Abstract
Consumption of a diet high in fat is a risk factor for a number of health problems, including obesity, type 2 diabetes and cardiovascular disease. Considerable pharmacological, genetic, and molecular evidence suggests that the hypothalamic melanocortin system plays a critical role in the control of food intake and body weight and, specifically, in fat ingestion. Administration of a melanocortin antagonist, agouti-related peptide (AgRP) (83–132) selectively increases intake of pure fat and high-fat mixed diets. Here, we examined possible mechanisms for this fat-specific effect of AgRP (83–132). In Experiment 1, we determined that intracerebroventricular administration of AgRP (83–132) selectively increased operant responding for a peanut oil, but not a sucrose, reinforcer when tested under a progressive ratio schedule. Experiment 2 employed a Pavlovian conditioning paradigm, in which icv AgRP enhanced appetitive responding toward stimuli that had previously been paired with peanut oil and reduced responding toward stimuli previously paired with sucrose, in the absence of consumption of either macronutrient. Finally, in Experiment 3, we tested the hypothesis that the MC system acts in anticipation of a fat consumption and found that hypothalamic AgRP mRNA was slightly, though not significantly, elevated in an environment predicting fat availability relative to one predicting carbohydrate availability. Collectively, these data indicate that, in addition to increasing free intake of dietary fats, AgRP (83–132) promotes responding for the opportunity to consume a fat reinforcer, as well as appetitive responding to fat-paired stimuli in the absence of ingestive stimulation. These results suggest a possible role for AgRP in the increased fat intake associated with obesity.
INTRODUCTION
Currently, two-thirds of the adult population in the United States is overweight, with half of those classified as obese (Ogden et al., 2006). Consumption of a high-fat diet is associated with a greater risk of obesity (Blundell et al., 1996, Bray & Popkin, 1998, Blundell & Cooling, 2000), as well as being a risk factor for a variety of other health conditions such as type 2 diabetes, cardiovascular disease, hypertension and some cancers(Srinath Reddy & Katan, 2004, Stoeckli & Keller, 2004). The fat content of foods is highly correlated with their energy density and consumption of foods high in energy density is strongly implicated in the development of overweight and obesity, largely attributed to passive overconsumption and low satiety values (Gerstein et al., 2004, Ledikwe et al., 2006). Further, a reduction in the energy density and fat content of the diet has been demonstrated to effectively reduce caloric intake and body weight and these dietary characteristics are also associated with successful weight loss maintenance (Bray & Popkin, 1998, Wing & Phelan, 2005). Clearly, an understanding of the biological and behavioral components that contribute to the selection of foods high in fat is critical to the study of body weight and energy regulation.
A compelling body of evidence implicates the hypothalamic melanocortin (MC) system as one of the central effectors controlling food intake and energy balance (Seeley et al., 2004, Lee & Wardlaw, 2007) and a number of findings suggest that the MC system effects food intake in a fat-specific way. When allowed to freely choose between separate sources of the three macronutrients -- fat, protein and carbohydrate -- agouti mice consumed a significantly greater proportion of their calories from fat and fewer from carbohydrates relative to their wild type counterparts (Koegler et al., 1999). Similarly, i3vt administration of exogenous AgRP (83–132) selectively increases intake of high-fat (40%), but not low-fat (4%) diet when given simultaneously (Hagan et al., 2001).
Peripheral administration of MTII, an MCR-3/4 agonist, selectively reduced fat intake in a three-macronutrient choice test (Samama et al., 2003). This effect appears to be mediated through the MCR-4, as the same result was observed using an agonist selective for this receptor and no effect of MTII treatment on macronutrient intake was observed when given to mice deficient for MCR-4 (MCR-4 −/−). Finally, MCR-4 −/− mice display marked and sustained increases in hyperphagia and body weight gain when shifted from a low-fat (12.8%) to a moderate-fat (25.1%) diet relative to their wild-type counterparts, an effect which is not observed in MCR-3−/− mice (Butler et al., 2001).
There are many factors which may influence macronutrient selection, including pre-ingestive components, such as taste, texture and palatability, and post-ingestive signaling, including differential release of gut peptides (Buchan, 1999). In addition, there are several behavioral components of ingestion that may be affected by the MC system and have an influence on food selection, including food seeking, appetitive and consummatory processes. Although previous work clearly indicates that the MC system alters intake of fats, important questions regarding the mechanism by which this occurs remain unanswered.
The experiments here were designed to address behavioral aspects of the selective increase in fat consumption by AgRP (83–132). We hypothesized that not only does AgRP increase intake of fats when they are freely available, but that it would also increase the effort expended to acquire fats and that it enhances responding to stimuli associated with fat consumption. To test these hypotheses we employed an operant responding task in Experiment 1 to assess whether AgRP would selectively increase active responding to obtain fats versus carbohydrates. Experiment 2 utilized a Pavlovian conditioning paradigm assessed the necessity of fat consumption for AgRP to facilitate fat-specific responding. Finally, in Experiment 3, we asked whether AgRP plays a role in the anticipation of fat consumption by presenting animals with cues that had been paired specifically with intake of either sucrose or peanut oil and measuring hypothalamic AgRP gene expression.
MATERIALS AND METHODS
Subjects
Subjects were male Long-Evans (Experiments 1 & 3) or Sprague-Dawley (Experiment 2) rats (Harlan Inc., Indianapolis, IN). The rats were approximately 90 days old and weighed 275–300 grams on arrival at the laboratory. Subjects were individually housed and maintained on a 12:12 light-dark cycle. All experimental procedures were conducted during the light phase. Water was available ad libitum in the home cage throughout all the experiments. For Experiments 1 & 2, subjects were maintained on standard rat chow at 85% of their free feeding body weight, except where noted. For Experiment 3, food was provided as described below. All procedures were approved by the University of Cincinnati Animal Care and Use Committee (Experiments 1 & 3) or the Purdue University Animal Care and Use Committee (Experiment 2).
Surgery (Experiments 1 & 2)
Each rat was anesthetized with ketamine/xylazine (10:6.5 solution, 1 ml/kg) and implanted with a cannula aimed at the 3rd-cerebral ventricle (i3vt). Surgery was performed as previously described with coordinates for cannula placement on the midline, 2.2 mm posterior to bregma, and 7.5 mm ventral to dura (Chavez et al., 1995). Animals were allowed to recover to pre-surgical body weight prior to beginning any experimental procedures.
Drugs
AgRP (83–132) was purchased from Phoenix Pharmaceuticals Inc. (Mountain View, CA). AgRP (83–132) was dissolved in physiological saline, which also served as the control solution. All i3vt injections were delivered in a volume of 2 μl. A dose of 1 nmol AgRP was used in all experiments. This dose has previously been demonstrated to significantly increase food intake and, more specifically, to selectively increase intake of a diet high in fat between 1 and 2 hr post-infusion (Rossi et al., 1998, Hagan et al., 2001).
Apparatus
Experiment 1
All conditioning and testing procedures were conducted in four identical conditioning chambers constructed of aluminum end walls and clear Plexiglas sides and measuring 21.6 × 21.6 × 27.9 cm. A grid of 0.48 cm in diameter stainless steel bars, spaced 1.9 cm apart, served as the floor of each chamber. A food cup was located on one end wall of each chamber inside a 5 × 5 cm recessed opening. Two levers were located approximately 3 cm to the left and right of the food cup, level with the top of the opening. Only the right lever was active during this experiment. All experimental events were controlled and recorded by computers located in an adjoining room running ABET software (Lafayette Instruments; Lafayette, IN).
Experiment 2
All conditioning and testing procedures were conducted in eight identical conditioning chambers constructed of aluminum end walls and clear Plexiglas sides and measuring 21.6 × 21.6 × 27.9 cm. A grid of 0.48 cm in diameter stainless steel bars, spaced 1.9 cm apart, served as the floor of each chamber. A food cup was located on one end wall of each chamber. The light CS was produced by a 6-W jeweled panel light located 6 cm above the food cup. The 300-Hz tone CS was produced by a Radio Shack Piezo Alerting Buzzer (catalog No. 273-068) located outside each chamber by the end wall with the food cup. All experimental events were controlled by computers located in an adjoining room.
Changes in appetitive behavior (behavior directed toward the food cup) were monitored by a computer-controlled infrared monitoring system. Sixteen electronic beams (an ENV 256C infrared Photobeam Controller and D16–712 Photobeam Input, Med Associates, Inc.) lined each cage from side wall to side wall. Interruptions of the beam directly in front of the food cup were monitored, and data analyzed, by software developed in the laboratory for the measure of appetitive behavior. The amount of appetitive behavior was determined by the percentage of time the beam directly in front of the food cup was broken. The computer and relay panel operating the beams were located in an adjoining room.
Experiment 3
Two contexts were used for this experiment. Context A was a suspended cage (16″ × 14″ × 7″) with solid stainless steel side and back walls and a wire grid front and floor. Food was made available through the front grid; water and nutrient solution bottles were placed such that the ball-bearing spouts protruded 1–2 inches inside the cage and 0.5–1 inch off the floor. Context B was a transparent plastic shoebox-style cage (10.5″ × 19″ × 8″) with a wire top and kitty litter (FMV brand) covering the floor. In addition, 4–5 drops of almond extract (Durkee, Ankeny, IA) were added to the kitty litter daily. Food was available in a hanging hopper on the front wall; bottles containing water and nutrient solutions were placed on the wire top such that the ball-bearing spouts protruded at a downward 45 angle approximately 1 inch into the cage.
Sucrose solution (15% sucrose, Kroger brand, Cincinnati, OH, in water) and peanut oil emulsion (6.6 % peanut oil, Planters brand/Nabisco, East Hanover, NJ, and 0.6% emulsifier, Emplex, American Ingredients Company, Kansas City, MO, in water) were prepared fresh daily. The two solutions were matched on caloric density and pH.
Procedure
Experiment 1
Following recovery from surgery, animals were divided into two weight-matched groups and all animals were gradually reduced to 85% of their free-feeding body weight over a period of approximately 7 days. For one group (n = 9), a 45 mg sucrose pellet (Test Diet, Richmond, IN) served as a reinforcer and for the second group (n = 10) a 0.3 ml drop of 100% peanut oil (Planters brand/Nabisco, East Hanover, NJ) served as the reinforcer. Training consisted of 22 daily, 60-min sessions. Subjects were run in squads of four. The first three sessions were autoshaping sessions in which each lever press earned a reinforcer and in addition for every 5 min that passed without earning a reinforcer, one was delivered in order to familiarize the animals to the location of the food cup and the food reinforcer itself. Animals were then exposed to a series of increasing fixed ratio (FR) schedules: FR1 (9 sessions), FR3 (3 sessions), FR5 (1 session). Following these FR sessions, progressive ratio (PR) training began. The response requirements of the PR schedule increased through the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 693, 737, 901. This type of PR schedule with an incrementally increasing requirement reduces inter-session variability and, by reducing the number of reinforcers received, minimizes the effects of satiety relative to a fixed increment PR (e.g., PR2). This PR schedule has been used in numerous studies assessing the effects of pharmacological treatments on responding for food reinforcement (Brown et al., 1998, Ward & Dykstra, 2005). PR sessions were terminated for an individual animal when 20 minutes had elapsed without earning a reinforcer. The last completed bar press requirement was termed the breakpoint. Seven PR sessions were conducted to achieve stable responding (± 10% with no consistent upward or downward trend over 3 sessions). Following the final PR training session, animals were returned to ad libitum chow for 4 days.
One hour before testing, food was removed from the home cages and i3vt injections were administered. Two test sessions were conducted with all animals receiving 1.0 nmol AgRP (83–132) (i3vt in 2 μl saline) on one test day and i3vt 2 μl saline on the other test day. Injections were counterbalanced such that half that animals in each reinforcer group received saline on test day 1 and AgRP on test day 2 and the other half received the opposite treatment order. The two test sessions were separated by 7 days without exposure to the operant chambers or food reinforcers to ensure that the effect of the previous treatment had washed out (food intake (g ± SEM) on Day 6 post-test: Sal = 22.23 ± 1.19, AgRP = 24.04 ± 1.56). Each test session was the same as the PR sessions during the training phase described above.
Immediately following the test session, rats were returned to their home cages and chow intake was measured at 1 h, 24 h and every subsequent 24 h until there were no longer significant differences between AgRP- and saline-treated animals. Intake at 1 h post-test was measured to ensure that the AgRP infusion was effective and only animals that increased their food intake in response to AgRP over this period (relative to their intake following saline administration) were retained for analysis, yielding final group sizes of n = 7 (peanut oil reinforcer) and n = 6 (sucrose reinforcer).
Experiment 2
The procedure for this experiment was based on a Pavlovian conditioning paradigm developed to assess the effects of pharmacological or other manipulations on macronutrient-associated stimuli(Davidson et al., 1997, Benoit et al., 1999). Each 30-min conditioning session contained 16 conditioning trials lasting 20 seconds each. For the first 10 s (pre-CS period) of each trial, neither CS was presented. However, appetitive behavior was recorded by the computer-controlled infrared monitoring system. In the second 10 s (CS period) of each trial the tone or light was presented and appetitive behavior was again measured. All trials terminated with the delivery of either 0.3 ml Planters brand 100% peanut oil or two 45-mg Noyes Formula F 100% sucrose pellets. For half the subject, the tone signaled delivery of sucrose pellets and the light signaled the delivery of peanut oil. The remaining subject received the reverse CS-US combinations.
The first 4 conditioning sessions contained trials with only one CS-US relationship. Two sessions contained tones followed by the appropriate US, while the remaining two sessions contained presentation of the light followed by appropriate US. In the remaining 13 sessions (10 pre-surgery and 3 post-surgery), both tone and light followed by the appropriate US occurred for 8 trials each. The mean ITI continued to be 120 s throughout conditioning, and the order of trial presentation was determined semi-randomly each day, with the qualification that no more than three of a single trial type could occur consecutively. Subjects were run in squads of eight. Following the final post-surgery training session, animals were returned to ad libitum lab chow for 4 days.
One hour before testing, food was removed from the home cages and i3vt injections were administered. Animals were divided into two equal groups matched with respect to performance at the end of training. One group of rats (n = 8) received i3vt 2 μl saline and the second group (n = 8) received 1.0 nmole AgRP (83–132) (i3vt in 2 μl saline). After 1 hr, the rats were taken to the conditioning chambers for test sessions. The 30-min sessions consisted of eight nonreinforced presentations of each CS following a pattern of TLLTLTTL. The mean ITI was 120 s, and appetitive behavior was measured during the pre-CS and CS periods.
Immediately following the extinction test session, rats were returned to the home cages and chow intake was measured at 1 and 24 h. Two AgRP-treated animals with intakes below the average for all animals that received saline were removed from the analysis, as this indicated that the AgRP was likely not effective. In order to reduce bias in the food intake analysis (as saline-treated animals were predicted to consume less food than AgRP-treated animals) and equate group sizes, the two saline-treated animals with the lowest intakes were then also removed, resulting in final group sizes of n = 6 per treatment condition.
Experiment 3
Prior to beginning the experiment, animals were divided into two weight-matched groups. One group received the 15% sucrose solution when in Context A and the 6.6% peanut oil emulsion in Context B (as described above), while the other received the opposite context-nutrient pairings. Food was removed from all animals’ home cages the night before beginning experimental procedures, water was available in the home cage and both experimental contexts at all times. Each day at the same time, animals were weighed and placed into an individual cage of the assigned context. After two hours, a bottle containing 50 mL of the appropriate nutrient solution was placed on the cage. One hour later, a hopper containing standard chow was placed on the cage. Four hours after receiving the food, both the chow hopper and the nutrient bottle were removed and all animals returned to their home cages. Daily intake of the nutrient solution was recorded. This procedure was repeated for 12 days according to the following context schedule: ABABBABAABAB. On the 13th day, animals were placed into Context A at the accustomed time, then removed from the context two hours later (the expected time to receive the nutrient bottle) and sacrificed. The brain was removed and a mediobasal portion of the hypothalamus dissected out (defined caudally by the mammillary bodies, rostrally by the optic chiasm, laterally by the optic tract, and superiorly by the apex of the hypothalamic third ventricle), quick-frozen on dry-ice and stored at −80°C.
The tissue was homogenized in 1 ml TriZol (MRC, Inc; Cincinnati, OH). RNA was isolated using the product insert protocol. cDNA was synthesized from 5 mg total RNA and diluted 1:2 with nuclease-free water. Primers for AgRP (5′-ATCTAGCACCTCTGCCAAA-3′) and NPY (5″-GGGGCATTTTCTGTGCTTT-3′) were obtained from Integrated DNA Technologies (Coralville, IA). Primer sets for L32, NPY, and AgRP were optimized for each gene to determine that correlation coefficients and PCR efficiency were within acceptable ranges according to manufacturer specification. L32 is a ribosomal RNA that is used as a reference control gene(Thellin et al., 1999). Real-Time PCR was performed in triplicate using an iCycler and iQ SYBER Green Mix (BioRad; Hercules, CA) according to the product insert with a 2-step amplification (95°C for 10 sec, annealing temp for 30 sec) × 40 cycles.
RESULTS & DISCUSSION
Experiment 1
Previous experiments demonstrating the effects of the MC system on macronutrient selection have employed paradigms in which all foods were freely available for consumption. In this experiment, we introduce a response requirement to obtain sucrose (100% carbohydrate) and peanut oil (100% fat) reinforcers using an operant conditioning paradigm. Here, animals were trained under food restricted conditions using a progressive ratio schedule until they achieved a stable breakpoint (defined as the final completed bar press requirement prior to a 20 minute period without earning a reinforcer). Using this type of schedule allows for the determination of the maximum number of responses that each individual animal will engage in for a specific reinforcer under a given set of conditions. After being returned to ad libitum feeding, all animals were administered AgRP (82–132) on one test day and saline on a second test day in order to assess the effects of AgRP (83–132) on bar pressing for fat and carbohydrate reinforcers on the PR schedule.
Operant training
During each level of FR training, mean earned reinforcers (± SEM) was significantly greater (p < 0.05) for animals receiving sucrose (autoshaping: 163.6 ± 41.79, FR1: 233.49 ± 19.88, FR3: 375.33 ± 40.81, FR5: 407.40 ± 59.96) than those responding for peanut oil (autoshaping: 32.05 ± 8.32, FR1: 42.83 ± 2.64, FR3: 51.48 ± 4.04, FR5: 43.14 ± 3.04). There was no significant difference in earned reinforcers across the different fixed ratios for animals receiving the peanut oil reinforcer, while there was a significant main effect of schedule for animals receiving sucrose (p < 0.05). Post-hoc tests indicated that only FR1 to FR3 showed a significant increase in earned reinforcers when each consecutive set of schedules was compared.
Figure 1a shows responding across the 7 PR training sessions. An ANOVA was run on these data including Reinforcer Type (sucrose vs. peanut oil) as a between-subjects variable and Session (1–7) as a within-subjects variable. No significant main effects or interactions involving Reinforcer Type indicates that animals were earning a similar number of peanut oil and sucrose reinforcers under the progressive ratio schedule. Further, there was no main effect of Session, demonstrating that responding was stable using this schedule prior to the test phase.
Figure 1.
a) Mean (± SEM) number of reinforcers earned during progressive ratio training sessions in Experiment 1. Solid circles represent sucrose reinforcers and open circles represent peanut oil reinforcers. b) Mean (± SEM) number of reinforcers earned during progressive ratio test sessions in Experiment 1. Solid bars represent saline treatment and hatched bars represent AgRP (83–132) treatment. * significantly different from saline, p < 0.05
AgRP PR test
Mean number of reinforcers earned when reaching breakpoint is depicted in Figure 1b. An mixed ANOVA including Reinforcer Type as a between-subjects variable and Drug Treatment as a within-subjects variable yielded significant main effects of both Reinforcer Type and Drug Treatment (p < 0.05), but no interaction of these factors (p = 0.08). However, one-way analyses, based on our a priori hypothesis, indicated a significant increase in peanut oil reinforcers earned when treated with AgRP (83–132) relative to saline treatment (p < 0.05), while there was no drug treatment effect on the number of sucrose reinforcers earned. The similarity in the level of responding for sucrose following either AgRP or saline administration, along with the fact that these were higher than levels of responding for peanut oil following either treatment, raises the question of whether this could be a ceiling effect. However, it is clear that the animals were capable of responding at much higher levels than seen during the test, as indicated by responses observed during training sessions (see Figure 1), making this a highly unlikely explanation for this result.
Food intake (post-test)
Administration of AgRP (83–132) increased 24-hr chow intake relative to saline (36.98 ± 5.12 versus 28.00 ± 2.81 g; p < 0.05) one day after the post-test. 24-hr intake was not different between animals treated with AgRP (24.24 ± 1.56 g) and saline (22.23 ± 1.19 g) 7 days following the test session (i.e., at the time of the second test session). Food intake effects were independent of the type of reinforcer received.
Experiment 2
The results of Experiment 1 demonstrate that, in addition to selectively increasing intake of freely available fat sources, AgRP (83–132) also increases the number of responses animals will make to receive a fat, but not a carbohydrate, reinforcer. During the test phase of Experiment 1, however, animals were still allowed to consume the fat reinforcers, leaving open the question of whether AgRP is increasing fat intake by directly influencing positive aspects of consumption (e.g., palatability, postingestive feeback) or by increasing approach responses to fat-related stimuli independent of consummatory behavior. Separating these two alternatives requires the use of a paradigm to assess the behavioral effects of AgRP (83–132) without the confounding measures of food intake itself. Prior work has demonstrated that rats are sensitive to metabolic modifications of behavior directed at stimuli that have been previously paired with either fat or carbohydrate (Davidson et al., 1997, Benoit et al., 1999). Under certain metabolic conditions (i.e., pharmacologic lipoprivation), rats will increase appetitive behavior toward stimuli that have been paired with fat, but not carbohydrate outcomes (Davidson et al., 1997). In the procedure used here, rats were trained to expect the delivery of peanut oil following one conditioned stimulus (CS) (e.g., a light) and to expect the presentation of sucrose pellets following a different CS (e.g., a tone). The oil or sucrose serves as an unconditioned stimulus (US). The animals learned to approach a food cup to obtain the oil or sucrose US when the appropriate CS (light or tone) is presented. After this training, the animals were treated with either saline or AgRP (83–132) and given test trials in which either the “oil” stimulus or the “sucrose” stimulus is presented, but no oil or sucrose is delivered. By measuring responding to each CS during this test session, Experiment 2 assessed the effects of AgRP (83–132) on responding to stimuli that had been previously paired with either fat or carbohydrate in the absence of consumption of these nutrients.
Pavlovian conditioning
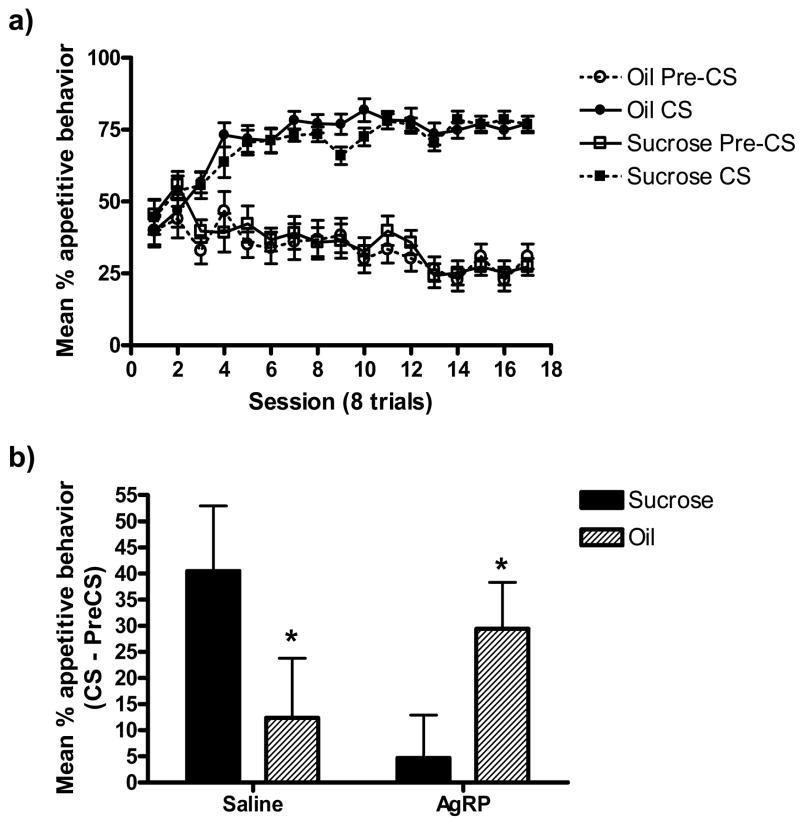
Increased appetitive behavior to both stimuli was observed across training sessions (Figure 2a). A Session × Period interaction (p < 0.05) indicates that learning occurred across trials, as there was no difference in responding during the two periods early in training, while by the final training sessions responding during the CS periods was greater than during the pre-CS periods (p < 0.05). That is, rats learned to increase their appetitive responding when the light or tone stimuli were presented. In the present experiment, there were no differences in responding between cues that predicted peanut oil and sucrose and there was no difference in responding during the three post-surgical sessions as compared to the three sessions immediately prior to surgery.
Figure 2.
a) Mean (± SEM) percent appetitive behavior (time at the food cup) during the Pavlovian training phase of Experiment 2. Solid symbols represent responding during the stimuli (CS period) and open symbols represent responding during the pre-CS period. b) Mean (± SEM) percent appetitive responding during the first 2 extinction test trials (i.e., no oil or sucrose delivered) of Experiment 2. Data are expressed as difference from pre-CS baseline. Solid bars represent responding during the oil-paired stimulus. Hatched bars represent responding during the sucrose-paired stimulus. Different letters denote significant differences (p < .05).
AgRP extinction test
Figure 2b depicts mean data from the first four trials of the test session (two of each trial type) in order to minimize the effects of extinction, expressed as a difference score (% CS minus % pre-CS-baseline time at the food cup). A mixed ANOVA with Drug Treatment (AgRP versus saline) as a between-subjects variable and US (peanut oil versus sucrose) as a within-subjects variable, yielded no significant main effects, but a significant interaction between these two factors (p < 0.05). One-way analyses revealed that rats injected with saline exhibited more responding during the sucrose stimulus, consistent with previous reports. Rats injected with AgRP (83–132), on the other hand, exhibited very little responding to the stimulus that predicted sucrose, but responded at much higher levels to the stimulus that predicted peanut oil (p’s < 0.05).
Food intake (post-test)
Consistent with Experiment 1, AgRP (83–132) increased chow intake during the 24-hr after the extinction test session, relative to saline treated rats (38.49 ± 2.90 versus 22.92 ± 2.86 g; p < 0.05).
Experiment 3
The results of Experiments 1 and 2 clearly indicate that activation of central MC receptors by AgRP selectively increases responding to cues that are predictive of fat availability. Other peptides that produce hyperphagic responses, such as ghrelin and neuropeptide Y (NPY), show endogenous increases in anticipation of a scheduled daily meal (Yoshihara et al., 1996, Drazen et al., 2006). Therefore, we designed Experiment 3 to assess the hypothesis that hypothalamic AgRP gene expression would be upregulated to a greater extent by anticipation of fat consumption than anticipation of carbohydrate consumption. To do this, we modified a previously used paradigm which employed spatial and temporal cues rats could use to predict the availabililty of food (Roitman et al., 2001). Here, animals were trained in two separate environments. Environment A consisted of a plastic cage with kitty litter on the floor and an almond odor, while Environment B was a stainless steel cage with a wire mesh floor. Each animal received a sucrose solution to drink in one environment and a peanut oil emulsion in the alternate environment at the same time each day. On the test day, all animals were placed in Environment A, then sacrificed at the time they had previously been presented with the nutrient solution in order to compare sucrose- and peanut oil-anticipatory hypothalamic gene expression using quantitative real-time PCR.
Training intake
Consumption of both nutrients increased across days of training (p < 0.01) and, overall, animals consumed more sucrose than peanut oil (17.84 ± 1.10 versus 14.18 ± 1.01 mL, p < 0.01). However, this difference was equivalent for animals trained to receive sucrose or peanut oil in Environment A (test environment) (p = 0.847).
AgRP mRNA expression
Animals anticipating the opportunity to consume peanut oil expressed slightly elevated levels of AgRP mRNA in the ARC compared to animals anticipating consumption of sucrose, although the increase was not significant (118.11 ± 17.71 versus 100.00 ± 10.21, p = 0.20). In comparison, there was no difference in NPY gene expression between the groups anticipating different nutrients (99.46 ±11.91 versus 100.00 ± 13.85, p = 0.49).
GENERAL DISCUSSION
The results of Experiments 1 and 2 demonstrate that central AgRP (83–132) administration increases active responding for the opportunity to consume a peanut oil reinforcer, but not a sucrose reinforcer and, furthermore, AgRP increases appetitive behaviors directed toward a conditioned stimulus that predicts the delivery of peanut oil, while reducing behaviors directed toward a conditioned stimulus that predicts the delivery of sucrose. These results are consistent with previous reports demonstrating the selective influence of melanocortins on intake of pure fats and mixed diets with a high fat content (Koegler et al., 1999, Butler et al., 2001, Hagan et al., 2001, Samama et al., 2003). The results of Experiment 2, in which animals displayed opposite behavioral responses during the oil-predicting and sucrose-predicting CSs even in the absence of food delivery, suggest that AgRP has macronutrient-related effects that are independent of the orosensory or postingestive consequences of fat or carbohydrate consumption.
There are a number of characteristics of the peanut oil and sucrose reinforcers used in these experiments that differ aside from the macronutrient aspect, including mouthfeel and texture (e.g., solid versus liquid, oiliness), taste (e.g., level of sweetness), caloric density, and smell. Any of these aspects of the stimuli may be involved in what is learned about these two foods and what animals are responsive to during training and testing. While our designs cannot identify the critical feature or feature of these reinforcers in our experiments, the data from Experiment 2 clearly demonstrate that the macronutrient-selective effect of AgRP can occur even in the absence of direct contact with that feature. Additionally, it is important to recognize that many of these characteristics are uniquely associated with a specific macronutrient (i.e., oiliness with fat and sweetness with carbohydrate), such that behaviors influenced by that specific factor will directly influence intake of that nutrient.
These data do not, however, eliminate the possibility that palatability and/or gut feedback is influenced by the MC system. In fact, hypothalamic MC neurons project to CNS areas important for GI satiety signals (i.e., dorsal vagal complex (Zheng et al., 2005)) and MC4-R is found in nuclei involved in taste processing (i.e., nucleus of the solitary tract) and those involved in food hedonics (i.e., ventral tegmental area, nucleus accumbens, substantia nigra (Kishi et al., 2003)). The hypothalamic MC system is also mediated by the opioid system (Hagan et al., 2001, Olszewski et al., 2001, Brugman et al., 2002), which has effects on food intake related to taste and palatability (Kelley et al., 2002, Li et al., 2003). However, the present results clearly indicate that AgRP has behavioral effects that can influence food selection via mechanisms that are independent of palatability, gastrointestinal feedback and other direct effects of nutrient consumption.
While AgRP treatment enhanced responses to both obtain and consume peanut oil and those directed toward an oil-predicting CS without oil ingestion, the effects on carbohydrate-based responses appear to be more dependent on the presence or absence of sucrose during the test. When the animals are allowed to consume the sucrose received during operant testing, AgRP has little effect on their bar-pressing behavior, whereas, in the absence of sucrose during the Pavlovian test, treatment with AgRP decreased appetitive responses toward that cue. This result may be due to the postitive consequences of sucrose ingestion overriding any reduction in “carbohydrate appetite” that occurs with AgRP treatment. It is also possible that a direct comparison between a carbohydrate and a fat source must occur in order for AgRP to have a carbohydrate-specific effect. In the training sessions for Experiment 2, all animals received both peanut oil and sucrose and responded to cues for both of these foods during the test session, while, in Experiment 1, each animal received only one of the two reinforcers throughout the study. These ideas are consistent with previous work demonstrating the capacity of AgRP to increase intake of standard lab chow, which is composed primarily of carbohydrates and is generally presented as the only available food source (Rossi et al., 1998, Hagan et al., 2001). Also, a number of the studies assessing melanocortin effects on macronutrient-based intake have employed paradigms in which foods with different levels of carbohydrates and fats are presented either simultaneously or sequentially to the same animals allowing for direct comparison of the nutrients (Koegler et al., 1999, Butler et al., 2001, Hagan et al., 2001, Samama et al., 2003).
Training under food restriction produced similar operant responding for both sucrose and peanut oil under the progressive ratio schedule, as well as similar Pavlovian approach responses to both the oil-predicting and sucrose-predicting CS. Yet in both experiments, testing animals under ad lib feeding conditions, in order to minimize endogenous AgRP levels, yielded increased responding for sucrose relative to fat under saline treatment. This result is consistent with previous data employing similar paradigms (Davidson et al., 1997, Benoit et al., 1999). One possible explanation for this outcome is that, while both peanut oil and sucrose are readily consumed by rats, sucrose may be the “preferred” of the two and the palatability of sucrose may maintain responding under conditions where feeding is occurring for reasons other than replenishing energy stores. Therefore, our data could be interpreted as an effect of AgRP on responding for a non-preferred reinforcer. However, in order for a relative preference to be established, the animals must experience both reinforcers and make a comparison. This was possible here only in Experiment 2, as animals in Experiment 1 received only one of either sucrose or peanut oil as a reinforcer for lever-press responses, yet in both cases AgRP significantly increased responding for the peanut oil, but not the sucrose, reinforcer.
On the other hand, under conditions of negative energy balance fat consumption may be driven by its higher caloric density relative to carbohydrate. Food restriction increases hypothalamic AgRP gene expression (Mizuno et al., 1999, Bi et al., 2003), which, based on the present data, is likely to drive up fat intake and responding for fat in comparison to ad lib fed conditions. In any case, our data are clear that, when tested under the same physiological conditions, AgRP administration counteracted the reduction in fat-associated responding that occurred when the animals were food sated, while having no effect on responding to obtain sucrose and actually further reducing behaviors directed toward a carbohydrate-paired cue. Importantly, the fact that in both experiments responding for oil and sucrose was similar during food-restricted training, but not when animals were treated with AgRP (83–132) indicates that AgRP does not simply induce a generalized hunger state, but rather seems to activate a specific “fat appetite”. This is consistent with our previous finding that administration of MTII, a synthetic MC3/4-R agonist, does not yield appetitive behaviors like those of food deprived animals (Benoit et al., 2001).
The effects of administration of exogenous AgRP on macronutrient-specific appetitive behaviors are evident from the results of Experiments 1 and 2. Experiment 3 was designed to test whether fat-predicting cues might also influence the expression of endogenous AgRP. Although there was only a slight increase in AgRP mRNA in animals’ anticipating fat availability relative to those anticipating carbohydrates, this difference was much larger than that observed for NPY, which is expressed in the same neuronal population as AgRP (Broberger et al., 1998, Hahn et al., 1998). This increase in AgRP gene expression was observed after only 6 exposures to each environment-nutrient pairing and was measured at only a single time point. It may be that increasing the strength of the association between the environment and the nutrient exposure through additional training, allowing more environment exposure prior to sacrifice on the day of testing, or taking measurements later relative to the anticipated nutrient presentation time would reduce the variability observed here and increase the magnitude of this effect.
In the current environment, foods with high fat content and, subsequently, high energy density, are readily available and the low satiety associated with consumption of these foods has been posited as a factor in the current epidemic of overweight and obesity. Studies indicate that a substantial portion of the United States population is attempting to lose weight at a given time (Serdula et al., 1999, Kruger et al., 2004), yet the majority are unsuccessful at losing weight and/or maintaining a weight loss over an extended period (Wing & Hill, 2001, Vogels et al., 2005). Following a period of food restriction, rats increase their intake of fat when given ad libitum access to separate macronutrient sources (Reed et al., 1988, Gerardo-Gettens et al., 1991, Welch et al., 1994), perhaps as a function of increased AgRP levels. This may be a contributing factor in human dieters succumbing to bouts of overeating following a period of restriction and possibly to the phenomenon of weight cycling, or “yo-yo dieting”, as the increased fat intake appears to be even greater subsequent to repeated cycles of restriction and refeeding (Gerardo-Gettens et al., 1991). Additional data suggest that decreased fat intake is a significant factor in reducing body weight, maintaining weight loss and reducing the risk of a number of health problems, such as type 2 diabetes and hypertension (Bray & Popkin, 1998, Delichatsios & Welty, 2005, Wing & Phelan, 2005, Lindstrom et al., 2006). Therefore, understanding the role that AgRP plays in behaviors related to fat consumption and nutrient-based food selection may lead to strategies for the prevention and treatment of overweight and obesity.
Acknowledgments
The authors would like to thank E. Air, J. Caldwell, R. Moore and J. Mak for assistance with performing surgeries and animal care and J. Heiman and C. Kemp for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benoit SC, Morrell JR, Davidson TL. Lesions of the amygdala central nucleus abolish lipoprivic-enhanced responding during oil-predicting conditioned stimuli. Behav Neurosci. 1999;113:1233–41. doi: 10.1037//0735-7044.113.6.1233. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Tracy AL, Air EL, Kinzig K, Seeley RJ, Davidson TL. The role of the hypothalamic melanocortin system in behavioral appetitive processes. Pharmacol Biochem Behav. 2001;69:603–9. doi: 10.1016/s0091-3057(01)00558-5. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–R6. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices and activity. Br J Nutr. 2000;83:S33–S8. doi: 10.1017/s0007114500000933. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. doi: 10.1146/annurev.nu.16.070196.001441. [DOI] [PubMed] [Google Scholar]
- Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–73. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Fletcher PJ, Coscina DV. Neuropeptide Y-induced operant responding for sucrose is not mediated by dopamine. Peptides. 1998;19:1667–73. doi: 10.1016/s0196-9781(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Brugman S, Clegg DJ, Woods SC, Seeley RJ. Combined blockade of both μ- and κ-opioid receptors prevents the acute orexigenic action of agouti-related protein. Endocrinology. 2002;143:4265–70. doi: 10.1210/en.2002-220230. [DOI] [PubMed] [Google Scholar]
- Buchan AM. Nutrient tasting and signaling mechanisms in the gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–G7. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–11. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci. 1995;109:528–31. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Altizer AM, Benoit SC, Walls EK, Powley TL. Encoding and selective activation of “metabolic memories” in the rat. Behav Neurosci. 1997;111:1014–30. doi: 10.1037//0735-7044.111.5.1014. [DOI] [PubMed] [Google Scholar]
- Delichatsios HK, Welty FK. Influence of the DASH diet and other low-fat, high-carbohydrate diets on blood pressure. Current Atherosclerosis Reports. 2005;7:446–54. doi: 10.1007/s11883-005-0061-x. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Gerardo-Gettens T, Miller GD, Horwitz BA, McDonald RB, Brownell KD, Greenwood MR, Rodin J, Stern JS. Exercise decreases fat selection in female rats during weight cycling. Am J Physiol Regul Integr Comp Physiol. 1991;260:R518–R24. doi: 10.1152/ajpregu.1991.260.3.R518. [DOI] [PubMed] [Google Scholar]
- Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients’ effects on satiation and satiety. J Am Diet Assoc. 2004;104:1151–3. doi: 10.1016/j.jada.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP-(83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol. 2001;280:R814–R21. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–35. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Koegler FH, Schaffhauser AO, Mynatt RL, York DA, Bray GA. Macronutrient diet intake of the lethal yellow agouti (Ay/a) mouse. Physiol Behav. 1999;67:809–12. doi: 10.1016/s0031-9384(99)00104-3. [DOI] [PubMed] [Google Scholar]
- Kruger J, Galuska DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med. 2004;26:402–6. doi: 10.1016/j.amepre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–8. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- Lee M, Wardlaw S. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010. doi: 10.2741/2366. [DOI] [PubMed] [Google Scholar]
- Li C-S, Davis BJ, Smith DV. Opioid modulation of taste responses in the nucleus of the solitary tract. Brain Res. 2003;965:21–34. doi: 10.1016/s0006-8993(02)03973-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, Tuomilehto J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia. 2006;49:912–20. doi: 10.1007/s00125-006-0198-3. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–7. doi: 10.1210/endo.140.10.6966. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Wirth MM, Grace MK, Levine AS, Giraudo SQ. Evidence of interactions between melanocortin and opioid systems in regulation of feeding. Neuroreport. 2001;12:1727–30. doi: 10.1097/00001756-200106130-00042. [DOI] [PubMed] [Google Scholar]
- Reed DR, Contreras RJ, Maggio C, Greenwood MR, Rodin J. Weight cycling in female rats increases dietary fat selection and adiposity. Physiol Behav. 1988;42:389–95. doi: 10.1016/0031-9384(88)90281-8. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–9. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim M, Morgan D, Small C, Edwards C, Sunter D, Abusnana S, Goldstone A, Russell S, Stanley S, Smith D, Yagaloff K, Ghatei M, Bloom S. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Samama P, Rumennik L, Grippo JF. The melanocortin receptor MCR4 controls fat consumption. Regul Pept. 2003;113:85–8. doi: 10.1016/s0167-0115(02)00299-9. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Drazen DL, Clegg DJ. The critical role of the melanocortin system in the control of energy balance. Annu Rev Nutr. 2004;24:133–49. doi: 10.1146/annurev.nutr.24.012003.132428. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999;282:1353–8. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- Srinath Reddy K, Katan M. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutrition. 2004;7:167–86. doi: 10.1079/phn2003587. [DOI] [PubMed] [Google Scholar]
- Stoeckli R, Keller U. Nutritional fats and the risk of type 2 diabetes and cancer. Physiol Behav. 2004;83:611–5. doi: 10.1016/j.physbeh.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291–5. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Vogels N, Diepvens K, Westerterp-Plantenga MS. Predictors of long-term weight maintenance. Obes Res. 2005;13:2162–8. doi: 10.1038/oby.2005.268. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–8. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol Regul Integr Comp Physiol. 1994;266:R426–R33. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- Wing RR, Phelan S. Long-term weight loss maintenence. Am J Clin Nutr. 2005;81:222S–5S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Honma S, Honma K. Effects of restricted daily feeding on neuropeptide Y release in the rat paraventricular nucleus. Am J Physiol Endocrinol Metab. 1996;270:E589–E95. doi: 10.1152/ajpendo.1996.270.4.E589. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud H-R. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–R58. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]