Abstract
The sentinel lymph node biopsy (SLNB) represents a minimal invasive surgical method for axillary staging in patients with primary breast cancer. In a prospective study, evaluation of quality of life (QOL) and arm morbidity was performed before surgery on a total of 56 breast cancer patients. The EORTC QLQ-C30 and EORTC QLQ-BR23 questionnaires were used for QOL assessment. Assessment of pain was additionally observed using the McGill Pain Questionnaire. Arm mobility was observed by goniometric measurement of arm movement. Data were collected before surgery (t1), 1 week after discharge (t2) and 9–12 months after surgery (t3).
The type of axillary surgery does not seem to affect global QOL at a short-time follow-up, but patients recover sooner after SLNB. Body image and sexual functioning remain stable in both types of axillary surgery. Arm/shoulder pain was reported in 36% of patients after SLNB in comparison to 68% receiving axillary lymph node dissection (ALND), and ‘numbness’ was reported only in 4% of patients in the SLNB group vs 19.3% after ALND. Abduction, flexion and horizontal adduction of the affected arm show significant impairment after ALND. Breast cancer patients should be counselled about the benefits of SLNB over ALND concerning QOL and postsurgery side effects in a short-term follow-up.
Keywords: quality of life, sentinel lymph node biopsy, breast cancer, morbidity
Axillary lymph node dissection (ALND) in breast cancer patients still represents the routine surgical method for axillary staging. Although the axillary node status is the most important prognostic factor for recurrence and survival (Fisher et al, 1984; Carter et al, 1989) and information obtained by axillary dissection is useful for planning adjuvant treatment, it is associated with substantial morbidity (Kissin et al, 1986; Ivens et al, 1992; Keramopoulos et al, 1993; Hack et al, 1999; Kakuda et al, 1999) and psychological distress (Maunsell et al, 1993; Tobin et al, 1993; Shimozuma et al, 1999). Hack et al, showed arm/shoulder pain, weakness or numbness in 72% and impaired range of motion in 73% of breast cancer patients after ALND, whereas high levels of quality of life (QOL) were reported. Moderate to severe pain was reported between 20, 23 and 32% (Van Dam et al, 1993; Kuehn et al, 2000; Ververs et al, 2001) and was not significantly related to time since surgery. Other reports suggest that arm problems after ALND are associated with a negative effect on the overall QOL of breast cancer patients (Maunsell et al, 1993; Kuehn et al, 2000). As a result of the need to reduce axillary morbidity, many investigations have been performed on sentinel lymph node biopsy (SLNB), an alternative procedure. Using vital dye and/or radiocolloid, the sentinel node/s as the first lymph node to receive lymphatic drainage from the primary tumour can be identified by a minimal invasive surgical technique. Recently, published data showed no sensory morbidity after SLNB (Giuliano et al, 2000) at a median follow-up of 39 months. Schrenk et al (2000) reported less postoperative arm pain, numbness and arm motion restriction after SLNB at a follow-up period of 15.4 months. The evaluation of morbidity after ALND vs SLNB is under investigation in ongoing randomised trials as the NSABP B-32 and the ALMANAC trial. The evaluation of QOL issues such as treatment side effects, patients satisfaction and symptom management are substantial parameters in decision making regarding surgical interventions. However, at this time little is known about the impact of SLNB on QOL in breast cancer patients.
The major objectives of this study are (1) to evaluate QOL differences in a short-term follow-up after two surgical procedures (ALND and SLNB) in breast cancer patients receiving breast-conserving treatment; (2) to determine the impact of SLNB on global QOL of breast cancer patients and (3) to compare morbidity end points (arm/shoulder mobility, pain, sensory morbidity) during different clinical phases.
MATERIALS AND METHODS
Selection of patients
In a prospective, longitudinal study between September 2000 and March 2002, we included 56 consecutive patients with newly diagnosed primary breast cancer. Study eligibility criteria included the following: (1) breast cancer stage I or II, (2) breast-conserving surgery in all patients, (3) patients' age between 18 and 80 years, (4) no severe physical and mental comorbidity, (5) performance status 0 and (6) informed consent.
Procedures
A total of 56 patients with invasive breast cancer received the sentinel node biopsy. In all, 25 patients receiving the SLNB only (Group I) were compared with 31 patients who underwent the standard level I and II ALND (Group II) when intraoperative frozen section showed metastatic disease. Before the study was started, a surgical protocol was implemented in order to minimise differences in technique. Similar incisions, similar anatomic dissections and similar drainage catheters were used. All patients received breast-conserving surgery. Our technique of SLNB has been described previously (Reitsamer et al, 2002). Briefly, SLNB was performed by the combined method using peritumoral injection of technetium-99m-labelled albumin (Nanocoll®, Sorin Biomedica, Saluggia, Italy) and subareolar subcutaneous injection of blue dye (Patent Blue V®, Laboratoire Guerbet, Aulnay-sous-Bois, France). Technetium-99m was injected 16–18 h before surgery and blue dye was injected 5 min prior to incision to identify the SLN. Hot and blue nodes were removed and frozen section was performed immediately. If SLN/s were negative in frozen section, patients had no further ALND. All patients received whole-breast irradiation after surgery. No radiotherapy to the axilla was performed. Adjuvant chemotherapy was administered before radiotherapy when indicated. Adjuvant endocrine treatment was initiated after surgery. The decision to use adjuvant chemotherapy or hormone therapy was mainly based on prognostic factors from the primary breast tumour such as tumour size, hormone receptor status and/or HER-2/neu status. Additionally, node-positive patients received adjuvant hormone therapy by participating in the national hormone treatment trial.
Assessments
Frequency
Data were collected at three time points: before surgery (t1), 1 week after discharge (t2), and 9–12 months after surgery (t3).
EORTC QLQ-C30
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire QLQ-C30, version 3.0, a cancer-specific questionnaire, is composed of five functional scales (physical, role, emotional, cognitive, social), the global health status and nine symptom scales (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, financial difficulties). The global health status correlates significantly with all the functional and symptom scales (Aaronson et al, 1993). For the functional and global QOL scales a higher score indicates a better level of functioning. All patients answered this questionnaire before surgery (t1), 1 week after discharge (t2) and 9–12 (t3) months after surgery.
EORTC QLQ-BR23
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire QLQ-BR23, the breast cancer module, incorporates four symptom scales (systemic therapy side effects, breast symptoms, arm symptoms, upset by hair loss) and four functional scales (body image, sexual functioning, sexual enjoyment, future perspective). All scores obtained from scales and single items range from 0 to 100. A higher score indicates a better level of functioning. All patients answered this questionnaire before surgery (t1), and 9–12 months after surgery (t3).
Range of arm/shoulder motion
All patients underwent goniometric measurement of the affected arm by a physiotherapist at every time point. Measurements of the following arm movements were obtained: shoulder flexion, shoulder extension, shoulder abduction, horizontal abduction and horizontal adduction.
McGill Pain Questionnaire
German version (Melzack, 1975; Stein and Mendl, 1988). This questionnaire is composed of sensory, affective, evaluative word descriptors in the form of 78 words grouped into 20 subclasses used by patients to specify subjective pain experience and of a visual analogue pain scale for measurement of pain intensity. The questionnaire provides information about the site of pain and the relative effects of a given manipulation on several dimensions of pain. All patients completed the questionnaire at every time point.
Karnofsky performance status scale (KPS)
The KPS scale consists of 11 components describing patients' mobility and ability to maintain employment, live at home and care for oneself. The scores used by clinicians range from 0 (worst physical condition) to 100 (best physical status).
Statistical analysis
Statistical methodology was used in accordance with The EORTC QLQ-C30 Scoring Manual (Fayers et al, 1999). In order to compare both types of surgery (ALND vs SLNB) nonparametric independent two-sided tests were applied (Wald–Wolfowitz test, Kolmogorov–Smirnov test, Mann–Whitney U-test) to all variables tested. Differences of the proportions of patients reporting pain after ALND over time were analysed using the Cochran's Q test. The same test was applied for analysis of the SLNB group. A P-value less than 5% was considered as significant.
RESULTS
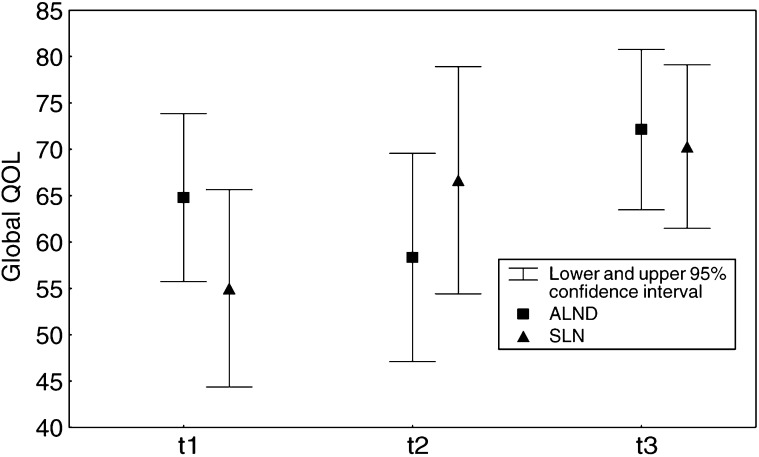
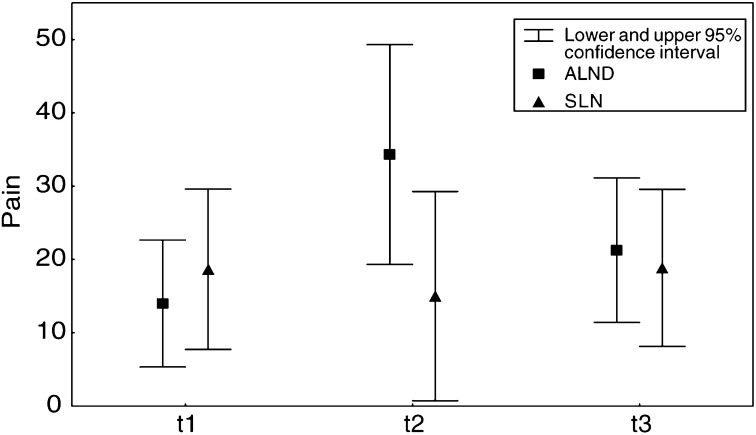
In all, 56 breast cancer patients participated in this study. Patients' clinical and demographic characteristics are summarized in Table 1 . The mean number of lymph nodes dissected was 2.2 in group I and 15.0 in group II. The percentage of postmenopausal patients was 71.4%. Preoperative arm symptoms, the Karnofsky performance status and QOL levels were comparable between both groups. Table 2 provides QOL levels (EORTC QLQ-C30) at all time points of assessment. The mean scores at baseline assessment (t1) showed that patients' global QOL and emotional functioning were more affected in both groups than physical functioning, role functioning, cognitive functioning and social functioning. However, significant improvement of global QOL (P=0.002) occurs at t2 only in patients after SLNB (Figure 1). Analysis of means of symptom scales shows significant higher levels of pain at t2 in patients after ALND (P=0.03) (Figure 2). Comparison of QOL dimensions assessed by the EORTC QLQ-C30 and QLQ-BR23 (Tables 2 and 3 ) shows no statistically significant differences among patients in both groups before surgery (t1). At t3, global QOL improved in both groups, but there were no statistically significant differences in any dimension of QOL. Karnofsky performance scores at baseline were high in both groups and showed no significant changes over time (Table 3).
Table 1. Patient characteristics.
| Total sample(n=56) | ALND(n=31) | SLNB(n=25) | P-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean | 60.1 | 57.7 | 61.4 | 0.27 |
| Site of axillary surgery (%) | ||||
| Right axilla | 51.7 | 54.8 | 48.0 | 0.62 |
| Left axilla | 48.3 | 45.2 | 52.0 | 0.6 |
| Cancer type (%) | ||||
| Invasive ductal | 62.5 | 64.5 | 56.0 | 0.52 |
| Invasive lobular | 14.3 | 6.5 | 24.0 | 0.07 |
| Invasive ductal and intraductal | 23.2 | 29.0 | 20.0 | 0.44 |
| Tumour size (%) | ||||
| pT1 | 71.4 | 74.2 | 68.0 | 0.62 |
| pT2 | 28.6 | 25.8 | 32.0 | 0.62 |
| Adjuvant treatment (%) | ||||
| HT and RT | 67.9 | 67.7 | 68.0 | 0.62 |
| CT and RT | 23.2 | 29.0 | 20.0 | 0.44 |
| RT alone | 8.9 | 3.3 | 12.0 | 0.22 |
| Menopausal status (%) | ||||
| Premenopausal | 28.6 | 35.5 | 20.0 | 0.20 |
| Postmenopausal | 71.4 | 64.4 | 80.0 | 0.20 |
| Civil status (%) | ||||
| Married/partnership | 53.6 | 58.1 | 48.0 | 0.45 |
| Single/widowed/divorced | 30.4 | 35.5 | 24.0 | 0.36 |
| Unknown | 16.0 | 6.4 | 28.0 | 0.03 |
| Education (%) | ||||
| Primary | 44.6 | 51.6 | 40.0 | 0.39 |
| Secondary/professional | 25.0 | 25.8 | 24.0 | 0.86 |
| Higher | 14.3 | 16.1 | 12.0 | 0.66 |
| Unknown | 16.1 | 6.5 | 24.0 | 0.06 |
HT=hormone therapy; RT=radiotherapy; CT=chemotherapy.
Table 2. Means (s.d.) of the EORTC QLQ-C30 scale scores.
| ALND | SLNB | P-value | |
|---|---|---|---|
| Before surgery (t1) | |||
| Global QOL | 63.8 (24.9) | 55.9 (25.9) | 0.87 |
| Physical functioning | 87.7 (19.2) | 90.8 (17.9) | 0.69 |
| Role functioning | 88.0 (26.1) | 90.9 (17.7) | 0.69 |
| Emotional functioning | 65.3 (21.6) | 53.8 (31.1) | 0.05 |
| Cognitive functioning | 80.6 (21.9) | 84.0 (23.8) | 0.41 |
| Social functioning | 85.9 (23.6) | 84.8 (22.3) | 0.75 |
| Pain | 14.1 (23.2) | 18.7 (27.0) | 0.90 |
| After discharge (t2) | |||
| Global QOL | 57.8 (20.5) | 68.5 (17.0) | 0.58 |
| Physical functioning | 82.3 (15.4) | 86.7 (14.9) | 0.37 |
| Role functioning | 60.2 (28.0) | 70.4 (30.9) | 0.37 |
| Emotional functioning | 75.6 (21.3) | 70.7 (27.3) | 0.07 |
| Cognitive functioning | 86.1 (22.3) | 90.7 (14.7) | 0.65 |
| Social functioning | 78.7 (28.4) | 83.4 (22.0) | 0.65 |
| Pain | 34.3 (29.2) | 16.7 (20.4) | <0.05a |
| 9–12 months after surgery (t3) | |||
| Global QOL | 72.1 (22.7) | 70.2 (20.3) | 0.45 |
| Physical functioning | 85.9 (21.4) | 87.2 (18.2) | 0.34 |
| Role functioning | 74.1 (27.6) | 78.3 (26.8) | 0.63 |
| Emotional functioning | 68.9 (19.8) | 70.5 (25.4) | 0.63 |
| Cognitive functioning | 77.6 (25.3) | 82.6 (24.8) | 0.21 |
| Social functioning | 86.2 (23.6) | 89.8 (19.9) | 0.50 |
| Pain | 21.3 (25.9) | 18.8 (24.8) | 0.29 |
Statistically significant.
Figure 1.
Comparison of global QOL (means) after ANLD vs SLNB over time.
Figure 2.
Comparison of pain (means) after ALND vs SLNB over time.
Table 3. Means (s.d.) of the EORTC QLQ-BR23 scale scores and Karnofsky performance status.
| ALND | SLNB | P-value | |
|---|---|---|---|
| Before surgery (t1) | |||
| Body image | 89.9 (18.3) | 83.2 (23.7) | 0.39 |
| Sexual functioning | 30.9 (31.9) | 28.7 (27.7) | 0.69 |
| Sexual enjoyment | 61.5 (38.1) | 52.3 (32.5) | 0.95 |
| Future perspective | 38.8 (33.9) | 31.8 (39.5) | 0.56 |
| Arm symptoms | 8.3 (14.4) | 19.5 (25.2) | 0.07 |
| KPS | 98.2 (4.6) | 99.1 (2.8) | 0.10 |
| 9–12 months after surgery (t3) | |||
| Body image | 87.9 (15.8) | 92.0 (13.6) | 0.45 |
| Sexual functioning | 33.4 (33.4) | 35.1 (24.8) | 0.45 |
| Sexual enjoyment | 63.9 (26.4) | 66.7 (23.5) | 0.20 |
| Future perspective | 56.7 (32.9) | 54.5 (34.9) | 0.18 |
| Arm symptoms | 21.2 (22.8) | 14.0 (18.4) | 0.26 |
| KPS | 96.1 (18.1) | 99.5 (2.0) | 0.21 |
Analysis of arm/shoulder mobility assessment data showed significant impairment of abduction and flexion in the operated arm at the time points t2, t3 and of horizontal adduction at the time point t3 in group II (Table 4 ).
Table 4. Means (s.d.) arm/shoulder motion, goniometric measurement.
| ALND | SLNB | P-value | |
|---|---|---|---|
| Before surgery (t1) | |||
| Abduction | 153.7 (19.3) | 160.4 (10.9) | 0.75 |
| Flexion | 152.1 (17.9) | 153.6 (13.6) | 0.58 |
| Extension | 50.7 (8.4) | 49.3 (5.8) | 0.55 |
| Horizontal abduction | 108.6 (12.8) | 108.1 (12.4) | 0.55 |
| Horizontal adduction | 35.1 (14.0) | 32.2 (8.2) | 0.98 |
| After discharge (t2) | |||
| Abduction | 128.3 (24.9) | 152.3 (13.7) | 0.013* |
| Flexion | 134.8 (21.9) | 150.6 (16.1) | 0.04* |
| Extension | 48.6 (11.5) | 51.7 (5.0) | 0.58 |
| Horizontal abduction | 106.1 (15.8) | 108.4 (13.2) | 0.72 |
| Horizontal adduction | 29.5 (14.4) | 29.4 (11.3) | 0.72 |
| 9–12 months after surgery (t3) | |||
| Abduction | 143.8 (22.8) | 158.9 (13.9) | 0.007* |
| Flexion | 146.0 (15.9) | 154.6 (15.0) | 0.03* |
| Extension | 47.1 (11.2) | 52.2 (27.1) | 0.39 |
| Horizontal abduction | 101.1 (15.9) | 106.5 (21.3) | 0.76 |
| Horizontal adduction | 34.5 (14.1) | 35.6 (19.1) | 0.011* |
P<0.05.
Analysis of data assessed by the McGill Pain Questionnaire showed significantly more sensory problems of the affected arm in group II at t3 when the number of words chosen (NWC) was compared with those of group I. Severity of pain measured by the visual analogue scale showed that women in group II reported significantly greater pain than in group I at t3 (Table 5 ).
Table 5. Means (s.d.) for pain.
| ALND | SLNB | P-value | |
|---|---|---|---|
| After discharge (t2) | |||
| Sensory (NWC) | 2.29 (2.67) | 0.88 (1.45) | 0.552 |
| Visual analogue scale | 1.45 (1.36) | 0.68 (1.03) | 0.823 |
| 9–12 months after surgery (t3) | |||
| Sensory (NWC) | 1.45 (2.29) | 0.96 (2.46) | 0.026* |
| Visual analogue scale | 1.13 (1.36) | 0.68 (1.63) | 0.012* |
P<0.05.
Arm/shoulder pain was reported in only 36% of patients after SLNB in comparison to 68% after ALND at t2. While the number of patients with pain decreased significantly over time in the ALND group at t3 (Cochran's Q, P=0.008), no significant changes could be found in the SLNB group (P=0.08). Arm symptoms assessment by the EORTC QLQ-BR23 questionnaire showed no significant difference in both the groups at t3 (Table 3).
DISCUSSION
The present study confirms previous observations suggesting that SLNB is associated with less arm/shoulder morbidity (Giuliano et al, 2000; Schrenk et al, 2000; Burak et al, 2002; Haid et al, 2002; Temple et al, 2002) than ALND. Evaluation and comparison of QOL outcomes in a short time follow-up in breast cancer patients undergoing ALND or SLNB after breast-conserving surgery provides additional observations:
(1) The type of axillary surgery does not seem to have an impact on global QOL, but may affect other QOL aspects as pain. (2) Body image and sexual functioning remain stable during the postsurgery follow-up in both types of axillary surgery. (3) The SLNB is associated with mild pain and mild sensory morbidity, significantly less than ALND, improving during the months following surgery. (4) Arm/shoulder abduction, flexion and horizontal adduction show significant impairment after ALND when compared with the preoperative range of motion.
The examination of postsurgery side effects after the different types of axillary surgery in our sample showed a significant difference in pain severity as well as in intensity of sensory morbidity of the affected arm after SLNB in comparison to ALND. Numbness was reported in 19.3% of the patients after ALND in contrast to 4% in the SLNB group, whereas ‘tugging’ was the most common complaint in both groups. The NWC shows that even after SLNB, a few patients experience substantial sensory complaints of the affected arm at t3. The properties of the McGill Pain Questionnaire in this matter are (1) exclusion of patients reporting breast pain, (2) specification of subjective pain intensity and (3) description of sensory qualities of pain by word descriptors as ‘numbing’, ‘tugging’, etc. Interestingly, evaluation of pain using the McGill Pain Questionnaire, the QLQ-C30 questionnaire and evaluation of ‘arm symptoms’ using the QLQ-BR23 questionnaire show some discrepancy. These results support the hypothesis, that current standard questionnaires do not cover all aspects of QOL (Janni et al, 2001). Several aspects of morbidity including pain, range of motion and sensory complaints of the affected arm have been reported to show significant difference in favour of SLNB (Giuliano et al, 2000; Schrenk et al, 2000; Burak et al, 2002; Haid et al, 2002; Temple et al, 2002). However, measuring instruments and scoring systems used in these studies differ widely.
In our study, patients' clinical and sociodemographic characteristics regarding age, tumour stage, adjuvant treatment were well balanced between the two groups. Using the EORTC QOL-C30 questionnaire, no significant difference could be detected in global QOL after ALND and SLNB at a short time follow-up. Interestingly, baseline assessment showed low levels of patients' global QOL in both groups increasing during follow-up. Statistically significant higher levels of global QOL are observed at t2 and t3 after SLNB, when compared with baseline levels. In contrast, impairment of global QOL at t2 after ALND clearly shows a difference in QOL improvement in favour of the SLNB group. We suggest that this is because patients in the SLNB group recover sooner than after ALND. However, patients having positive nodes in the ALND group reflect a more advanced disease. Randomised trials, as the ALMANAC trial, can possibly demonstrate the impact of axillary status on QOL. In addition to global QOL, assessment of emotional functioning shows low levels at baseline too, with no significant changes during follow-up in both groups. An explanation for low levels at baseline is that patients being informed about the breast cancer diagnosis before surgery induced psychological distress (Fallowfield et al, 1986; Ganz et al, 1992; Coscarelli Schag et al, 1993). In this study the Karnofsky performance status score was, for most patients, over 90 at baseline and showed no differences in patients' physical condition in both groups during follow-up. Using the EORTC QLQ-BR23 questionnaire comparison of body image and sexual functioning showed no difference between the two groups.
In the present study, we used a variety of validated measurement instruments to specify reliably patients' subjective experience of postoperative morbidity and QOL after SLNB in comparison to ALND. To our knowledge, this is one of the first reports to compare various aspects of QOL and arm/shoulder morbidity after different types of axillary surgery considering presurgery assessments. However, despite the analysis of many covariates with different measurement instruments a potential limitation of our study may be the small sample size.
In conclusion, the SLNB as a minimal invasive technique for axillary staging seems to be an alternative to ALND associated with a better postsurgery arm/shoulder mobility, with less pain and less sensory morbidity of the affected arm in a short-time follow-up. Severity of post-treatment side effects and QOL aspects should be considered when counselling breast cancer patients.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez N, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F, for the European Organization for Research Treatment of Cancer Study Group on Quality of Life (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 [DOI] [PubMed] [Google Scholar]
- Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC (2002) Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg 183: 23–27 [DOI] [PubMed] [Google Scholar]
- Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status and survival in 24,740 breast cancer cases. Cancer 63: 181–187 [DOI] [PubMed] [Google Scholar]
- Coscarelli Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L (1993) Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol 11: 783–793 [DOI] [PubMed] [Google Scholar]
- Fallowfield LJ, Baum M, Maguire GP (1986) Effects of breast conservation on psychological morbidity associated with diagnosis and treatment of early breast cancer. Br Med J 293: 1331–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayers PM, Aaronson NK, Bjordal K, Curran D, Groenvold M (1999) The EORTC QLQ-C30 Scoring Manual, 2nd edn. Brussels: European Organization for Research and Treatment of Cancer, Pamphlet [Google Scholar]
- Fisher ER, Sass R, Fisher B (1984) Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (Protocol No. 4). Cancer 53: 712–723 [DOI] [PubMed] [Google Scholar]
- Ganz PA, Schag CAC, Lee JJ, Polinsky ML, Tan SJ (1992) Breast conservation versus mastectomy. Is there a difference in psychological adjustment or quality of life in the year after surgery? Cancer 69: 1729–1738 [DOI] [PubMed] [Google Scholar]
- Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, Glass EC, Turner RR (2000) Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 18: 2553–2559 [DOI] [PubMed] [Google Scholar]
- Hack TF, Cohen L, Katz J, Robson L, Goss P (1999) Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol 17: 143–149 [DOI] [PubMed] [Google Scholar]
- Haid A, Koberle-Wuhrer R, Knauer M, Burtscher J, Fritzsche H, Peschina W, Jasarevic Z, Ammann M, Hergan K, Sturn H, Zimmermann G (2002) Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: a comparative evaluation. Breast Cancer Res Treat 73: 31–36 [DOI] [PubMed] [Google Scholar]
- Ivens D, Hoe AL, Podd TJ, Hamilton CR, Taylor I, Royle GT (1992) Assessment of morbidity from complete axillary dissection. Br J Cancer 66: 136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janni W, Rjosk D, Dimpfl T, Haertl K, Strobl B, Hepp F, Hanke A, Bergauer F, Sommer H (2001) Quality of life influenced by primary surgical treatment for stage I–III breast cancer – Long-term follow-up of a matched-pair analysis. Ann Surg Oncol 8: 542–548 [DOI] [PubMed] [Google Scholar]
- Kakuda JT, Stuntz M, Trivedi V, Klein SR, Vargas HI (1999) Objective assessment of axillary morbidity in breast cancer treatment. Am Surgeon 65: 995–998 [PubMed] [Google Scholar]
- Keramopoulos A, Tsionou C, Minaretzis D, Michalas S, Aravantinos D (1993) Arm morbidity following treatment of breast cancer with total axillary dissection: a multivariated approach. Oncology 50: 445–449 [DOI] [PubMed] [Google Scholar]
- Kissin MW, Querci della Rovere G, Easton D, Westbury G (1986) Risk of lympoedema following the treatment of breast cancer. Br J Surg 73: 580–584 [DOI] [PubMed] [Google Scholar]
- Kuehn T, Klauss W, Darsow M, regele S, Flock F, Maiterth C, Dahlbender R, Wendt I, Kreienberg R (2000) Long-term morbidity following axillary dissection in breast cancer patients – clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat 64: 275–286 [DOI] [PubMed] [Google Scholar]
- Maunsell E, Brisson J, Deschenes L (1993) Arm problems and psychological distress after surgery for breast cancer. Can J Surg 36: 315–320 [PubMed] [Google Scholar]
- Melzack R (1975) The McGill pain questionnaire: major properties and scoring methods. Pain 1: 277–299 [DOI] [PubMed] [Google Scholar]
- Reitsamer R, Peintinger F, Prokop E, Menzel C, Cimpoca W, Rettenbacher L (2003) Sentinel lymph node biopsy alone without axillary lymph node dissection – follow up of sentinel lymph node negative breast cancer patients. Eur J Surg Oncol 29: 221–223 [DOI] [PubMed] [Google Scholar]
- Schrenk P, Rieger R, Shamiyeh A, Wayand W (2000) Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 88: 608–614 [DOI] [PubMed] [Google Scholar]
- Shimozuma K, Ganz PA, Petersen L, Hirji K (1999) Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat 56: 45–57 [DOI] [PubMed] [Google Scholar]
- Stein C, Mendl G (1988) The German counterpart to McGill pain questionnaire. Pain 32: 251–255 [DOI] [PubMed] [Google Scholar]
- Temple LK, Baron R, Cody HS, Fey JV, Thaler HAT, Borgen PI, Heerdt AS, montgomery LL, Petrek JA, Van Zee KJ (2002) Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol 9: 654–662 [DOI] [PubMed] [Google Scholar]
- Tobin MB, Lacey HJ, Meyer L, Mortimer PS (1993) The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphoedema. Cancer 72: 3248–3252 [DOI] [PubMed] [Google Scholar]
- Van Dam MS, Hennipman A, de Kruif JT, van der Tweel I, de Graaf PW (1993) Complications following axillary dissection for breast carcinoma. Ned Tijdschr Geneesk 137: 2395–2398 [PubMed] [Google Scholar]
- Ververs JM, Roumen RM, Vingerhoets AJ, Veugdenhil G, Coebergh JW, Crommelin MA, Luiten EJ, Repelaer van Driel OJ, Schijven M, Wissing JC, Voogd AC (2001) Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancer. Eur J Cancer 37: 991–999 [DOI] [PubMed] [Google Scholar]