Abstract
Stage IIIA endometrial cancer includes patients with serosal or adnexal invasion and patients with positive peritoneal cytology only. In this study, we assessed the impact of peritoneal cytology on endometrial cancer survival. All endometrial cancer patients receiving surgery and radiotherapy at the Geneva University Hospitals between 1980 and 1993 were included. Stage lllA cancers were categorised into ‘cytological’ stage lllA (only positive peritoneal cytology) and ‘histological’ stage lllA (serosal or adnexal infiltration). Survival rates were analysed by Kaplan–Meier method and compared using log-rank test. The prognostic importance of peritoneal cytology was analysed by multivariate regression analysis. This study included 170 endometrial cancers (112 stage I, 17 cytological stage IIIA, 18 histological stage IIIA, 9 stage lllB+). Disease-specific survival of cytological stage IIIA was not different from stage I (94 vs 88% respectively, P=0.5) but better than histological stage IIIA (94 vs 51% respectively, P<0.01). Histological stage IIIA patients were at increased risk to die from cancer compared to stage I patients (HR 2.7, 95% CI 1.0–7.7), while cytological stage IIIA patients were not (HR 0.3, 95% CI 0.3–2.0). Cytological stage lllA endometrial cancer has similar prognosis as stage l and better prognosis than histological stage IIIA. Additional research, definitively separating stage and cytology is warranted.
Keywords: endometrial cancer, peritoneal cytology; survival, radiotherapy
Endometrial cancer is the most common genital tract malignancy in Western countries (Grady and Ernster, 1996; Burke et al, 1997; Parkin et al, 1997; Greenlee et al, 2000). The long-term survival of patients with endometrial cancer is related to the stage at diagnosis. Disease-specific 5-year survival for tumours confined to the uterus is over 80%, but decreases to approximately 40% for tumours invading the serosa, the fallopian tube or the ovary (AJCC, 2002).
Before 1988, endometrial cancer was staged clinically (International Federation of Gynaecology & Obstetrics, FIGO 1971). This staging system was based on the fractional biopsy of the endometrium, the size of the uterine cavity and physical examination. The clinical system was abandoned because newly accumulated data from surgical staging reports, among others including depth of myometrial invasion, allowed stratification. A new surgical staging system was approved at the 1988 FIGO meeting (Grady and Ernster, 1996; Burke et al., 1997; FIGO, 2000; AJCC, 2002). In addition to pathological extension, the result of cytology obtained by peritoneal washings became an important determinant of staging. In particular, stage l patients with positive peritoneal cytology were upstaged to stage lllA (Konski et al, 1988). Stage IIIA therefore covers both patients with only positive peritoneal cytology and patients with macroscopic/histological invasion of serosa or adnexal tissues.
As some studies have suggested that cytology is an important prognostic factor, while others did not (Grimshaw et al, 1990; Morrow et al, 1991; Kadar et al, 1992; Obermair et al, 2001; Preyer et al, 2002; Kasamatsu et al, 2003), the value of peritoneal cytology in the staging of endometrial cancer is still controversial (Kasamatsu et al, 2003). In this study, we evaluated the impact of positive peritoneal cytology on the survival of patients operated for endometrial carcinoma and treated with adjuvant brachytherapy and/or external radiotherapy.
METHODS
Patients
The current study included women who received surgery followed by adjuvant external radiotherapy and/or brachytherapy for endometrial cancer between 1980 and 1993 at the departments of Gynaecology and Radiation Oncology of the Geneva University Hospitals (n=295). We excluded patients with stage I or stage II endometrial cancer without peritoneal cytology assessment (n=111), women with previous malignancy occurring within 5 years prior to the diagnosis of endometrial cancer (n=2), or patients who were not resident in the canton (n=12). The final study population included 170 patients. According to the Geneva cancer registry, these women represented approximately 50% of all endometrial cancer patients treated in the public sector during the period considered.
Peritoneal washing was performed by either collecting liquid present in the peritoneal cavity, or by rinsing the cavity with 100 cm3 of physiological saline. The liquid was centrifuged and assessed for the presence of malignant cells. Surgical treatment generally included hysterectomy and oophorosalpingectomy, except for three patients (<2%) who received only simple hysterectomy. A total of 12 patients (7%) underwent lymph node dissection. All surgical procedures were followed by external radiotherapy and/or brachytherapy.
Variables
Histologic type and differentiation of the endometrial tumours were coded according to the International Classification of Diseases for Oncology (World Health Organization, 1990). A patient was considered to have positive peritoneal cytology if adenocarcinoma cells were detected, regardless of the number of cancer cells. Stages were coded according to the 1988 FIGO staging system: stage I, tumour confined to uterus; stage II, tumour invading cervix; stage IIIA, tumour associated with positive peritoneal cytology or macroscopic or histological involvement of serosa or adnexa and stage IIIB+, tumour invading vagina, mucosa of bladder/bowel, regional lymph node or distant metastases. For the purpose of the present study, stage lllA cancers were further categorised as: ‘cytological’ stage lllA defined as cases with only positive peritoneal cytology and ‘histological’ stage lllA defined as cases with histological or macroscopic infiltration of serosa or adnexal tissues.
Other variables of interest were: age at diagnosis (<50, 50–69, ⩾70 years), period of diagnosis (1980–87, 1988–93), differentiation (good, moderate, poor, unknown), degree of myometrial invasion (<50%, ⩾50%), type of surgery (hysterectomy and oophorosalpingectomy with and without lymphadenectomy), and type of radiotherapy (external with brachytherapy, external only, brachytherapy only).
Data on survival and follow-up were derived from the Geneva cancer registry and included vital status and date of death or departure from the canton (regularly and systematically obtained from the Cantonal Population Office) and cause of death (retrieved from medical files).
Statistical analyses
The 5-year overall and disease-specific survival rates were calculated. Disease-specific survival was calculated considering death from endometrial cancer only. Survival curves were obtained by Kaplan–Meier method and were compared by nonparametric survival analyses using log-rank test. The effect of peritoneal cytology on endometrial cancer mortality was analysed by multivariate Cox's proportional-hazards regression analysis, taking into account other variables significantly linked to survival. Analyses were performed using SPSS (Hull and Nie, 1995). Differences were considered statistically significant at P<0.05.
RESULTS
Of the 170 endometrial cancer patients included in the study, 112 were diagnosed as stage I, 14 as stage II, 17 as cytological stage IIIA, 18 as histological stage IIIA, and nine were diagnosed as stage IIIB+. If peritoneal cytology had not been incorporated in the staging of the endometrial cancers, 15 of the 17 cytological stage IIIA tumours would have been classified as stage I and two as stage II.
Table 1 presents the characteristics of these 170 patients according to stage of endometrial cancer. The mean age of the patients was 64.7 years and was similar for the different stages. Compared to histological stage IIIA tumours, cytological stage IIIA tumours invaded the myometrium to a lesser extent, were better differentiated and received less aggressive radiotherapy.
Table 1. Characteristics of 170 endometrial cancer patients according to stage.
| Stage I (n=112) n (%) | Stage II (n=14) n (%) | Stage IIIA cyt (n=17) n (%) | Stage IIIA hist (n=18) n (%) | Stage IIIB+(n=9) n (%) | |
|---|---|---|---|---|---|
| Mean age (range) years | 64 (33–81) | 66 (55–78) | 66 (51–81) | 65 (42–90) | 65 (37–77) |
| Age category (years) | |||||
| <50 | 7 (6) | — | — | 2 (11) | 1 (11) |
| 50–69 | 71 (63) | 10 (71) | 12 (71) | 10 (56) | 4 (44) |
| 70+ | 34 (31) | 4 (29) | 5 (29) | 6 (33) | 4 (44) |
| Period of diagnosis | |||||
| 1980–1987 | 65 (58) | 4 (29) | 5 (29) | 5 (28) | 7 (78) |
| 1988–1993 | 47 (42) | 10 (71) | 12 (71) | 13 (72) | 2 (22) |
| Invasion of myometrium | |||||
| <50% | 70 (63) | 4 (29) | 8 (47) | 5 (28) | 4 (44) |
| ⩾50% | 42 (37) | 10 (71) | 9 (53) | 13 (72) | 5 (56) |
| Differentiation | |||||
| Good | 67 (60) | 5 (45) | 8 (57) | 6 (38) | 2 (22) |
| Moderate | 30 (27) | 4 (36) | 3 (21) | 4 (25) | 4 (44) |
| Poor | 15 (13) | 2 (18) | 3 (21) | 6 (38) | 3 (33) |
| Unknown | — | 3 | 3 | 2 | — |
| Surgical treatment | |||||
| Hysterectomy | 3 (3) | — | — | — | — |
| Hysterectomy+unilat/Bilateral annexectomy Idem plus lymphadenectomy | 105 (94) | 13 (93) | 16 (94) | 17 (94) | 4 (44) |
| 4 (3) | 1 (7) | 1 (6) | 1 (6) | 5 (56) | |
| Radiotherapy | |||||
| External and brachy | 46 (42) | 11 (92) | 11 (58) | 15 (83) | 7 (78) |
| External only | 8 (7) | — | 3 (21) | 3 (17) | 2 (22) |
| Brachy only | 56 (51) | 1 (8) | 3 (21) | — | — |
| Unknown | 2 | 2 | — | — | — |
Stage I=tumour confined to uterus; stage II=tumour invading cervix; stage IIIA cyt=positive peritoneal cytology; stage IIIA hist=histological involvement of serosa or adnexa; stage IIIB+=tumour invading vagina, mucosa of bladder/bowel, regional lymph node or distant metastases.
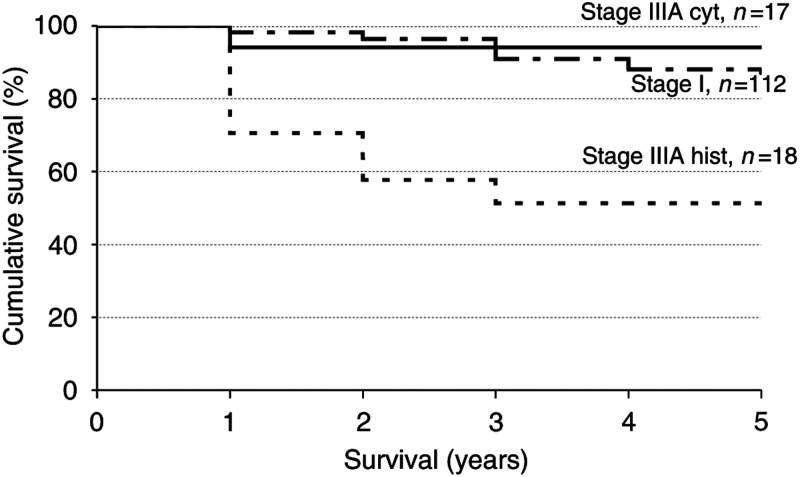
The median duration of follow-up was 126 months and 14 patients (8%) were lost to follow-up. Table 2 presents overall and disease-specific 5-year survival rates per stage. Figure 1 presents disease-specific survival curves for stage I, cytological stage IIIA and histological stage IIIA endometrial cancer. The disease specific 5-year survival of patients with cytological stage IIIA tumours was not significantly different from that of patients with stage I tumours (94 vs 88% respectively, log-rank test: P=0.5). In contrast, the 5-year disease-specific survival of patients with histological stage IIIA tumours was significantly worse (51 vs 94% for histological vs cytological stage IIIA cancers, log-rank test: P<0.01). A similar pattern was observed when considering the overall survival rates accounting for all deaths, including death from other causes than endometrial cancer.
Table 2. Observed and disease-specific 5-year survival after endometrial cancer according to stage.
| n | Number of endometrial cancer deaths | Median follow-up (days) | Overall 5-year survivala (%) | Disease specific 5-year survivalb (%) | |
|---|---|---|---|---|---|
| Stage I | 112 | 14 | 1825 | 85 | 88 |
| Stage II | 14 | 8 | 1176 | 36 | 43 |
| Stage III cyt | 17 | 1 | 1825 | 94 | 94 |
| Stage IIIA hist | 18 | 8 | 1048 | 44 | 51 |
| Stage IIIB + | 9 | 5 | 1224 | 44 | 44 |
Stage I=tumour confined to uterus; stage II=tumour invading cervix; stage IIIA cyt=positive peritoneal cytology; Stage IIIA hist=histological involvement of serosa or adnexa; stage IIIB+=tumour invading vagina, mucosa of bladder/bowel, regional lymph node or distant metastases.
All cases of death.
Death from endometrial cancer.
Figure 1.
Disease-specific survival curves of endometrial cancer for the stages I, cytological IIIA and histological IIIA. Stage I=tumour confined to uterus; stage IIIA cyt=positive peritoneal cytology; stage IIIA hist=histological involvement of serosa or adnexa. Log-rank test of comparison of survival of stage I vs cytological stage IIIA: P=0.5; log-rank test of comparison of survival of cytological stage IIIA vs stage histological IIIA: P=0.008.
After adjustment for factors significantly linked to survival from endometrial cancer (i.e. age, tumour differentiation and type of radiotherapy) as shown in Table 3 , the risk to die from endometrial cancer was still not significantly different in patients with cytological stage IIIA compared with patients with stage I cancer (HR 0.3, 95% CI 0.3–2.0). Compared to stage I, patients with stage II, histological IIIA, and IIIB+ tumours were at respectively 3.2-fold (95% CI 1.2–8.6), 2.7-fold (95% CI 1.0–7.7) and 3.5-fold (95% CI 1.2–10.4) increased risk to die from their endometrial cancer. The results were not modified when excluding the two patients in the cytological stage IIIA category who had cervical invasion (i.e. who would have been staged as stage II in the absence of positive peritoneal cytology).
Table 3. Hazard ratioa of death from endometrial cancer according to stage.
| HRa | 95% CI | |
|---|---|---|
| Stage I | 1b | |
| Stage II | 3.2* | 1.2–8.6 |
| Stage IIIA cytological | 0.3 | 0.3–2.0 |
| Stage IIIA histological | 2.7c | 1.0–7.7 |
| Stage IIIB and more | 3.5* | 1.2–10.4 |
Hazard ratio are derived from Cox model adjusted for age, tumour differentiation and type of radiotherapy (external radiation therapy, brachytherapy or both).
reference category.
P borderline significant (0.057).
P<0.05. Stage I=tumour confined to uterus; stage II=tumour invading cervix; stage IIIA cyt=positive peritoneal cytology; Stage IIIA hist=histological involvement of serosa or adnexa; stage IIIB+=tumour invading vagina, mucosa of bladder/bowel, regional lymph node or distant metastases.
DISCUSSION
This study is the first to compare the prognosis of patients with stage I, cytological stage IIIA, and histological stage IIIA endometrial cancer in the same patient population. It suggests that positive peritoneal cytology is not an independent prognostic factor in patients with endometrial cancer treated with surgery and radiotherapy. Irradiated women with only positive cytology (cytological stage IIIA) had the same prognosis as women with endometrial cancer confined to the uterus (stage I) and a much better prognosis than women with histological stage IIIA cancer.
However, our study has several limitations: 111 women had no peritoneal cytology and were therefore excluded, patients were selected for inclusion in the study on the basis of administration of radiotherapy, and the study has a relatively low statistical power due to the limited number of patients included. Nonetheless, the lack of significant survival difference between the stage I and cytological stage IIIA groups suggest that the FIGO classification system classifies patients with positive peritoneal cytology into an inappropriately high-risk category. However, this finding might also be explained by a disproportionate benefit derived from radiotherapy in patients with positive peritoneal cytology.
Table 4 shows that similarly high survival rates (88–94% 3–5 year survival) in women with stage I and cytological stage IIIA cancer have been observed in series where, as in our study, the large majority of cytological stage IIIA patients underwent either pelvic radiotherapy or total pelvic lymphadenectomy (Kadar et al, 1992; Kasamatsu et al, 2003). In contrast, the relatively poor results reported by other investigators (64–67% 3–5 year survival) in cytological stage IIIA patients might be explained by the fact that in these studies, only a minority of patients received treatment to pelvic lymph nodes (Morrow et al, 1991; Obermair et al, 2001; Preyer et al, 2002). Pelvic radiotherapy or lymphadenectomy might be especially important for patients with positive peritoneal cytology, as it has been shown by Morrow et al (1991) that the presence of positive peritoneal cytology increases the risk of para-aortic lymph node invasion (15 vs 4%), and by inference increases the likelihood of pelvic node involvement.
Table 4. Overview of studies on survival in women with stage l, cytological stage lllA and histological stage lllA endometrial cancer.
|
Survival (%) |
|||||
|---|---|---|---|---|---|
| Study | Stage I | Stage IIIA cyt | Stage IIIA hist | P-value | Remarks |
| Preyer et al (2002) | — | 64a | 38a | =0.05 | Exact number of patients with cytological stage IIIA receiving RT unclear. Estimated that less than 50% received ext RT or LND |
| Morrow et al (1991) | 93a | 65a | — | <0.05 | No routine LND. Exact number of cyt stage IIIA patients treated with RT unclear. Estimated that less than 50% received ext RT |
| Obermair et al (2001) | 96c | 67c | — | <0.05 | No routine LND. Exact number of patients with cyt stage IIIA receiving RT unclear. Estimated that two of 13 received ext RT |
| Kasamatsu et al (2003) | 94c | 90c | — | NS | All patients underwent LND |
| Kadar et al (1992) | 93b,* | 88b,* | — | NS | All patients underwent LND |
| Tebeu et al (this study) | 88b | 94b | 51b | <0.05 | 80% of patients with cyt stage IIIA received ext RT |
5-year disease-free survival.
5-year disease-specific survival.
3-year disease-free survival. LND=lymph node dissection; ext RT=external radiotherapy.
Estimated from the survival curve.
The proportion of cytological stage IIIA cancer patients with cervical involvement is unknown in the majority of studies, except for the study of Kasamatsu et al and ours, in which the proportions of cytological stage IIIA patients with cervical involvement were relatively low (0 and 12%, respectively). As cervical involvement is known to affect the survival negatively, a higher proportion of cytological stage IIIA patients with cervical involvement might explain unfavourable survival rates. Therefore, the unknown proportions of patients with cervical involvement complicate interpretation of outcomes and impair comparison between the different studies.
In conclusion, this study found comparable survival rates for patients with cytological IIIA and stage I endometrial cancer, which were significantly superior to that of patients with histological stage IIIA endometrial cancer. Because all studies, including ours, have important shortcomings, no definitive conclusion on the importance of positive peritoneal cytology can be drawn and therefore, additional research is warranted. We strongly recommend that stage and cytology be separated in future studies, in order to better define the impact of peritoneal cytology on outcome of endometrial cancer.
Acknowledgments
We thank Ms Stina Blagojevic for her editorial assistance, Professor Félix Krauer and Professor John Kurtz for their excellent assistance throughout the study. Dr Tebeu was financially supported by the Yaounde-Geneva Cooperation. This work was supported in part by the Swiss National Science Foundation, Bern, Switzerland, by the Fonds de Péréquation, Geneva, Switzerland and by Schering AG, Berlin, Germany.
References
- AJCC (2002) AJCC Cancer Staging Handbook From the AJCC Cancer Staging Manual. New York: Springer [Google Scholar]
- Burke TW, Eifel PJ, Muggia FM (1997) Cancer of the uterine body. In Cancer Principles & Pratice of Oncology, DeVita Jr VT, Hellman S, Rosenberg SA (eds) pp 1478–1499. Philadelphia New York: Lippincott-Raven [Google Scholar]
- FIGO (2000) Staging Classifications and Clinical Practice Guidelines of Gynecological Cancers by the FIGO Committee on Gynecologic Oncology. Oxford: Elsevier [PubMed] [Google Scholar]
- Grady D, Ernster VL (1996) Endometrial cancer. In Cancer Epidemiology and Prevention, Schottenfeld D, Fraumeni Jr JF, (eds) pp 1058–1089. New York: Oxford University Press [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50: 7–33 [DOI] [PubMed] [Google Scholar]
- Grimshaw RN, Tupper WC, Fraser RC, Tompkins MG, Jeffrey JF (1990) Prognostic value of peritoneal cytology in endometrial carcinoma. Gynecol Oncol 36: 97–100 [DOI] [PubMed] [Google Scholar]
- Hull CH, Nie NH (1995) 1995 SPSS update 7-9 New procedures for releases 7-9. New York: McGraw-Hill Book Company [Google Scholar]
- Kadar N, Homesley HD, Malfetano JH (1992) Positive peritoneal cytology is an adverse factor in endometrial carcinoma only if there is other evidence of extrauterine disease. Gynecol Oncol 46: 145–149 [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Onda T, Katsumata N, Sawada M, Yamada T, Tsunematsu R, Ohmi K, Sasajima Y, Matsuno Y (2003) Prognostic significance of positive peritoneal cytology in endometrial carcinoma confined to the uterus. Br J Cancer 88: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konski A, Poulter C, Keys H, Rubin P, Beecham J, Doane K (1988) Absence of prognostic significance, peritoneal dissemination and treatment advantage in endometrial cancer patients with positive peritoneal cytology. Int J Radiat Oncol Biol Phys 14: 49–55 [DOI] [PubMed] [Google Scholar]
- Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE (1991) Relationship between surgical–pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40: 55–65 [DOI] [PubMed] [Google Scholar]
- Obermair A, Geramou M, Tripcony L, Nicklin JL, Perrin L, Crandon AJ (2001) Peritoneal cytology: impact on disease-free survival in clinical stage I endometrioid adenocarcinoma of the uterus. Cancer Lett 164: 105–110 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J (eds) (1997) Cancer Incidence in Five Continents, Vol. Vll. IARC Scientific Publications no 143, Lyon: International Agency for Research on Cancer [Google Scholar]
- Preyer O, Obermair A, Formann E, Schmid W, Perrin LC, Ward BG, Crandon AJ, Nicklin JL (2002) The impact of positive peritoneal washings and serosal and adnexal involvement on survival in patients with stage IIIA uterine cancer. Gynecol Oncol 86: 269–273 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1990) ICD-O International Classification of Diseases for Oncology. Geneva: WHO [Google Scholar]