Abstract
Objectives. We assessed educational disparities in smoking rates among adults with diabetes in managed care settings.
Methods. We used a cross-sectional, survey-based (2002–2003) observational study among 6538 diabetic patients older than 25 years across multiple managed care health plans and states. For smoking at each level of self-reported educational attainment, predicted probabilities were estimated by means of hierarchical logistic regression models with random intercepts for health plan, adjusted for potential confounders.
Results. Overall, 15% the participants reported current smoking. An educational gradient in smoking was observed that varied significantly (P<.003) across age groups, with the educational gradient being strong in those aged 25 to 44 years, modest in those aged 45 to 64 years, and nonexistent in those aged 65 years or older. Of particular note, the prevalence of smoking observed in adults aged 25–44 years with less than a high school education was 50% (95% confidence interval: 36% to 63%).
Conclusions. Approximately half of poorly educated young adults with diabetes smoke, magnifying the health risk associated with early-onset diabetes. Targeted public health interventions for smoking prevention and cessation among young, poorly educated people with diabetes are needed.
Smoking is recognized as the leading preventable cause of death and one of the most potent risk factors for cardiovascular disease and cancer. The total annual direct and indirect costs of smoking in the United States for 1995–1999 were estimated to be $158 billion.1 In the United States during 1997–2001, cigarette smoking and tobacco exposure resulted in approximately 438000 premature deaths, 5.5 million years of potential life lost, and $92 billion in productivity losses annually.2 Diabetes confers a similar burden in annual health care expenditures ($132 billion).3
Smoking may be a particularly important risk multiplier for adults with diabetes, because it is associated with hyperglycemia, microvascular complications, insulin resistance, and microalbuminuria4–6 and greatly increases an already elevated risk of cardiovascular disease,7,8 end-stage renal disease,9,10 and death.11,12 Moreover, although quitting smoking reduces the mortality risk, the detrimental effects can persist for years after quitting, especially for smokers with diabetes.13
In the general patient population, poverty and lower educational attainment are linked to a higher prevalence of smoking.14 Nonetheless, relatively little is known about smoking patterns among adults with diabetes and, in particular, about the influence of social disparities, such as educational differences, on the prevalence of smoking in this group. Understanding which subpopulations are most at risk for smoking would help health plans and policymakers target their smoking cessation and prevention interventions among enrollees with diabetes.
Translating Research Into Action for Diabetes (TRIAD) is an ongoing study of quality of care and self-care for people with diabetes in managed care settings in 7 US states that began in 2000.15 As part of TRIAD, we examined the relation between socioeconomic status and various health behaviors among people with diabetes. TRIAD surveyed a large cohort of adults with diabetes enrolled in managed care, enabling a detailed examination of smoking and social factors in addition to other factors that contribute to risk for future complications. Here we focus on the relation between educational attainment and smoking.
METHODS
Study Setting
In brief, TRIAD is a multicenter, prospective, cohort study that collects and analyzes data on health plans, provider groups, and diabetic patients. The study’s methods have been detailed previously.15 The primary objective of TRIAD is to determine how managed care systems influence processes and outcomes of diabetes care.
There are 6 translational research centers: Pacific Health Research Institute in Honolulu, Hawaii; Indiana University Translational Research Center in Indianapolis; Kaiser Foundation Research Institute in Oakland, California; University of California, Los Angeles, School of Medicine; University of Medicine and Dentistry of New Jersey in New Brunswick and Newark; and University of Michigan in Ann Arbor.
These translational research centers collaborate with 10 health plans and 68 provider groups, which in 2002 served approximately 180000 people with diabetes across the United States. The TRIAD study had 8785 participants in 2002–2003, when this second TRIAD survey was conducted. The populations served were ethnically diverse and came from Hawaii, California, Texas, Indiana, Michigan, Pennsylvania, and New Jersey.
Managed care health plans are defined as entities that deliver, administer, or assume risk for health services to influence the quality, access, cost, and outcomes of health care for a defined population.16 Health plans participating in TRIAD included staff model health maintenance organizations, network and independent practice association model health maintenance organizations, point-of-service plans, and preferred provider organizations; plans were for-profit or not-for-profit and included Medicare and Medicaid products.
Study Design and Data Collection
Participants were eligible for TRIAD if they were aged 18 years or older, were not pregnant, had diabetes (on the basis of physician diagnosis) for at least 1 year, were continuously enrolled in the health plan for at least 1.5 years at baseline, and used health services during that time.
For this analysis, as in previous studies of socioeconomic position and health,17 we excluded participants younger than 25 years (31 participants in this analysis), because they may not have completed their education (our primary exposure of interest). Because of the language constraints of our interviewers, we restricted the study to participants who spoke either English or Spanish.
Ninety-seven percent (n=8785) of the 8972 eligible people who were contacted responded to the survey. When we used the Council of American Survey Research Organizations’ definition of response rate (which assumes those unable to be contacted had the same rate of eligibility as those contacted and had been counted in the denominator), the survey response rate was 69%. Of the 8785 participants who initially responded to the survey by a computer-assisted telephone interview or mail during 2002–2003, 6538 participants provided complete data on smoking and the covariates included in our analysis.
Among the relations between socioeconomic status and various health behaviors we examined, we focus here on smoking. Smoking was based on self-report, shown previously to be a reliable measure in the general population,18 and was defined as the respondent having answered “everyday” or “some days” to the question: “in the past year, have you smoked cigarettes every day, some days, or not at all?” Educational attainment was selected as the measure of socioeconomic status because it is easily ascertained in population surveys and is associated with important related factors (such as functional health literacy19) that affect health. Moreover, education is usually completed early in the life course, is stable thereafter, and is less subject to reverse causality by health status than income or occupation, both of which may be affected by declining health status.
Statistical Analysis
We calculated predicted probabilities of smoking at each level of education. These probabilities were derived from hierarchical logistic regression models using the SAS 8.02 GLIMMIX macro and the penalized quasi-likelihood estimation method (SAS Institute Inc, Cary, NC), with random intercepts for health plan, to account for the clustering of observations within health plans.
As our effect measure (i.e., adjusted smoking prevalence), we calculated the predicted probabilities rather than rely on odds ratios, because the latter become poor estimates of relative risk when outcomes are common (i.e., prevalent in more than 30% of the sample).20
We stratified educational attainment into 4 levels: some high school or less, high school graduate, some college, and 4-year college graduate or greater. We adjusted the hierarchical model for suspected confounders including gender, age group (25 to 44 years, 45 to 64 years, and 65 years and older), race/ethnicity (Asian/Pacific Islander, Hispanic, non-Hispanic African American, non-Hispanic White, Other), Spanish-speaking, employment status (employed; retired; or unemployed, student, or homemaker), time since diabetes diagnosis (0 to 5 years, 5 to 10 years, 10 or more years), type of diabetes treatment (diet only, oral medications only, insulin), health education class attendance in past year, presence of depressive symptoms (i.e., respondent reported having depressive symptoms on more than half the days within the past 2 weeks or was taking antidepressants at the time of the interview), and cardiovascular risk score.
For cardiovascular risk, we used a summary score (0 to 3), summing indicators of (1) physician-diagnosed myocardial infarction, or having ever had bypass surgery or angioplasty; (2) physician-diagnosed stroke, cerebrovascular accident, or a transient ischemic attack or “ministroke”; and (3) having ever had a toe, foot, or leg amputation. Because only 36 participants had all 3 indicators, we grouped them with participants having 2 indicators.
We did not adjust for income, because it may have acted as a mediating rather than a confounding variable in our conceptual framework.
To evaluate whether the education–smoking association differed demographically (e.g., hypothesized effect modifiers such as age), we tested the statistical significance of crossproduct terms (e.g., age group interaction with education stratum).
To further understand the extent to which historical patterns of smoking initiation and quitting rates may have shaped the current prevalence of smoking (i.e., cohort effect), we evaluated self-reported former smoking in the 1 health plan that collected such data by survey (Kaiser Permanente, California).
RESULTS
Sample Characteristics
The sample was racially diverse and included a balance of men and women across a range of ages older than 25 years (Table 1 ▶ shows unadjusted proportions). Almost all the participants (97%) responded to the survey in English. The majority of participants were treated with oral hypoglycemic agents, insulin, or insulin plus oral therapy; less than 10% controlled their diabetes with diet and exercise alone. One fifth attended health education classes during the previous year.
TABLE 1—
Unadjusted Participant Characteristics of Adults with Diabetes, by Current Smoking Status: Translating Research Into Action for Diabetes Study, 2002–2003
| Sample, No. (%) | Smokers, No. (% Prevalence) | P | |
| Overall sample | 6578 (100) | 1003 (15) | . . . |
| Gender | .65 | ||
| Women | 3537 (54) | 536 (15) | |
| Men | 3001 (46) | 467 (16) | |
| Age, y | < .001 | ||
| 25–44 | 628 (10) | 160 (25) | |
| 45–64 | 3226 (49) | 647 (20) | |
| ≥ 65 | 2684 (41) | 196 (7) | |
| Education | < .001 | ||
| Some high school or less | 1239 (19) | 234 (19) | |
| High school graduate | 1911 (29) | 306 (16) | |
| At least some college | 2001 (31) | 330 (16) | |
| College graduate | 1387 (21) | 133 (10) | |
| Race/ethnicity | < .001 | ||
| Asian/Pacific Islander | 947 (14) | 138 (15) | |
| Hispanic | 1040 (16) | 127 (12) | |
| Non-Hispanic African American | 948 (14) | 206 (22) | |
| Non-Hispanic White | 3029 (46) | 424 (14) | |
| Other | 574 (9) | 108 (19) | |
| Interviewed in Spanish | 200 (3) | 21 (11) | .054 |
| Diabetes treatment | .18 | ||
| Diet and exercise only | 449 (7) | 68 (15) | |
| Oral agents | 3894 (60) | 573 (15) | |
| Insulin | 2195 (34) | 362 (16) | |
| Time since diagnosis of diabetes, y | < .001 | ||
| < 5 | 1209 (18) | 206 (17) | |
| 5–10 | 1997 (31) | 342 (17) | |
| ≥ 10 | 3332 (51) | 455 (14) | |
| Attended health education class in past year | 1066 (16) | 113 (11) | < .001 |
| Reported having depressive symptoms in the last few weeks | 2869 (44) | 556 (19) | < .001 |
| Employment status | < .001 | ||
| Employed | 2786 (43) | 483 (17) | |
| Retired | 2590 (40) | 248 (10) | |
| Homemaker, student, or not employed | 1162 (18) | 272 (23) | |
| Cardiovascular comorbidity score | .1 | ||
| No comorbidities | 4870 (74) | 769 (16) | |
| 1 comorbidity | 1350 (21) | 182 (13) | |
| ≥ 2 comorbidities | 318 (5) | 52 (16) | |
| Health plan location | |||
| California | 1259 (19) | 142 (11) | < .001 |
| Hawaii | 1363 (21) | 224 (16) | |
| Indiana | 721 (11) | 208 (29) | |
| Michigan | 1013 (15) | 133 (13) | |
| New Jersey | 859 (13) | 153 (18) | |
| Texas | 1323 (20) | 143 (11) | |
| Education stratified by age | |||
| Some high school or less | < .001 | ||
| 25–44 | 56 (5) | 30 (54) | |
| 45–64 | 418 (34) | 139 (33) | |
| ≥ 65 | 765 (62) | 65 (9) | |
| High school graduate | < .001 | ||
| 25–44 | 174 (9) | 57 (33) | |
| 45–64 | 921 (48) | 195 (21) | |
| ≥ 65 | 816 (43) | 54 (7) | |
| At least some college | < .001 | ||
| 25–44 | 241 (12) | 57 (24) | |
| 45–64 | 1117 (56) | 223 (20) | |
| ≥ 65 | 643 (32) | 50 (8) | |
| College graduate | .004 | ||
| 25–44 | 157 (11) | 16 (10) | |
| 45–64 | 770 (56) | 90 (12) | |
| ≥ 65 | 460 (33) | 27 (6) | |
Overall, 15% of the sample reported to be currently smoking. The prevalence of smoking varied substantially across the 6 translational research centers (11% in California and Texas plans, 13% in the Michigan plan, 16% in the Hawaii plan, 18% in the New Jersey plan, and 29% in the Indiana plan).
In bivariate analyses, smoking rates were similarly distributed among men and women but differed significantly across age groups, with those aged 25–44 years having the highest rates, those aged 45–64 years having the second-highest rates, and those aged 65 years and older having the lowest rates. Moreover, smoking was more common in non-Hispanic African Americans, those with lower educational attainment, those with shorter duration of diabetes, those not attending health education classes, and those with depressive symptoms. There was very little difference in the smoking prevalence between participants with 1 or more serious comorbidities (myocardial infarction, stroke, amputation) and participants with no comorbidities.
Education and Smoking
In multivariate analyses including all participants, we observed a significant association between higher smoking prevalence and lower educational attainment. We observed a gradient of smoking prevalence across levels of education in the crude prevalences (Table 1 ▶) and after adjustment for potential confounders (listed previously) and accounting for clustering within health plans.
However, the adjusted point estimates (predicted probabilities) differed from the crude prevalences. After adjustment, the predicted probability of smoking was 24% (95% confidence interval [CI] = 21%, 28%) for participants with some high school or less; 18% (95% CI = 16%, 20%) for high school graduates including those with some college; and 10% (95% CI = 9%, 13%) among college graduates (P < .001).
The relation between education and smoking did not differ significantly by gender or race (not shown). However, the relation between education and smoking varied markedly across age groups (age by education interaction term P < .003), with the relation very strong in those aged 25 to 44 years, modest for those aged 45 to 64 years, and nonexistent for those aged 65 years and older (Figure 1 ▶). The highest prevalence of smoking (50% [95% CI = 36%, 63%]) was observed among the young adults with less than a high school education.
FIGURE 1—
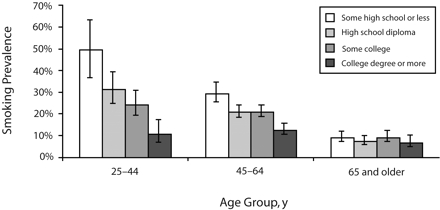
Prevalence of smoking among adults with diabetes, across age groups and educational attainment: Translating Research Into Action for Diabetes (TRIAD) study survey, 2002–2003.
Note. Prevalences (95% confidence intervals) are based on predicted probabilities generated from the hierarchical logistic regression model (accounting for clustering within health plan) and adjusted for age group, gender, race/ethnicity, Spanish-speaking, employment status, time since diabetes diagnosis, type of diabetes treatment, health education class attendance in past year, presence of depressive symptoms, and presence of a cardiovascular risk factor.There was a significant age-by-education interaction (P<.003).
DISCUSSION
This is the first large, multicenter study of the relation between educational attainment and smoking behaviors among adults with diabetes in managed care settings across the United States. We observed striking social disparities in smoking among adults aged 25–64 years, but not among those aged 65 years and older.
Alarmingly high smoking rates (approximately 50%) were observed in poorly educated managed care enrollees with diabetes and aged 25–44 years. A population-based sample (1988 Behavioral Risk Factor Surveillance System survey) has also reported greater smoking rates among diabetic respondents with lower levels of education or income.21 However, we are not aware of recent large studies that have specifically examined smoking prevalence in young diabetic adults.
Our findings are also consistent with an international comparison in 12 European countries from 1986 to 1994 that found, in most countries, greater educational differences in smoking rates in a younger (aged 20 to 44 years) group and higher smoking rates among those who were less educated.22 People with diabetes, particularly early-onset diabetes (i.e., with a longer exposure to diabetes), are at elevated risk of micro- and macrovascular disease. Smoking further increases this risk, along with the risk of other smoking-related diseases (e.g., lung cancer, emphysema), adding to the overall health care costs of this patient group that already has high morbidity.
These studies suggest that intensive interventions and support should be targeted toward the most vulnerable group—young people with diabetes—and should be tailored to reach patients with the lowest educational attainment.
The interaction between age and education we observed in this study may be because of differential survival (i.e., survival bias) or a cohort effect. Higher mortality rates among poorly educated smokers compared with well-educated smokers could have attenuated any educational gradient historically observed in younger smokers. However, considerable evidence also supports a cohort effect.
Smoking is usually initiated early in life, and thus, the presence of the educational gradient in smoking we observed in young adults (aged 25–44 years) likely reflects current patterns of social acceptability: greater smoking initiation in less educated, poorer, and minority youths14 and a greater and increasing cessation among those with higher levels of education.23
Current smoking patterns in elderly persons may reflect historical social norms, which persist to some extent over time, in addition to selective mortality and differential quitting rates. Before the extensive campaigns for smoking cessation (e.g., the 1968 “Fairness Doctrine”), smoking was more universally acceptable.24 Highly educated people tend to adopt innovative behavior earlier, and thus, the diffusion of smoking in the first half of the 20th century was thought to have begun among the young and highly educated who considered it an innovative behavior that challenged taboos.24
However, in our analyses, the prevalence of self-reported former smoking among currently nonsmoking participants (defined as those who had not smoked at all in the year prior to the survey) older than 45 years was uniform across educational attainment, although there was an educational gradient for those younger than 45 years (with greater former smoking rates in poorly educated participants). Thus, among older (older than 65 years) smokers, smoking initiation was likely more equally distributed across social strata at the time these now-elderly smokers initiated smoking (especially before the 1960s). Less social stratification in smoking initiation in the past may have formed the smoking patterns that persisted and are now reflected in the current and former smoking patterns that were reported in the elderly participants.
This hypothesis is consistent with other studies suggesting a cohort effect, with widening social disparities in smoking initiation and cessation starting in the 1970s to the present.14,25,26
Researchers in European countries have shown that social gradients in smoking rates vary from country to country, and they have related these differences to the theory of a 4-stage smoking epidemic.22,24 Consistent with diffusion theory, smoking is initially an exceptional behavior concentrated in higher socioeconomic groups (stage 1), becomes common (stage 2), declines in men as it simultaneously reaches its peak in women (stage 3), and finally declines in the whole population while aggregating in the lower socioeconomic groups (stage 4).
The relation between educational differences and smoking status is becoming more pronounced in the United States,14 suggesting that this country is moving into or is currently at stage 4. This trend was likely shaped by more prominent public antismoking efforts conferring a greater benefit on those with higher levels of education. Moreover, smoking itself is now well recognized as a risk factor for diabetes,27 and thus, the social gradient in smoking before the onset of diabetes may further amplify the existing social disparities in diabetes prevalence.
We have shown that poor educational attainment is associated with greatly amplified smoking prevalence in adults aged 25–44 years with diabetes. Men and women diagnosed with diabetes before the age of 40 years regardless of smoking status are predicted to lose at least 19 and 22 quality-adjusted life-years, respectively.28
The combined effect of diabetes and smoking creates an urgent need for heightened awareness of and a renewed focus on smoking cessation in young and poorly educated people with diabetes. Although the many health benefits of stopping smoking have long been accepted, only recently has a randomized trial of a smoking cessation intervention in a clinical setting demonstrated a reduction in mortality.12
Brief, novel cessation interventions (e.g., using telephone quit lines29 and mobile telephone text messaging30) have demonstrated cost-effectiveness. Rigorous studies have shown that no other clinical intervention can offer as large an effectiveness as when telephone quit lines are integrated into existing health system smoking cessation efforts.31
A recent report from the United Kingdom found that cigarette smokers with diabetes underutilized and had poor awareness of aids to smoking cessation (e.g., nicotine replacement therapy).32 Managed care is well positioned to apply targeted smoking prevention and cessation interventions for their patients with diabetes and to use communication techniques appropriate for patients with low levels of education and inadequate functional health literacy.19
Limitations
Limitations to this study include the fact that our sample came from the managed care populations participating in the 6-state TRIAD study. Thus, participants were not randomly selected from all people with diabetes in the United States and may not represent the larger population of managed care settings.
Smoking status was based on self-report, which, although considered a reliable measure,18 may underestimate actual smoking prevalence.
The value of education may differ across ethnic groups (e.g., higher education among minorities was historically not associated with similar levels of material wealth as among Whites because of limited job opportunities and discrimination in the workforce).
Despite these limitations, we consider education to be less vulnerable to reverse causality than income (poor health can limit future earning potential) and much more stable over the life course.
Conclusions
Smoking and early-onset diabetes put patients in double jeopardy, dramatically increasing their risk of morbidity and mortality. Renewed efforts must be made to target smoking cessation and prevention efforts toward young, poorly educated diabetic patients. The high prevalence of smoking in this at-risk population and the low rates of use of smoking cessation interventions may continue to widen social disparities in diabetes health outcomes for decades to come.
Acknowledgments
This study was funded by Translating Research Into Action for Diabetes (TRIAD; grant U–48-CCU916373) from the Centers for Disease Control and Prevention (CDC) and the National Institute of Child Human Development (grant R01 HD046113–01).
We thank the TRIAD participants, other TRIAD investigators, and staff and health plan partners that made this study possible. We also acknowledge Stanton Glanz for his insights into our findings.
Note. The study is solely the responsibility of the authors and do not necessarily represent the official views of the CDC or National Institutes of Health.
Human Participant Protection The TRIAD study protocol was reviewed and approved by the CDC institutional review board and the institutional review boards at all 6 translational research centers.
Peer Reviewed
Contributors A. J. Karter originated the study and supervised all aspects of its implementation. M. R. Stevens completed the analyses. All authors helped to conceptualize ideas, interpret findings, and review drafts of the article.
References
- 1.Centers for Disease Control and Prevention. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, Ga: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- 2.Armour BS, Woollery T, Malarcher A, Pechacek TF, Husten C. Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- 3.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26: 917–932. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi N, Stephenson JM, Fuller JH. The relationship between smoking and microvascular complications in the EURODIAB IDDM Complications Study. Diabetes Care. 1995;18:785–792. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Alberiche M, Zenere MB, Bonadonna RC, Muggeo M, Bonora E. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82: 3619–3624. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson PM, Gudbjornsdottir S, Eliasson B, Cederholm J. Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes—data from the National Diabetes Register in Sweden. Diabetes Metab. 2004;30:261–268. [DOI] [PubMed] [Google Scholar]
- 7.Karim R, Buchanan TA, Hodis HN, Li Y, Mack WJ. The association of smoking and subclinical atherosclerosis in type 2 diabetes: modification by duration of diabetes. Diabet Med. 2005;22:81–87. [DOI] [PubMed] [Google Scholar]
- 8.Al Delaimy WK, Manson JE, Solomon CG, et al. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med. 2002;162:273–279. [DOI] [PubMed] [Google Scholar]
- 9.Chuahirun T, Simoni J, Hudson C, et al. Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am J Med Sci. 2004;327: 57–67. [DOI] [PubMed] [Google Scholar]
- 10.Gambaro G, Bax G, Fusaro M, et al. Cigarette smoking is a risk factor for nephropathy and its progression in type 2 diabetes mellitus. Diabetes Nutr Metab. 2001;14:337–342. [PubMed] [Google Scholar]
- 11.Al Delaimy WK, Willett WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes: the Nurses’ Health Study cohort. Diabetes Care. 2001;24:2043–2048. [DOI] [PubMed] [Google Scholar]
- 12.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care. 1997;20:1266–1272. [DOI] [PubMed] [Google Scholar]
- 14.Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the United States. Educational differences are increasing. JAMA. 1989;261:56–60. [PubMed] [Google Scholar]
- 15.The Translating Research Into Action for Diabetes (TRIAD) Study: a multicenter study of diabetes in managed care. Diabetes Care. 2002;25:386–389. [DOI] [PubMed] [Google Scholar]
- 16.Kongstvedt PR. Essentials of Managed Health Care. Gaithersburg, Md: Aspen; 1995.
- 17.Berkman LF, Macintyre S. The measurement of social class in health studies: old measures and new formulations. IARC Sci Publ. 1997; 138:51–64. [PubMed] [Google Scholar]
- 18.Huerta M, Chodick G, Balicer RD, Davidovitch N, Grotto I. Reliability of self-reported smoking history and age at initial tobacco use. Prev Med. 2005;41: 646–650. [DOI] [PubMed] [Google Scholar]
- 19.Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163:83–90. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Newman J. Smoking and diabetes mellitus. Findings from 1988 Behavioral Risk Factor Surveillance System. Diabetes Care. 1991;14:871–874. [DOI] [PubMed] [Google Scholar]
- 22.Cavelaars AE, Kunst AE, Geurts JJ, et al. Educational differences in smoking: international comparison. BMJ. 2000;320:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilpin EA, Pierce JP. Demographic differences in patterns in the incidence of smoking cessation: United States 1950–1990. Ann Epidemiol. 2002;12:141–150. [DOI] [PubMed] [Google Scholar]
- 24.Pampel FC. Age and education patterns of smoking among women in high-income nations. Soc Sci Med. 2003;57:1505–1514. [DOI] [PubMed] [Google Scholar]
- 25.Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the United States. Projections to the year 2000. JAMA. 1989;261: 61–65. [PubMed] [Google Scholar]
- 26.Winkleby MA, Cubbin C, Ahn DK, Kraemer HC. Pathways by which SES and ethnicity influence cardiovascular disease risk factors. Ann N Y Acad Sci. 1999; 896:191–209. [DOI] [PubMed] [Google Scholar]
- 27.Foy CG, Bell RA, Farmer DF, Goff DC Jr, Wagenknecht LE. Smoking and incidence of diabetes among US adults: findings from the insulin resistance atherosclerosis study. Diabetes Care. 2005;28: 2501–2507. [DOI] [PubMed] [Google Scholar]
- 28.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. [DOI] [PubMed] [Google Scholar]
- 29.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quit-line for smokers. N Engl J Med. 2002;347: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers A, Corbett T, Bramley D, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294:482–487. [DOI] [PubMed] [Google Scholar]
- 32.Gill GV, Morgan C, MacFarlane IA. Awareness and use of smoking cessation treatments among diabetic patients. Diabet Med. 2005;22:658–660. [DOI] [PubMed] [Google Scholar]


