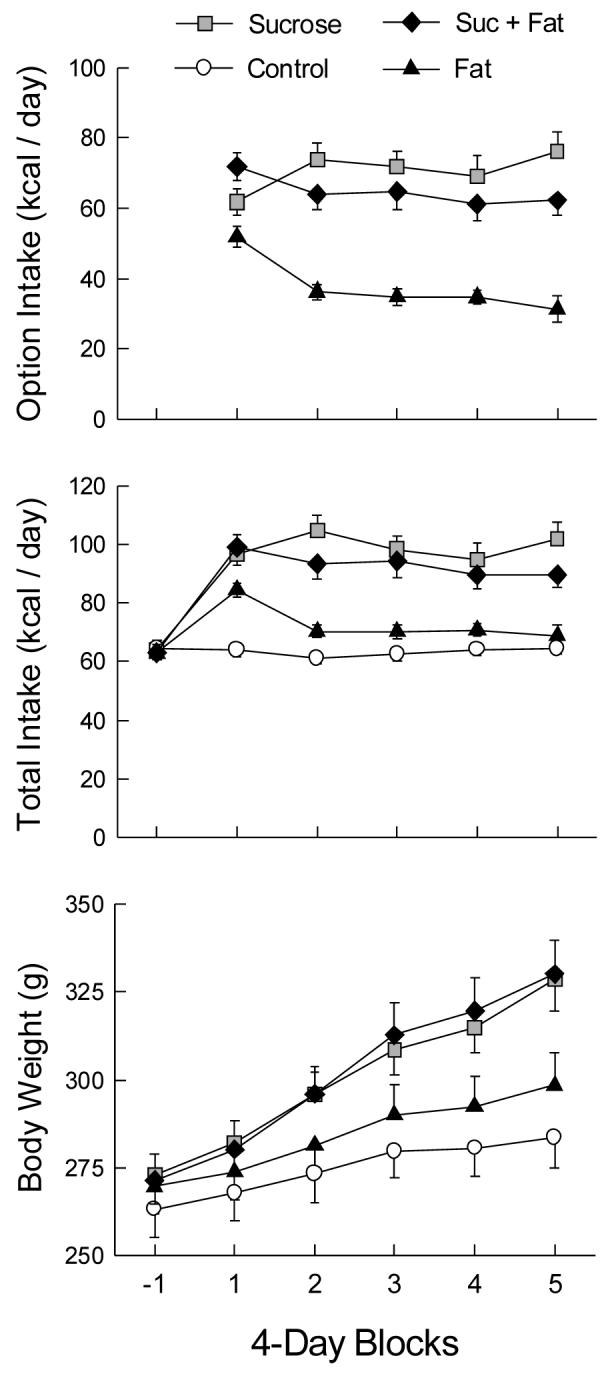
Mean (± sem) option and total energy intakes and body weights in 4-day blocks of Experiment 3A. Block -1 is the baseline measure prior to the introduction of option access. During blocks 1-5, the Sucrose group received a bottle of 32% sucrose solution, the Fat group received a jar of shortening, and the Sucrose+Fat group received both options. The Control group did not receive any optional foods.
Table 2.
Energy intake (kcal) of each food, and proportion of energy by macronutrient in Experiment 3A
| Group | ||||
|---|---|---|---|---|
| Control | Sucrose | Fat | Sucrose+Fat | |
| Food: | ||||
| Chow | 63.2 | 28.7 | 35.1 | 28.3 |
| Sucrose | 70.6 | 39.3 | ||
| Shortening | 37.7 | 25.5 | ||
| Total kcal | 63.2 | 99.3 | 72.8 | 94.1 |
| % Nutrient: | ||||
| Protein | 28.0 a | 8.2 b | 13.5 c | 8.6 b |
| Carbohydrate | 59.9 a | 88.2 b | 28.8 c | 59.7 a |
| Fat | 12.1 a | 3.5 b | 57.7 c | 31.7 d |
Within rows, values with different superscripts differed (percentage protein, p < 0.05; others, p < 0.001).
Total energy intake increased markedly from chow baseline to the first block of the option period, then declined to constant levels (Block F(5,175) = 59.72, p < 0.001). The groups differed in total energy intake during the option period but not during the chow baseline (Group × Block F(15,175) = 15.15, p < 0.001). Intakes of the Sucrose and Sucrose+Fat groups were similar and exceeded those of the Fat and control groups, which consumed similar total energy, (Group F(3,35) = 18.10, p < 0.001). An exception was the first block: the Fat group’s intake was intermediate between the controls and the other groups, F(3,35) = 20.98, p < 0.001.
Body weight gains varied by group, F(3,35) = 18.52, p < 0.001; in parallel with daily energy intakes, the Sucrose and Sucrose+Fat groups were similar and exceeded the weight gains of the Fat and control groups, which did not differ significantly. The group differences in weight gain were apparent by day 4 of option access and were sustained through the option period, Group × Block F(12,140) = 13.16, p < 0.001). Cumulative energy intake was strongly correlated with 20-day weight gain (r = 0.917, p < 0.001). After the access to optional foods was removed, the option groups lost weight. At the end of the 20-day period, the groups no longer differed in body weight (mean 307 g).
Experiment 3A found that a 32% sucrose solution was superior to solid fat in stimulating overeating. Although offering fat as well as sucrose changed the nutrient content of the diet consumed by the Sucrose+Fat animals relative to those with only the sucrose option, overeating and weight gain were very similar in the two groups. Thus the possibility that a ceiling effect limits sucrose or total intake was not resolved by adding a separate fat option: the rats reduced sucrose intake in compensation for the added fat. In a previous study [23], groups of rats maintained on chow were offered liquid options: sucrose solution (8% w/w), oil emulsion (35% w/w), or a mixed emulsion (8% sucrose + 35% oil). In spite of big differences in sucrose concentration and in fat type and form, the results were fairly similar to the present study, with one major exception: the fixed-proportion sugar-oil mixture led to greater overeating and weight gain (relative to sucrose) than did the separate options in Experiment 3A. The mixture group consumed a diet that was 72% fat energy, with the oil in the mixture contributing 90% of its energy. In contrast, the present Sucrose+Fat group selected a diet that was only 32% fat, and took only 40% of option energy as fat. Together these data suggest that an optional mixed food high in fat is the most efficient of the tested conditions for promoting overeating and weight gain. Experiment 3B tested this possibility by taking advantage of well-studied high-fat foods, a high-fat milk diet and chocolate chip cookies. Besides the comparison of sucrose and milk as single options, we tested whether two high-fat mixed foods were more potent than one in promoting overeating.
Experiment 3B
Foods
In addition to chow and 32% sucrose solution as in the previous experiments, some rats were given cookies (Chips Ahoy, Kraft Foods, East Hanover, NJ) and high-fat milk. The milk was first described by Warwick and Weingarten [48] and has been used in many studies of high-fat hyperphagia [e.g., 2,24,49,51]. It was prepared by making a corn oil emulsion in water with Emplex (sodium stearoyl lactylate, American Ingredients, Grandview, MO) and adding evaporated milk (Carnation, Nestle USA, Solon, OH) and maltodextrin (Maltrin 580, Grain Processing, Muscatine, IA). Table 1 lists the energy density and proportional macronutrient composition of the foods.
Table 1.
Energy density and proportion of energy by macronutrient of the foods
| Chow | Sucrose | Shortening | Milk | Cookie | |
|---|---|---|---|---|---|
| kcal/g: | 3.3 | 1.28 | 9.0 | 1.96 | 5.0 |
| % Nutrient: | |||||
| Protein | 28.0 | 0 | 0 | 6.4 | 5.0 |
| Carbohydrate | 59.8 | 100 | 0 | 34.0 | 51.0 |
| Fat | 12.1 | 0 | 100 | 59.6 | 44.0 |
The animals of Experiment 3A were divided into new groups equated for prior group membership and weight gains during option access and the subsequent chow period. The control group (n=9) continued to receive chow and water. In addition to chow and water, the Sucrose group also received a bottle of 32% sucrose, the Milk group was given a bottle of high-fat milk, and the Milk+Cookie group received the same milk and a jar of chocolate chip cookies processed into crumbs. The milk and cookies occupied the same respective locations in the cage as the sucrose and shortening in Experiment 3A. The three experimental groups each had 10 rats. Intakes were measured daily for 20 days.
The primary data for Experiment 3B are shown in Figure 6. Option intake of the Sucrose group was less than that of the Milk and Milk+Cookie groups, F(2,27) = 30.72, p < 0.001. Option intake increased from the first to the second block and then declined in the last two blocks, F(4,108) = 26.33, p < 0.001. Option intakes of the Milk and Milk+Cookie groups remained similar and exceeded that of the Sucrose group throughout the access period (Group × Block F(8,108) = 7.90, p < 0.001). Average intakes of chow and the option foods are shown in Table 3, along with proportional nutrient intake. The Milk and Milk+Cookie group intakes were very similar in overall nutrient composition. The Milk+Cookie group selected 69% of their option energy as milk.
Figure 6.
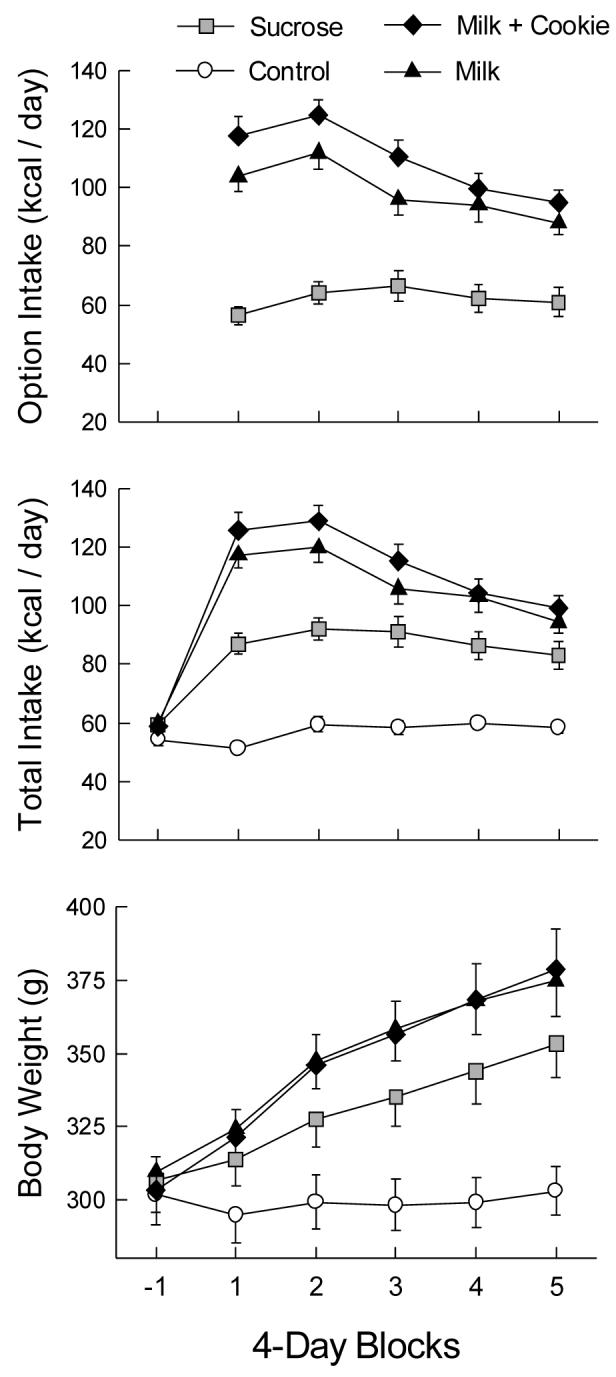
Mean (± sem) option and total energy intakes and body weights in 4-day blocks of Experiment 3B. Block -1 is the baseline measure prior to the introduction of option access. During blocks 1-5, the Sucrose group received a bottle of 32% sucrose solution, the Milk group received a bottle of high-fat milk, and the Milk+Cookie group received high-fat milk and a jar of chocolate chip cookies. The Control group did not receive any optional foods.
Table 3.
Energy intake (kcal) of each food, and proportion of energy by macronutrient in Experiment 3B
| Group | ||||
|---|---|---|---|---|
| Control | Sucrose | Fat | Sucrose+Fat | |
| Food: | ||||
| Chow | 57.4 | 26.0 | 9.3 | 5.1 |
| Sucrose | 61.9 | |||
| Milk | 98.7 | 75.9 | ||
| Cookie | 33.5 | |||
| Total kcal | 57.4 | 87.9 | 109.0 | 114.5 |
| % Nutrient: | ||||
| Protein | 28.0 a | 8.4 b | 8.3 b | 7.0 b |
| Carbohydrate | 59.9 a | 88.0 b | 36.1 a | 40.2 a |
| Fat | 12.1 a | 3.6 b | 55.2 c | 52.9 c |
Within rows, values with different superscripts differed (p < 0.001).
Total energy intake increased from chow baseline to the option period, Block F(5,175) = 127.93, p < 0.001. During the option period, the groups differed in mean energy intake, F(3.35) = 39.53, p < 0.001; intakes of the Milk and Milk+Cookie groups were similar and exceeded those of the Sucrose group, with all three exceeding the control energy intake. Control intake did not change over the option period, and the Milk and Milk+Cookie group intakes differed from those of the Sucrose group throughout option access (Group × Block F(15,175) = 18.21, p < 0.001). Milk and Milk+Cookie energy intake was highest in the first two blocks, and then declined in each subsequent block.
Body weight gain varied by group, F(3,35) = 31.59, p < 0.001; the Milk and Milk+Cookie groups were similar and weighed more than the Sucrose group, and all three markedly exceeded the control weight. Weights differed at each 4-day block (Block F(4,140) = 196.32, p < 0.001). Control weight did not change over the option period, the option groups exceeded controls after only 4 days of option access, and the lesser gain of the Sucrose group than the others was already apparent (Group × Block F(12,140)= 16.29, p < 0.001). A separate analysis of the weight gains of the option groups in blocks 2-5 showed no interaction, indicating that the rate of growth was the same for all three groups during this period. Cumulative energy intake was strongly correlated with 20-day weight gain (r = 0.873, p < 0.001).
Experiment 3B refutes the hypothesis of a ceiling effect on total intake with high-energy options to chow. It remains possible that daily sucrose intake is limited by a ceiling effect, which could reflect a greater satiating effect of sucrose than the high-fat mixed foods. This is consistent with findings that intragastric delivery of high-carbohydrate milk diet is more satiating than that of the high-fat milk diet [24].
General Discussion
This series of experiments was stimulated by the report [43] that offering multiple sources of 32% sucrose solution, an attractive alternative to chow, produced greater stimulation of overeating and weight gain than offering a single source. We began by attempting to replicate the basic finding, and did not obtain it in three experiments. Animals given six bottles of fluid consumed similar amounts of sucrose whether it was available in one or five of the bottles. We tested the effect of flavor variety, hypothesizing that it might increase intake above that of unvarying unflavored sucrose, but this manipulation was also without effect. Finally, we compared sucrose access to that of other palatable optional foods, and found it more effective than pure fat but less effective than a liquid high-fat mixed diet.
Number of sources
Unlike the effects observed by Tordoff for sucrose and for alcohol intake [43,44], increasing the number of bottles of 32% sucrose offered to rats in our experiments did not increase total intake of sucrose or lead to differential excess weight gain. The reason for this discrepancy is not clear; the animals and the basic conditions were very similar between laboratories, and the possibility that larger cages were responsible for Tordoff’s result was not borne out. Our measurements of spillage from sucrose bottles were used to correct the intake values, whereas Tordoff reports no correction in the sucrose study. He discusses spillage elsewhere [44,45], indicating that it might account for 20-30% of effect size in mice and less in rats. By that criterion spillage is unlikely to account for the difference between the sucrose intakes of his one- and five-bottle sucrose groups, and certainly cannot explain the greater weight gain of the five-bottle group. The possible limitation on intakes of the one-bottle group, as described in the discussion of Experiment 1A, is the simplest candidate explanation.
An apparent effect of expanding the number of sucrose bottles from one to five in Experiment 2 suggested that we were detecting an availability effect within rather than between groups. But the increase in intake was no different from that observed during the first few access days in animals that always had only one bottle of sucrose. Even this effect of initial increases in sucrose intake is quite different from Tordoff’s one bottle sucrose group, which consumed only half the amount of sucrose taken by the five bottle group. All groups in Experiment 2 initially drank as much as Tordoff’s five bottle animals.
In offering explanations for the effect of availability in several of his studies Tordoff has suggested that the larger number of containers of food might be analogous to greater abundance of that food in the animal’s environment, a greater rate of encountering food sources. Animals might respond to more frequent encounters with a food by consuming more of it. In agreement with this idea, laboratory simulations of foraging, in which chow is freely available but access to a single bottle of sucrose is constrained by lever-pressing cost, have shown that sucrose intake is greatest when costs are minimal and declines systematically as costs rise and sucrose becomes harder to obtain [4,7]. However, a larger cage with more dispersed bottles may not be comparable to greater difficulty in gaining access to sucrose sources, given their unchanging locations within the cage. Furthermore, other manipulations of availability, such as intermittent access to options [e.g., 10,12,25], can affect meal size but usually do not affect daily intake.
Despite our failure to replicate Tordoff’s finding, it is clear that in some circumstances greater intake is observed from multiple sources than a single source. Tordoff’s sucrose paper includes a nutrient selection experiment showing greater intake of fat or carbohydrate sources when extra cups of those nutrients were provided. Tordoff’s mouse studies revealed greater intake of alcohol [44] and other taste solutions [45] when extra bottles were offered. The number of fluid sources influenced starlings’ intake in a study of chemical repellents [14]. Rats given four cups of standard chow ate more than those given only one, but intakes were equal in other groups given one or four cups of high-fat chow [25]. Rats that were offered two cups of a high-sucrose diet initially overate compared to those with a single cup, though the intakes converged after a few weeks [30]. We did observe an effect of number of sources of water, and perhaps even much larger enclosures with multiple chambers and dispersed sucrose and water sources would be effective. While the cages used in Experiment 1B are large relative to the typical laboratory cage, they are still small compared to the semi-natural foraging environments used in some studies (e.g., 12 m2) [13] or the natural home range of wild rats [20]. Thus, the present results do not argue against the possibility that widespread food availability contributes to human obesity [e.g., soda vending machines in schools, fast-food restaurants 52].
The variety effect
Our impetus for these experiments was to explore the possibility that offering differently flavored sucrose solutions might lead to further increases in intake. However, in Experiment 2 providing five bottles of sucrose with different flavors (e.g., one plain and four with added flavoring) did not alter total intake relative to five bottles of unflavored sucrose, suggesting that this means of producing “variety” was ineffective.
Previous studies of simple variety in flavor added to a constant maintenance diet have found increased intake in single meals [6,17,46]. When nutritionally identical foods were continuously available for several weeks [28], rats given a balanced diet did not eat more when given three different flavors at a time than rats given one cup of unflavored food. Other groups given three flavors of high-fat high-sucrose food ate more than animals given an unflavored version, but this result was not replicated in the same report [28]. In this experiment, the rats in unflavored groups had only one food cup, so the nature of the effect, number of sources vs flavor variety, is not certain. An “isocafeteria” of standard chow plus three rodent chows with added flavors generated greater intake and weight gain than four cups of standard chow [21]. However, the foods varied in nutrient content as well as flavor, so the factors promoting overeating are not clear in this case either.
In Experiment 3 we tested the effect of more pronounced variety, by offering foods that differed in nutrient content and orosensory qualities, and did obtain differential excess energy intake and weight gain. This is not a new result per se; many other investigators have used cafeteria techniques to produce overeating [e.g., 34,36,37]. The new information is a direct comparison of the effect of sucrose to that of other optional foods. Relative to a semisolid source of fat, sucrose was more effective, and adding fat access along with sucrose did not generate additional intake. However, sucrose was less effective than offering mixed high-fat, high-carbohydrate foods, and the high-fat milk was the most effective option. Increasing variety by offering both milk and cookies did not increase overeating and weight gain relative to milk alone.
Other comparisons of overeating induced by separate sources of sucrose and fat have usually found more potent effects of sucrose than fat. Rats adapted to a macronutrient selection diet (casein, starch, and shortening) were consuming almost half their energy as fat, but reduced fat intake when a 32% sucrose option was added, taking half their energy as sucrose and overconsuming total energy [1]. This shift in food choice has an orosensory component: rats given brief choice tests with dilute solutions (to minimize postingestive effects) prefer sucrose to corn oil in the absence of food deprivation [41]. Rats given sucrose solution and chow consumed more option and total energy than rats given corn oil emulsion and chow, but the weight gains of these groups were similar and did not differ from those of the chow controls [23]. In contrast, intake differences were smaller in a comparison of 32% sucrose and pure corn oil options, with only slightly greater sucrose than oil intakes, and similar weight gains [9]. This is surprising given the prior finding of greater intake and weight gain with emulsion than pure oil options [22], but may be explained by the longer period of access in that study, since the intake differences emerged gradually and did not differ significantly in the first few weeks of access. In further comparisons among fat option types and forms, shortening was more effective than pure or emulsified oil in promoting overeating and weight gain [22]. Yet in the present study shortening was not very potent as a single option compared to sucrose, and did not lead to greater overeating when offered in addition to sucrose.
Another study compared 20% sucrose and shortening options offered to male rats fed a high-fat (53% energy) purified diet and found no differences in option or total energy intake and weight gain [47]. The differences from the present study in sucrose concentration, sex of animals and composition of maintenance diet make it difficult to specify the reason for the differential outcomes. However, another study of male rats fed high-fat purified diets of similar density and composition (40-60% fat energy) also found similar weight gains with and without a 37.5% sucrose option; other groups fed a purified diet with macronutrient composition similar to chow gained more weight when given the sucrose option [31]. Together these data suggest that differences in relative sucrose solution potency may be somewhat dependent on the composition of the maintenance diet. But the use of male rats, which were young adults still growing during the study period, makes the data difficult to compare with that of adult females, used here and in the majority of studies cited.
The most powerful stimulus for overeating and weight gain in this study was the high-fat milk diet. Like the sucrose solution, its hydrated form may be important. Rats offered a carbohydrate (sucrose, glucose, Polycose) option in dry form consumed less carbohydrate and gained less weight than rats given the same saccharides in hydrated form (as a solution or gel) [39]. Offering a high-sucrose complete diet in hydrated and dry forms also yielded different energy intakes: animals consuming the hydrated diet ate more and gained more weight than those given dry food, and animals given both forms preferred the hydrated version [30]. A moderate-fat liquid diet (Ensure), which at 1 kcal/ml has a lower energy density than 32% sucrose, can increase weight gain and is eaten preferentially when offered as an option to a high-energy solid diet designed to produce obesity [18,19]. Other studies offering one or more solid foods (e.g., crackers, cookies, chocolate) as options to chow obtained total intakes of about 100 kcal/d [34,35], which is less than the total intake of the milk groups in the present study.
Potential reasons for the superiority of high-fat milk to sucrose solution in stimulating overeating are its greater energy density, possibly greater palatability, and mixed-nutrient composition. The energy density difference between the milk diet and 32% sucrose does not appear to be a critical variable: rats maintained on this diet at 1.15 kcal/ml (close to that of 32% sucrose) gained the same amount of weight as rats given the 2.3 kcal/ml diet [50]. The milk diet is palatable to rats [48], but the relative attractiveness of milk diet and 32% sucrose solution has not been compared directly. As a more complex substance with attractive components (oil emulsion, maltodextrin), the milk may be more palatable than 32% sucrose; a two-bottle sham-feeding test would be informative on this issue. Some findings suggest, however, that post-oral factors may be more important. In separate studies, chow-fed rats given a flavored saccharin solution paired with intragastric infusions of the high-fat milk diet consumed more calories than did rats given the flavored saccharin solution paired with intragastric infusions of 32% sucrose (112.8 vs. 91.0 kcal/day [3,24]. The milk diet’s high-fat mixed-nutrient composition (which reduces the dilution of essential nutrients, such as protein, in the overall diet) may be the most important difference that elevates its effectiveness relative to the sucrose solution.
The present experiments reinforce the notion that stimulation of overeating and weight gain by palatable foods can depend on multiple factors. The number of discrete sources of attractive foods may be less important than their physical form and nutrient composition. One combination that could be quite potent is to offer a dual option: high-fat milk and concentrated sucrose solution. This would bring together the effects of palatability, liquid form, and nutrient variety, and provide another test of the effect of orosensory variety. The sustained overeating in response to optional foods remains a powerful technique for studying diet-induced obesity in rodents.
References
- [1].Ackroff K, Sclafani A. Sucrose-induced hyperphagia and obesity in rats fed a macronutrient self-selection diet. Physiol Behav. 1988;44:181–7. doi: 10.1016/0031-9384(88)90135-7. [DOI] [PubMed] [Google Scholar]
- [2].Ackroff K, Sclafani A. Energy density and macronutrient composition determine flavor preference conditioned by intragastric infusions of mixed diets. Physiol Behav. 2006;89:250–60. doi: 10.1016/j.physbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [3].Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–41. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- [4].Castonguay TW, Philips S, Collier GH. Sucrose procurement cost and dietary selection. Nutr Behav. 1985;2:201–22. [Google Scholar]
- [5].Cezard JP, Broyart JP, Cuisinier-Gleizes P, Mathieu H. Sucrase-isomaltase regulation by dietary sucrose in the rat. Gastroenterology. 1983;84:18–25. [PubMed] [Google Scholar]
- [6].Clifton PG, Burton MJ, Sharp C. Rapid loss of stimulus-specific satiety after consumption of a second food. Appetite. 1987;9:149–56. doi: 10.1016/0195-6663(87)90044-4. [DOI] [PubMed] [Google Scholar]
- [7].Collier G, Johnson DF. Sucrose intake as a function of its cost and the cost of chow. Physiol Behav. 2000;70:477–87. doi: 10.1016/s0031-9384(00)00303-6. [DOI] [PubMed] [Google Scholar]
- [8].Collier GH, Johnson DF, CyBulski KA, McHale CA. Activity patterns in rats (Rattus norvegicus) as a function of the cost of access to four resources. J Comp Psychol. 1990;104:53–65. doi: 10.1037/0735-7036.104.1.53. [DOI] [PubMed] [Google Scholar]
- [9].Corwin RL, Rice HB. Effects of enterostatin in non-food-deprived rats with limited or continuous access to oil or sucrose. Physiol Behav. 1998;65:1–10. doi: 10.1016/s0031-9384(98)00078-x. [DOI] [PubMed] [Google Scholar]
- [10].Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- [11].Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. In: Crawley J, Gerfen C, McKay R, Rogawski M, Sibley D, Skolnick P, editors. Current protocols in neuroscience. Wiley; New York: 2006. [DOI] [PubMed] [Google Scholar]
- [12].Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [13].Ellison G. Stress and alcohol intake: the socio-pharmacological approach. Physiol Behav. 1987;40:387–92. doi: 10.1016/0031-9384(87)90066-7. [DOI] [PubMed] [Google Scholar]
- [14].Hile AG, Tordoff MG. Influence of the number of repellent-treated and untreated food or water containers on intake by the European starling. Appetite. 2005;45:81–5. doi: 10.1016/j.appet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [15].Hirsch E, Ball E, Godkin L. Sex differences in the effects of voluntary activity on sucrose-induced obesity. Physiol Behav. 1982;29:253–62. doi: 10.1016/0031-9384(82)90012-9. [DOI] [PubMed] [Google Scholar]
- [16].Kanarek RB, Hirsch E. Dietary-induced overeating in experimental animals. Fed Proc. 1977;36:154–8. [PubMed] [Google Scholar]
- [17].Le Magnen J. Increased food intake induced in rats by changes in the satiating sensory input from food (first published in French in 1956) Appetite. 1999;33:33–5. doi: 10.1006/appe.1999.0257. [DOI] [PubMed] [Google Scholar]
- [18].Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–9. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- [19].Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol. 2002;282:R46–54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- [20].Lore R, Flannelly K. Habitat selection and burrow construction by wild Rattus norvegicus in a landfill. J Comp Physiol Psychol. 1978;92:888–96. [Google Scholar]
- [21].Louis-Sylvestre J, Giachetti I, Le Magnen J. Sensory versus dietary factors in cafeteria-induced overweight. Physiol Behav. 1984;32:901–5. doi: 10.1016/0031-9384(84)90275-0. [DOI] [PubMed] [Google Scholar]
- [22].Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 1989;46:937–46. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- [23].Lucas F, Sclafani A. Hyperphagia in rats produced by a mixture of fat and sugar. Physiol Behav. 1990;47:51–5. doi: 10.1016/0031-9384(90)90041-2. [DOI] [PubMed] [Google Scholar]
- [24].Lucas F, Ackroff K, Sclafani A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am J Physiol. 1998;275:R1511–R22. doi: 10.1152/ajpregu.1998.275.5.R1511. [DOI] [PubMed] [Google Scholar]
- [25].Mathes CM, Rowland NE. Effect of increased food availability on food intake and body weights in rats. Appetite. 2004;42:383. [Google Scholar]
- [26].McCrory MA, Fuss PJ, McCallum JE, Yao M, Vinken AG, Hays NP, Roberts SB. Dietary variety within food groups: association with energy intake and body fatness in men and women. Am J Clin Nutr. 1999;69:440–7. doi: 10.1093/ajcn/69.3.440. [DOI] [PubMed] [Google Scholar]
- [27].McCrory MA, Fuss PJ, Saltzman E, Roberts SB. Dietary determinants of energy intake and weight regulation in healthy adults. J Nutr. 2000;130:276S–9S. doi: 10.1093/jn/130.2.276S. [DOI] [PubMed] [Google Scholar]
- [28].Naim M, Brand JG, Kare MR, Carpenter RG. Energy intake, weight gain and fat deposition in rats fed flavored, nutritionally controlled diets in a multi-choice (“cafeteria”) design. J Nutr. 1985;115:1447–58. doi: 10.1093/jn/115.11.1447. [DOI] [PubMed] [Google Scholar]
- [29].Ramirez I. When does sucrose increase appetite and adiposity? Appetite. 1987;9:1–19. doi: 10.1016/0195-6663(87)90049-3. [DOI] [PubMed] [Google Scholar]
- [30].Ramirez I. Feeding a liquid diet increases caloric intake, weight gain and body fat in rats. J Nutr. 1987;117:2127–34. doi: 10.1093/jn/117.12.2127. [DOI] [PubMed] [Google Scholar]
- [31].Rattigan S, Clark MG. Effect of sucrose solution drinking option on the development of obesity in rats. J Nutr. 1984;114:1971–7. doi: 10.1093/jn/114.10.1971. [DOI] [PubMed] [Google Scholar]
- [32].Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychol Bull. 2001;127:325–41. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- [33].Riby JE, Kretchmer N. Effect of dietary sucrose on synthesis and degradation of intestinal sucrase. Am J Physiol. 1984;246:G757–63. doi: 10.1152/ajpgi.1984.246.6.G757. [DOI] [PubMed] [Google Scholar]
- [34].Rogers PJ, Blundell JE. Meal patterns and food selection during the development of obesity in rats fed a cafeteria diet. Neurosci Biobehav Rev. 1984;8:441–53. doi: 10.1016/0149-7634(84)90003-4. [DOI] [PubMed] [Google Scholar]
- [35].Rolls BJ, Rowe EA, Turner RC. Persistent obesity in rats following a period of consumption of a mixed, high-energy diet. J Physiol. 1980;298:415–27. doi: 10.1113/jphysiol.1980.sp013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rolls BJ, Van Duijvenvoorde PM, Rowe EA. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol Behav. 1983;31:21–7. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- [37].Sclafani A, Springer D. Dietary obesity in adult rats: Similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–71. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- [38].Sclafani A, Aravich PF, Landman M. Vagotomy blocks hypothalamic hyperphagia in rats on a chow diet and sucrose solution, but not on a palatable mixed diet. J Comp Physiol Psychol. 1981;95:720–34. doi: 10.1037/h0077830. [DOI] [PubMed] [Google Scholar]
- [39].Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: Effects of saccharide type, form, and taste. Neurosci Biobehav Rev. 1987;11:155–62. doi: 10.1016/s0149-7634(87)80020-9. [DOI] [PubMed] [Google Scholar]
- [40].Sclafani A. Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev. 1987;11:131–53. [PubMed] [Google Scholar]
- [41].Sclafani A, Ackroff K. Deprivation alters rats’ flavor preferences for carbohydrates and fats. Physiol Behav. 1993;53:1091–9. doi: 10.1016/0031-9384(93)90364-l. [DOI] [PubMed] [Google Scholar]
- [42].Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–9. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- [43].Tordoff MG. Obesity by choice: the powerful influence of nutrient availability on nutrient intake. Am J Physiol. 2002;282:R1536–9. doi: 10.1152/ajpregu.00739.2001. [DOI] [PubMed] [Google Scholar]
- [44].Tordoff MG, Bachmanov A. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin Exp Res. 2003;27:600–6. doi: 10.1097/01.ALC.0000060529.30157.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tordoff MG, Bachmanov A. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–24. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Treit D, Spetch ML, Deutsch JA. Variety in the flavor of food enhances eating in the rat: A controlled demonstration. Physiol Behav. 1983;30:201–11. doi: 10.1016/0031-9384(83)90007-0. [DOI] [PubMed] [Google Scholar]
- [47].Trout DL, Moy NL, Putney JD, Johnson DA. Dietary regimens for inducing mild hyperphagia, obesity and hyperinsulinemia in rats. Nutr Rep Int. 1978;18:227–33. [Google Scholar]
- [48].Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–R7. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- [49].Warwick ZS, McGuire CM, Bowen KJ, Synowski SJ. Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. Am J Physiol. 2000;278:R196–200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- [50].Warwick ZS, Synowski SJ, Bell KR. Dietary fat content affects energy intake and weight gain independent of diet caloric density in rats. Physiol Behav. 2002;77:85–90. doi: 10.1016/s0031-9384(02)00816-8. [DOI] [PubMed] [Google Scholar]
- [51].Warwick ZS. Dietary fat dose dependently increases spontaneous caloric intake in rat. Obes Res. 2003;11:859–64. doi: 10.1038/oby.2003.118. [DOI] [PubMed] [Google Scholar]
- [52].Wiecha JL, Finkelstein D, Troped PJ, Fragala M, Peterson KE. School vending machine use and fast-food restaurant use are associated with sugar-sweetened beverage intake in youth. J Am Diet Assoc. 2006;106:1624–30. doi: 10.1016/j.jada.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [53].Woods SC, Kenney NJ. Alternatives to homeostasis. Behav Brain Sci. 1979;1:123–4. [Google Scholar]


