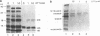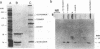Abstract
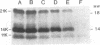
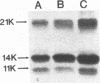
The mechanism by which the 14-kDa fusion protein of vaccinia virus (VV) is anchored in the envelope of intracellular naked virions (INV) is not understood. In this investigation, we demonstrate that the 14-kDa protein interacts with another virus protein with an apparent molecular mass of 21 kDa. Microsequence analysis of the N terminus of the 21-kDa protein revealed that this protein is encoded by the VV A17L gene. The 21-kDa protein is processed from a 23-kDa precursor, by cleavage at amino acid position 16, at the consensus motif Ala-Gly-Ala, previously identified as a cleavage site for several VV structural proteins. The 21-kDa protein contains two large internal hydrophobic domains characteristic of membrane proteins. Pulse-chase analysis showed that within 1 h after synthesis, the 14-kDa protein forms a stable complex with the 21-kDa protein. Formation of the complex was not inhibited by rifampin, indicating that the interaction between these two proteins occurs prior to virion morphogenesis. Immunoprecipitation analysis of disrupted virions showed the presence of the 21-kDa protein in the viral particle. Release of the 14-kDa-21-kDa protein complex from INV required treatment with the nonionic detergent Nonidet P-40 and a reducing agent. The protein complex consisted of 14-kDa trimers and of 21-kDa dimers. Since the 14-kDa fusion protein lacks a signal sequence and a large hydrophobic domain characteristic of membrane proteins, our findings suggest that the 21-kDa protein serves to anchor the 14-kDa protein to the envelope of INV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasco R., Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991 Nov;65(11):5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S., Esteban M. Isolation and characterization of attenuated mutants of vaccinia virus. Virology. 1987 Aug;159(2):408–422. doi: 10.1016/0042-6822(87)90480-6. [DOI] [PubMed] [Google Scholar]
- Dallo S., Rodriguez J. F., Esteban M. A 14K envelope protein of vaccinia virus with an important role in virus-host cell interactions is altered during virus persistence and determines the plaque size phenotype of the virus. Virology. 1987 Aug;159(2):423–432. doi: 10.1016/0042-6822(87)90481-8. [DOI] [PubMed] [Google Scholar]
- Demkowicz W. E., Maa J. S., Esteban M. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol. 1992 Jan;66(1):386–398. doi: 10.1128/jvi.66.1.386-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Blumenthal R., Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990 Oct;64(10):4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. A., Smith G. L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992 Mar;66(3):1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Essani K., Dales S. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology. 1979 Jun;95(2):385–394. doi: 10.1016/0042-6822(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Gong S. C., Lai C. F., Dallo S., Esteban M. A single point mutation of Ala-25 to Asp in the 14,000-Mr envelope protein of vaccinia virus induces a size change that leads to the small plaque size phenotype of the virus. J Virol. 1989 Nov;63(11):4507–4514. doi: 10.1128/jvi.63.11.4507-4514.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S. C., Lai C. F., Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990 Sep;178(1):81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985 Sep;55(3):651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lai C. F., Gong S. C., Esteban M. Structural and functional properties of the 14-kDa envelope protein of vaccinia virus synthesized in Escherichia coli. J Biol Chem. 1990 Dec 25;265(36):22174–22180. [PubMed] [Google Scholar]
- Lai C. F., Gong S. C., Esteban M. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J Virol. 1991 Oct;65(10):5631–5635. doi: 10.1128/jvi.65.10.5631-5635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., McKenzie M., Parce J. W. Subunit interactions of vesicular stomatitis virus envelope glycoprotein stabilized by binding to viral matrix protein. J Virol. 1992 Jan;66(1):349–358. doi: 10.1128/jvi.66.1.349-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Grimley P. M. Assembly of vaccinia virus particles from polypeptides made in the presence of rifampicin. Virology. 1971 Jul;45(1):123–134. doi: 10.1016/0042-6822(71)90119-x. [DOI] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- Oie M., Ichihashi Y. Characterization of vaccinia polypeptides. Virology. 1981 Aug;113(1):263–276. doi: 10.1016/0042-6822(81)90153-7. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Kristensson K. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J Gen Virol. 1985 Mar;66(Pt 3):643–646. doi: 10.1099/0022-1317-66-3-643. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A., Szilágyi J. F. Events in vaccinia virus-infected cells following the reversal of the antiviral action of rifampicin. J Gen Virol. 1970 Dec;9(3):225–237. doi: 10.1099/0022-1317-9-3-225. [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Rodriguez J. R., Rodriguez J. F., Trauber D., Esteban M. Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1287–1291. doi: 10.1073/pnas.86.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987 Nov;61(11):3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990 Sep 25;18(18):5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz C., Payne L. G., Gubser J., Wittek R. A mutation in the gene encoding the vaccinia virus 37,000-M(r) protein confers resistance to an inhibitor of virus envelopment and release. J Virol. 1991 Jul;65(7):3435–3442. doi: 10.1128/jvi.65.7.3435-3442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke J. K., Franke C. A., Hruby D. E. Proteolytic maturation of vaccinia virus core proteins: identification of a conserved motif at the N termini of the 4b and 25K virion proteins. J Gen Virol. 1991 Feb;72(Pt 2):411–416. doi: 10.1099/0022-1317-72-2-411. [DOI] [PubMed] [Google Scholar]