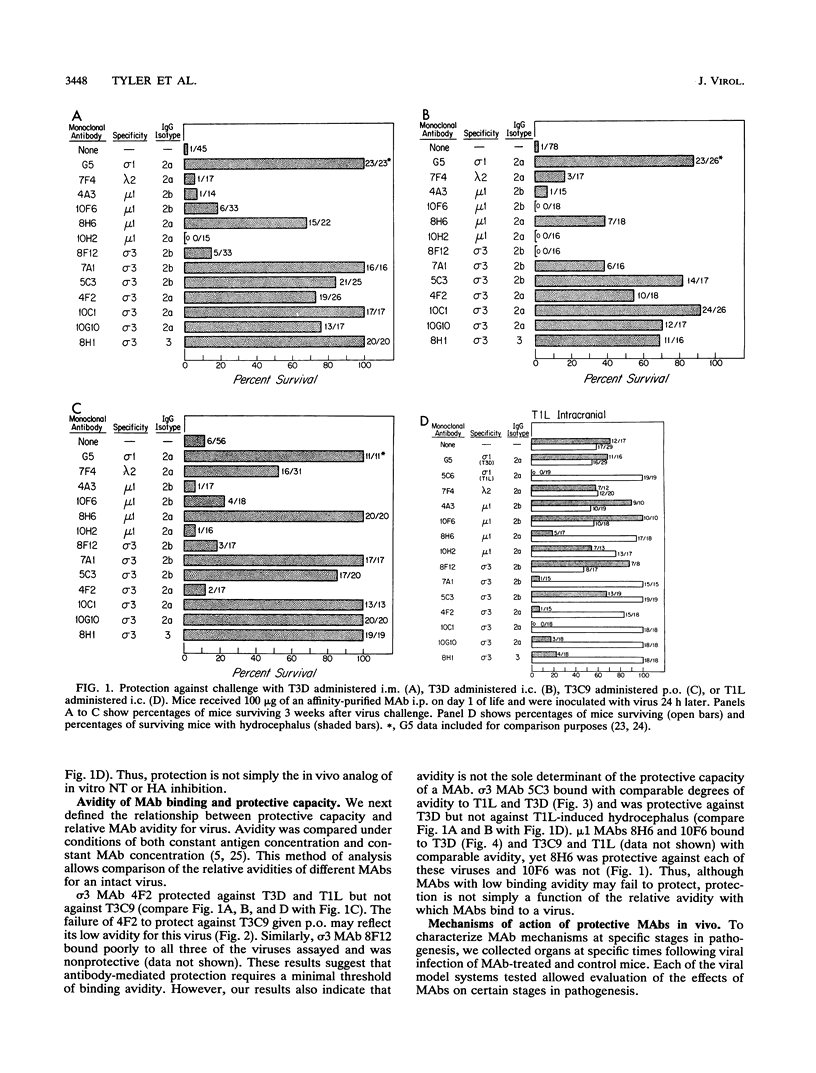
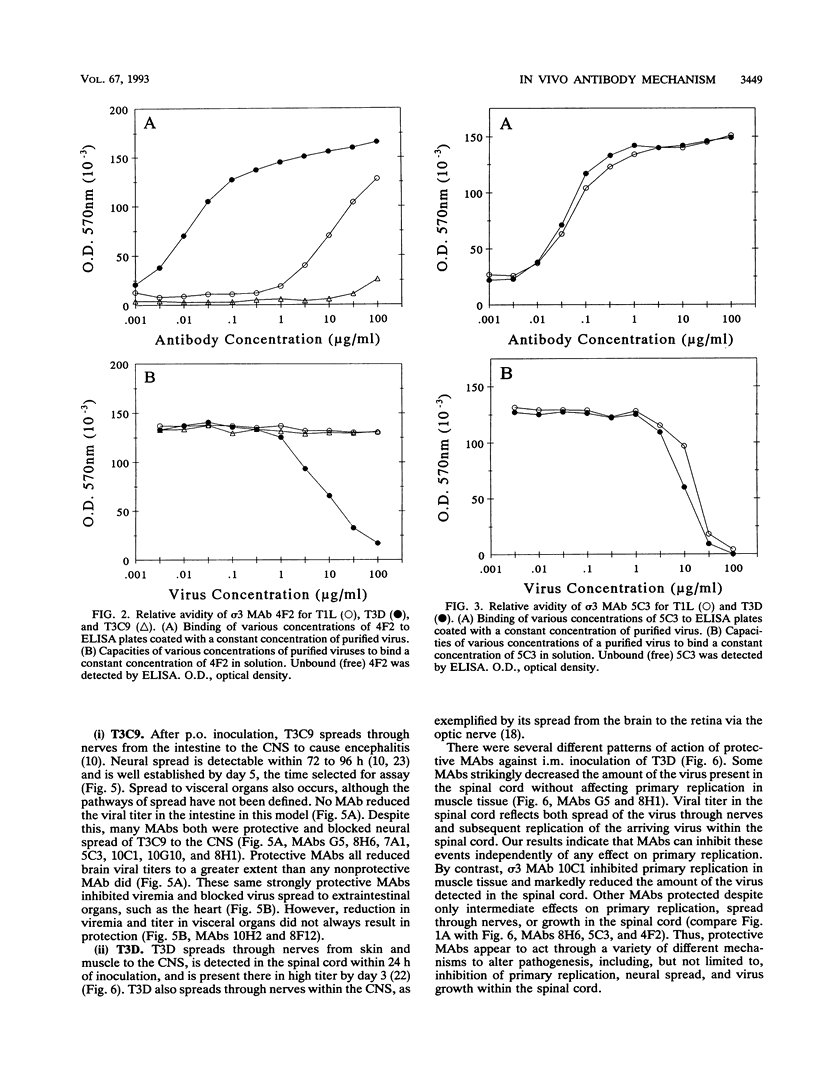
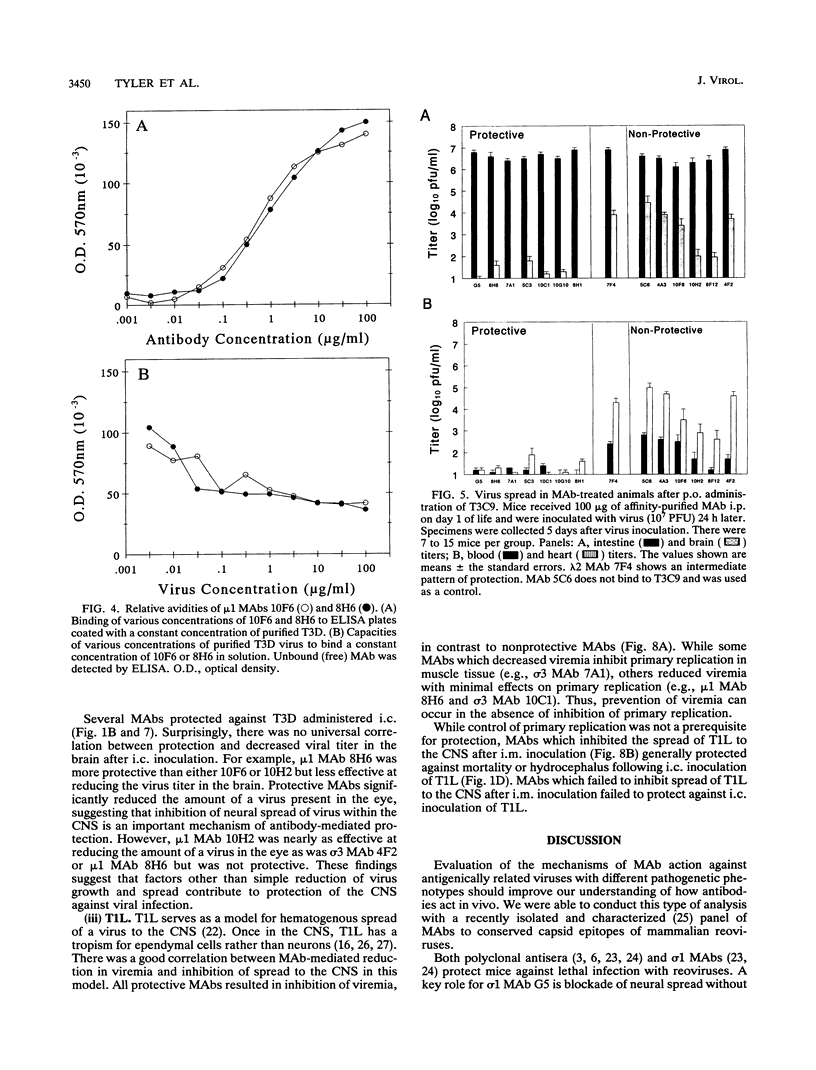
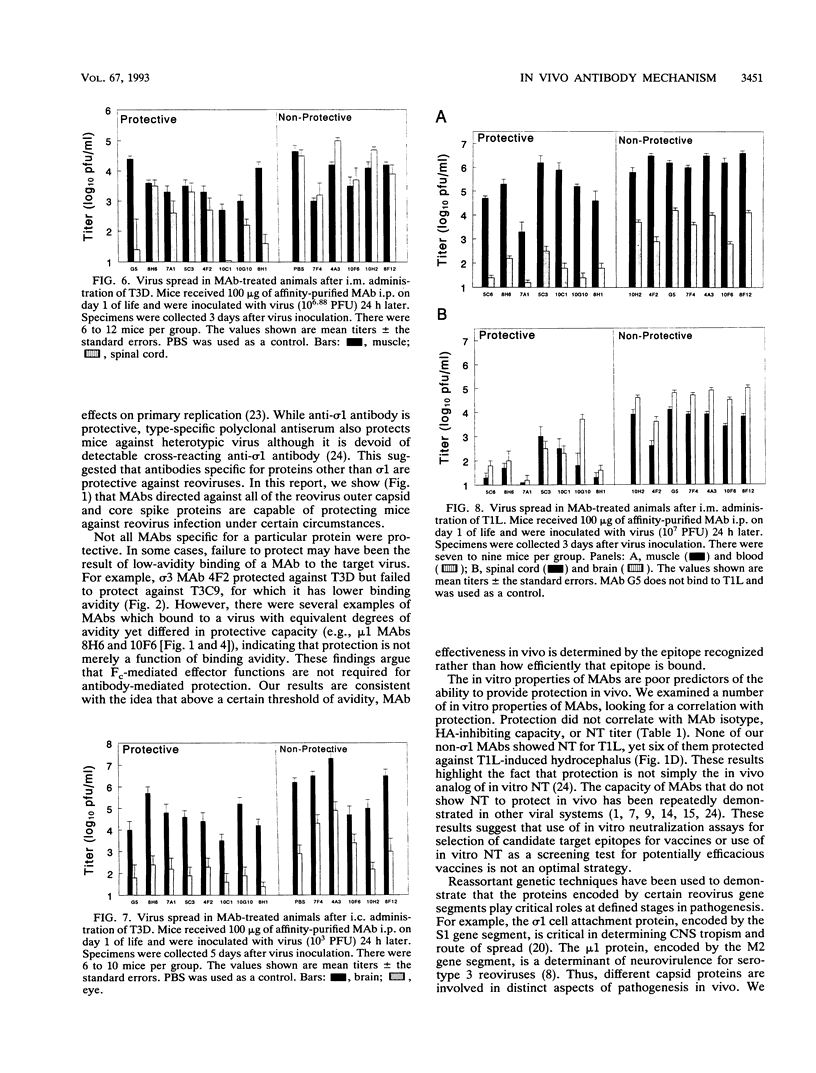
Abstract
We used a recently isolated and characterized panel of monoclonal antibodies (MAbs) specific for cross-reactive determinants on reovirus outer capsid proteins to define mechanisms of antibody-mediated protection in vivo. We studied the capacities of MAbs to protect against lethal infection with reoviruses which differ in site of primary replication, route of spread, and central nervous system tropism. We found the following. (i) MAbs specific for each of the viral outer capsid proteins (sigma 1, sigma 3, and mu 1) and the core spike protein (lambda 2) were protective under certain circumstances. (ii) In vitro properties of MAbs, including isotype, neutralization of viral infectivity, inhibition of virus-induced hemagglutination, and avidity of binding, were poorly predictive of the capacities of MAbs to protect in vivo. (iii) MAbs did not act at a single stage during pathogenesis to mediate protection; instead, protective MAbs were capable of altering a variety of stages in reovirus pathogenesis. (iv) MAbs protective against one reovirus also protected against other reoviruses that utilized different pathogenetic strategies, suggesting that the viral epitope bound by an antibody rather than the pathogenetic strategy employed by the virus is a critical determinant of antibody-mediated protection in vivo. (v) A prominent mechanism of protective MAb action is inhibition of viral spread through nerves from a site of primary replication (e.g., the intestine or muscle tissue) to the central nervous system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J Virol. 1985 May;54(2):546–551. doi: 10.1128/jvi.54.2.546-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Cuff C. F., Lavi E., Cebra C. K., Cebra J. J., Rubin D. H. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J Virol. 1990 Mar;64(3):1256–1263. doi: 10.1128/jvi.64.3.1256-1263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Gagner J. P., Morrison L. A., Fields B. N. Penetration of the nervous systems of suckling mice by mammalian reoviruses. J Virol. 1991 Jan;65(1):123–131. doi: 10.1128/jvi.65.1.123-131.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- Grosfeld H., Velan B., Leitner M., Cohen S., Lustig S., Lachmi B. E., Shafferman A. Semliki Forest virus E2 envelope epitopes induce a nonneutralizing humoral response which protects mice against lethal challenge. J Virol. 1989 Aug;63(8):3416–3422. doi: 10.1128/jvi.63.8.3416-3422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester J. C., Rouse B. T. The mouse model and understanding immunity to herpes simplex virus. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S935–S945. doi: 10.1093/clind/13.supplement_11.s935. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Sidman R. L., Fields B. N. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3852–3856. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P. L. Mucosal immune response to poliovirus vaccines in childhood. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S361–S368. doi: 10.1093/clinids/6.supplement_2.s361. [DOI] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Weiner H. L. Viral receptors on isolated murine and human ependymal cells. Science. 1982 Jan 22;215(4531):419–421. doi: 10.1126/science.6276976. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., Bronson R. T., Byers K. B., Fields B. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985 Jan;35(1):88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., Virgin H. W., 4th, Bassel-Duby R., Fields B. N. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J Exp Med. 1989 Sep 1;170(3):887–900. doi: 10.1084/jem.170.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Mann M. A., Fields B. N., Tyler K. L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991 Dec;65(12):6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]



