Summary
Background
Zinc supplementation can reduce subsequent morbidity in children recovering from diarrhoea and respiratory illness in developing countries. However, whether routine supplementation would decrease morbidity and mortality in populations with zinc deficiency is unclear. We assessed the effect of daily zinc supplementation on children in southern Nepal.
Methods
We did a community-based, cluster-randomised, double-masked, placebo-controlled, 2×2 factorial trial in children aged 1−35 months. Treatment groups were placebo, iron and folic acid, zinc, and iron and folic acid with zinc, with daily doses of 12·5 mg iron, 50 μg folic acid, and 10 mg zinc. Study staff gave children tablets on 2 days each week and left tablets with caregivers for other days. All children received vitamin A supplementation twice per year. Results of the iron arm of the trial have been reported previously. Between October, 2001, and January, 2006, 41 276 children were enrolled into the placebo (n=20 308) or zinc (n=20 968) groups and were followed-up for 60 636·3 person-years. The primary outcome was child mortality, and analyses were by intention to treat. Daily reports of signs and symptoms of common morbidities in stratified random subsamples of children were assessed every week for 12 months. This study is registered at ClinicalTrials.gov, number NCT00109551.
Findings
2505 children refused to continue the trial and 3219 children were lost to follow-up. There was no significant difference in mortality between the zinc and placebo groups (316 vs 333 deaths; hazard ratio 0·92, 95% CI 0·75−1·12). Zinc had no effect on mortality in children younger than 12 months (181 vs 168 deaths; 1·04, 0·83−1·31); mortality was lower, but not statistically significantly so, in older children receiving zinc (135 vs 165; 0·80, 0·60−1·06). The frequency and duration of diarrhoea, persistent diarrhoea, dysentery, and acute lower respiratory infections did not differ between the groups.
Interpretation
Total mortality of children receiving zinc supplementation was not significantly different from that of children receiving placebo. Further data are needed from other populations with endemic zinc deficiency to confirm the potential age-specific effects reported in this study.
Introduction
Despite substantial progress in reducing deaths of young children over the past 30 years, more than 10 million children die each year in developing countries, and most of these deaths are preventable.1 The WHO Global Burden of Disease Project estimates that malnutrition, which includes micronutrient deficiencies, is the leading risk factor for child mortality in low-income and middle-income countries.2–4
Zinc deficiency is one of the most common micronutrient deficiencies in children under the age of 5 years in developing countries. Deficiency of zinc is associated with dysfunction of the immune system, growth retardation, and a high risk of morbidities such as diarrhoea and acute respiratory infections.5–8 Clinical trials have shown that zinc supplementation, as an adjuvant to oral rehydration therapy for the treatment of diarrhoea, can reduce the duration of episodes and stool volume,9 and there is evidence that zinc supplementation can reduce the risk of mortality in low-birthweight infants and in children who are 1−4 years of age.10–13 Few data are available on the degree of zinc deficiency in young children in south Asia, but low dietary intake of zinc and low zinc concentrations in the serum of women of reproductive age suggest that most young children in this area are zinc deficient.14,15 On the basis of this probable high rate of deficiency and the evidence that zinc intake reduces mortality in subsets of young children, our aim was to test the effect of daily supplementation with zinc on mortality and morbidity of children in the southern plains of Nepal. This study formed part of the Nepal Nutrition Intervention Project, Sarlahi-4 (NNIPS-4); the results of the arms of this trial that assessed the effects of iron and folic acid have been reported previously.16
Methods
Participants
NNIPS-4 was a cluster-randomised, placebo-controlled, community-based, 2×2 factorial trial done between October, 2001, and January, 2006, in rural southern Nepal. The study population consisted of children aged 1−35 months residing in households in the NNIPS-4 study area of 30 Village Development Committees (VDCs) in Sarlahi District. Sarlahi District is in the low-lying plains of Nepal that border India to the south, within the catchment of the Ganges river and its tributaries that drain from the Himalayas to the Bay of Bengal. We chose this area because it typifies much of the Indian subcontinent. The VDCs included in this study are similar to other VDCs in the district and are characterised by traditional rural Hindu culture. Most workers in the district are peasant farmers or labourers, and this area is thought of as poor within Nepal: about 67% of the population is classified as having a total household income that is less than adequate.17 VDCs are each divided into nine wards by the government, and we further divided wards into sectors that were each of a size that one female community worker could manage in roughly 20 h per week. 425 sectors in the 270 wards formed the units of randomisation. All of the sectors were mapped and the houses numbered.
All children who were 1−35 months of age living in households in the study area during the baseline enrolment round were eligible for participation. Additionally, all children born into households in the study area were eligible once they became 1 month of age if that house was their primary residence. Verbal informed consent was obtained on a community basis during meetings with community leaders, and from the parents of each eligible child at the time of enrolment. Some parents who initially refused to allow their children to be enrolled changed their minds during the follow-up phase of the study, and these children were enrolled when consent was received. This study received ethical approval from the Nepal Health Research Council, the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA, and the Institutional Review Board of Cornell University, Ithaca, New York, NY, USA. An independent data and safety monitoring board that included a paediatrician, a biostatistician, and the Member Secretary of the Nepal Health Research Council met five times to review the protocol and the data for safety and efficacy.
Procedures
Sectors were randomly assigned to treatment groups in blocks of four, stratified by VDC and geographical area. Randomisation at the sector level protected the study from unintentional crossover because workers within a sector dispensed only one type of coded supplement. All possible orders of the four treatment groups were written on 107 paper slips, with roughly equal numbers of slips for each order. One slip was randomly drawn to assign treatment codes to four sectors within a VDC. This continued until all sectors were assigned.
The treatment groups were placebo, iron and folic acid, zinc, and iron and folic acid with zinc. The doses of the nutrients used were given daily in tablets that were placebos or that contained 12·5 mg elemental iron as ferrous sulphate and 50 μg folic acid, 10 mg elemental zinc as zinc sulphate, or both. Children under 12 months received half a tablet. The supplements were formulated and manufactured as dispersible tablets by Nutriset (Malaunay, France) in conjunction with the Department of Child and Adolescent Health and Development (WHO, Geneva, Switzerland). The tablets were packaged in blister packs of 14 tablets, and the foil backing on the pack was imprinted with the treatment code. Independent laboratories checked the supplements regularly for content and potency. All children were visited twice every week by study staff, who gave tablets for those days directly to the child. Adequate tablets for daily doses until the next visit were left with the child's caregiver. Older children took the tablets directly and caregivers were instructed to dissolve the tablets in clean water or breastmilk for younger children. During the visits, compliance with supplementation was assessed, and the number of tablets consumed in the preceding week was recorded for each child. All investigators, study staff, and participants were unaware of the assigned treatment. Treatment codes were kept by the Department of Child and Adolescent Health and Development. All children received vitamin A supplementation (200 000 IU for children 12 months or older, 100 000 IU for those 6−12 months, and none for children under 6 months) twice per year, through the national programme distribution system or from study staff.
On the basis of recommendations from the data and safety monitoring board, the arms of the trial in which children were given iron and folic acid were stopped in November, 2003, and children in those sectors were randomly reassigned to either placebo or zinc.16 The iron and folic acid arms of the trial were stopped because there was no effect on mortality or morbidity; the data and safety monitoring board advised that further enrolment to the zinc and placebo arms would be more productive. Consent was again obtained before children began to take the newly assigned supplements. All children enrolled after November, 2003, received only either placebo or zinc supplementation.
Four independent samples, each of about 1200 children younger than 24 months of age who were enrolled in the trial, were randomly selected for participation in a morbidity substudy. Children in these samples were visited every week for 12 months to assess signs and symptoms of selected morbidities. Caregivers were asked about the onset and length of specific signs and symptoms for each day in the preceding week. The morbidities assessed were cough, fever, difficult or rapid breathing, diarrhoea, and dysentery. A further stratified random sample of children of 24 months or older was selected for assessment of zinc status after 12 months of supplementation. Venous blood was drawn and centrifuged, and the serum separated and collected. Serum was stored at −10°C in Nepal and then shipped in liquid nitrogen to Baltimore, USA, where it was stored at −70°C until analysis. Serum samples were run on an AAnalyst 800 (Perkin Elmer) atomic absorption spectrophotometer in the graphite furnace mode at 213·9 nm and 324·8 nm for zinc and copper, respectively, with a spectral bandwidth of 0·7 nm for peak areas.
At the community level, the presence of economic, educational, and health facilities was assessed during interviews with local leaders before the start of enrolment. At the household level, socioeconomic status indicators, health indicators, household structure, and housing material were assessed during a census taken before enrolment began. All households that entered the trial after the initial interviews because of a recent birth had their household interview at the time of child enrolment. Children were discharged from the study when they reached 36 months of age. Data on birthweight and gestational age were available for 16 195 children who were enrolled at birth in a trial of how skin and umbilical cord cleansing with chlorhexidine affected neonatal mortality.18,19
The primary outcome was all-cause mortality. Deaths were identified during the twice-weekly home visits; report of a death prompted verification and a cause-of-death assessment by verbal autopsy with members of the immediate family. Causes of death were determined by independent review of the verbal autopsy information by two physicians, and by an algorithm-based approach that used information from the direct questions on the verbal autopsy and a review of the description of events surrounding the death provided by the family. The combination of an algorithmic approach with review by physicians meant that fewer deaths were classified as uncertain than would have been with either process alone.
Secondary outcomes were: cause-specific mortality; the incidence and severity of diarrhoea, dysentery, and acute respiratory illness as assessed in the morbidity substudy; growth; and motor and cognitive development as assessed in a subgroup of the population. An episode of diarrhoea was defined as one or more consecutive days with four or more loose watery stools per day, but we used additional definitions of five or more, six or more, and seven or more stools per day. Persistent diarrhoea was defined as an episode that lasted for longer than 14 days. Dysentery was defined as an episode of diarrhoea with at least 1 day with blood or mucus in the stool. Individual episodes of diarrhoea and dysentery were separated by at least three symptom-free days. Episodes of acute respiratory illness were defined as one or more consecutive days of fever, cough, or difficulty breathing, with all three symptoms on at least 1 day during the episode and at least 7 days between episodes. In children who had more than one morbid episode, the number of episodes was also estimated. Results of the growth, motor development, and cognitive development substudies will be reported separately.
Statistical analysis
We designed the study to detect a reduction in child mortality in the active treatment group of at least 20%, with a baseline mortality rate of 19·4 per 1000 in the control group, 80% power, a two-sided type I error of 5%, 10% loss to follow-up, a design effect of 1·23, and the assumption that the effects of iron and folic acid do not interact with those of zinc. We calculated that a sample size of about 24 000 person-years in each row (column) of the marginal comparisons (ie, zinc vs placebo, or iron and folic acid vs placebo) would be necessary. Roughly 14 months into recruitment, the mortality rate of children was lower than expected and the sample size was recalculated with a target of 30 000−33 000 person-years in each row (column) of the marginal comparisons.
We used SAS (version 9) and Stata (version 9) to compare the baseline household, maternal, and child characteristics of the treatment groups to assess imbalance after randomisation. We estimated SEs for relative risks taking into account the clustered randomisation using the generalised estimating equations approach.20 The effect of treatment on mortality was analysed by an incidence density approach with person-time as the denominator of the rates, and by a survival analysis approach with Kaplan-Meier mortality curves and Cox proportional hazard models. Hazard ratios were estimated from a proportional hazards regression model calculated as the hazard in children given zinc divided by the hazard in children given placebo. Robust variance estimation was used to calculate SEs of the hazard ratios from proportional hazards models to account for the clustered randomisation.21
All analyses were done by intention to treat for children whose parents gave consent. Children who migrated out of the study area or whose parents refused further participation after originally agreeing were right censored when they left the study. Children who were enrolled in the iron-containing arms when these were discontinued were reassigned to either placebo or zinc according to random reassignment of their sector.
We analysed two sets of children. The first consisted of those who were originally assigned to receive either zinc or placebo alone. The second set consisted of the children from the first set plus the children who were originally assigned to iron and folic acid or to iron and folic acid with zinc who were reassigned to receive either zinc or placebo. Person-time for the children who were reassigned was counted only after the switch-over in November, 2003. Results were similar for both sets, so only those from the merged set are presented. No pre-established statistical stopping rule was adopted by the data and safety monitoring board.
Data from the morbidity subsamples were combined. Incidence density rates of diarrhoea, dysentery, and acute lower respiratory illness were calculated with days during episodes excluded from the denominator of person-time at risk. We calculated all point estimates and CIs for the relative risks taking into account the clustered randomisation using generalised estimating equations.
To summarise evidence for the effect of zinc supplementation on mortality in children under the age of 4 years, we did a meta-analysis of our trial and the three others that have enrolled unselected children and have data on mortality outcomes.13,22,23 Data were available from all four studies to estimate overall treatment effects, from three studies to estimate treatment effects for children younger than 12 months of age, and from two studies for children of 12 months or older. We calculated pooled estimates of relative risks using inverse variance weights.
This study is registered at ClinicalTrials.gov, number NCT00109551.
Role of the funding source
The financial supporters of this trial had no role in the study design, data analysis, data interpretation, or in the writing, reviewing, or approving of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
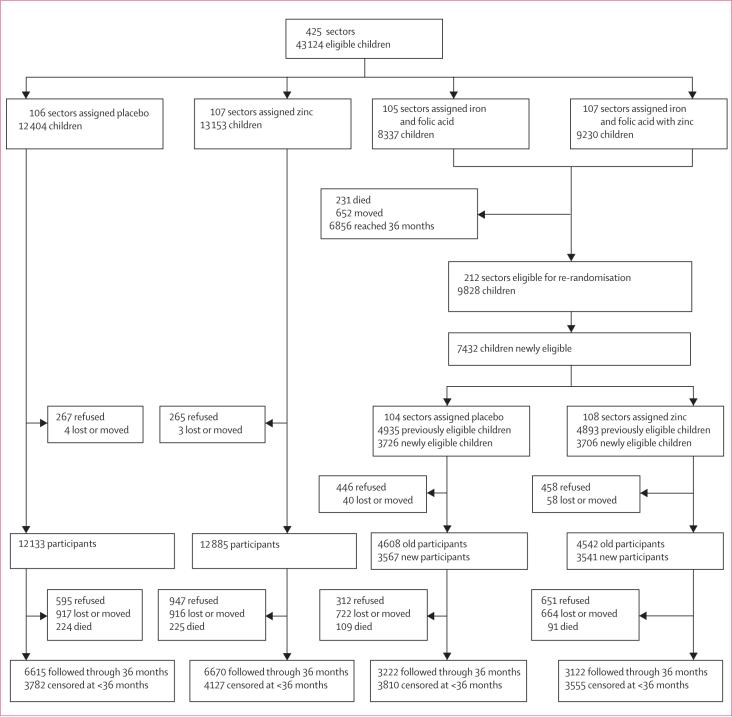
Figure 1 shows the trial profile. Families with 42 817 eligible children residing in 425 sectors were approached for participation. This group consisted of: 25 557 children in sectors originally assigned to either placebo or zinc; 9828 children from sectors originally assigned to iron and folic acid or to iron and folic acid with zinc who were reassigned to placebo or zinc; and 7432 children from sectors originally assigned to iron and folic acid or to iron and folic acid with zinc who were not eligible for the original allocation, but were subsequently randomly assigned to either placebo or zinc.
Figure 1.
Trial profile
The families of 1436 children (3%) refused consent and 105 (<1%) moved out of the study area or were lost between the time of initial recruitment and enrolment (figure 1). This left 41 276 children who enrolled: 20 308 and 20 968 in the placebo and zinc groups, respectively. 2505 of the children who enrolled (6%) refused to continue participation and 3219 (8%) moved or were lost during the follow-up period.
Baseline characteristics, including demographic descriptors of the family, indicators of socioeconomic status, and underlying risk of mortality, were similar in the zinc and placebo groups (table 1). About 70% of children enrolled were from Madeshi families (an ethnic group of north Indian origin) and about 30% were from Pahadi families (an ethnic group that originated from the hill areas of Nepal). Less than one in eight families were from the higher castes (Brahmins and Chetris). Only about a quarter of mothers and half of the fathers were literate, and this literacy was usually at a basic level. Roughly three-quarters of fathers were farmers or labourers, and only about a quarter of households had electricity.
Table 1.
Baseline comparisons by treatment group
| Placebo (n=20 308) | Zinc (n=20 968) | |
|---|---|---|
| Age (months) | ||
| 1−5 | 11824 (58%) | 12237 (58%) |
| 6−11 | 1832 (9%) | 1909 (9%) |
| 12−23 | 3298 (16%) | 3480 (17%) |
| 24−35 | 3354 (17%) | 3342 (16%) |
| Sex | ||
| Male | 10375 (51%) | 10873 (52%) |
| Female | 9933 (49%) | 10095 (48%) |
| Ethnic group (NR=227) | ||
| Pahadi | 6196 (31%) | 6513 (31%) |
| Madeshi | 14011 (69%) | 14329 (69%) |
| Caste (NR=195) | ||
| Brahmin | 1499 (7%) | 1211 (6%) |
| Chetri | 1483 (7%) | 1339 (6%) |
| Vaishya | 12490 (62%) | 13520 (65%) |
| Shudra | 2676 (13%) | 2833 (14%) |
| Muslim or other | 2073 (10%) | 1957 (9%) |
| Parental literacy | ||
| Paternal literacy (NR=224) | 11094 (55%) | 11142 (53%) |
| Maternal literacy (NR=206) | 5083 (25%) | 4790 (23%) |
| Paternal occupation (NR=285) | ||
| Farmer | 9256 (46%) | 9782 (47%) |
| Labourer | 5996 (30%) | 6112 (29%) |
| Business | 2713 (13%) | 2477 (12%) |
| Private service | 1437 (7%) | 1618 (8%) |
| Government | 540 (3%) | 478 (2%) |
| None | 243 (1%) | 340 (2%) |
| Previous child mortality (NR=536) | ||
| Rate per 1000 livebirths | 80·7 | 81·9 |
| ≥1 previous child | 4497 (22%) | 4784 (23%) |
| Electricity in house (NR=198) | ||
| Yes | 4700 (23%) | 4628 (22%) |
| Ownership | ||
| Bicycle (NR=194) | 10014 (50%) | 10622 (51%) |
| Cart (NR=191) | 2452 (12%) | 2396 (12%) |
| Radio (NR=197) | 5797 (29%) | 5718 (27%) |
| TV (NR=212) | 3563 (18%) | 3267 (16%) |
| Paddy farm land (NR=372) | 9844 (49%) | 10399 (50%) |
| Non-paddy farm land (NR=358) | 9055 (45%) | 9391 (45%) |
| Cattle (NR=194) | 12399 (61%) | 13004 (62%) |
| Goats (NR=198) | 9405 (47%) | 9921 (48%) |
| House wall material (NR=193) | ||
| None | 282 (1%) | 276 (1%) |
| Thatch | 5389 (27%) | 5916 (28%) |
| Mud | 11022 (55%) | 11325 (54%) |
| Wood | 585 (3%) | 598 (3%) |
| Concrete | 2943 (15%) | 2747 (13%) |
| House roof material (NR=202) | ||
| None | 96 (1%) | 83 (<1%) |
| Thatch | 3524 (17%) | 3422 (16%) |
| Tile or tin | 15696 (78%) | 16623 (80%) |
| Concrete | 904 (5%) | 726 (4%) |
| Latrine at house (NR=262) | ||
| Yes | 2466 (12%) | 1962 (9%) |
| Water source (NR=197) | ||
| Tube well | 16694 (83%) | 17428 (84%) |
| Ring well | 3026 (15%) | 3071 (15%) |
| Other | 499 (3%) | 361 (2%) |
| Mid-upper-arm circumference (cm) (NR=848) | ||
| Mean (SD) | 10·98 (1·96) | 10·98 (1·96) |
| Median (IQR) | 10·50 (7·3−13·7) | 10·50 (7·3−13·7) |
| ≤11·5 | 12082 (61%) | 12486 (61%) |
| 11·5−12·4 | 2327 (12%) | 2461 (12%) |
| 12·5−13·4 | 2664 (13%) | 2764 (13%) |
| ≥13·5 | 2758 (14%) | 2886 (14%) |
Data are number (% of children for whom that category of information was reported), unless otherwise indicated. NR=not reported.
After 12 months of supplementation, serum zinc concentrations were higher (table 2) and the proportion of children with concentrations below 9·2 μmol/L was 32% lower (95% CI −62 % to 22%) in the zinc group than in the placebo group, which shows that the supplements contained bioavailable zinc. There was no difference in serum copper concentrations, which suggests that zinc supplementation did not interfere with copper absorption (table 2).
Table 2.
Zinc and copper status by treatment group, 12 months after supplementation began
| Placebo | Zinc | |
|---|---|---|
| Serum zinc (μmol/L) | ||
| Number assessed | 146 | 152 |
| Mean (SD) | 11·0 (2·1) | 11·8 (2·4) |
| Median (IQR) | 10·8 (8·0 to 13·6) | 11·3 (8·4 to 14·2) |
| Difference (95% CI)* | .. | 0·8 (0·08 to 1·51) |
| <9·2 | 24 (16%) | 17 (11%) |
| 9·2−10·7 | 46 (32%) | 41 (27%) |
| ≥10·7 | 76 (52%) | 94 (62%) |
| Serum copper (μmol/L) | ||
| Number assessed | 145 | 152 |
| Mean (SD) | 20·4 (3·7) | 20·1 (3·8) |
| Median (IQR) | 20·2 (15·3 to 25·1) | 19·6 (15·1 to 24·1) |
| Difference (95% CI)* | .. | −0·6 (−1·3 to 0·5) |
| <9·4 | 1 (1%) | 1 (1%) |
| 9·4−11·0 | 0 (0%) | 0 (0%) |
| ≥11·0 | 144 (99%) | 151 (99%) |
Difference and 95% CI calculated using generalised estimating equations to adjust for clustered randomisation.
Compliance was good, but refusal to continue participation was more common in the zinc group than in the placebo group (1598 [8%] vs 907 [5%]; exact difference 3·15%, 95% CI 2·69−3·61). Supplements were taken on 76·8% of assigned days in the placebo group versus 72·9% of days for the zinc group.
Overall, there was no significant difference in mortality between the zinc and placebo groups (table 3). There was evidence of effect modification by age (test for interaction, p=0·12). Mortality rates were equal in the zinc and placebo groups for children under 12 months of age, but there was non-significant 20% lower mortality among children 12 months or older in the zinc group. Figure 2 shows a similar pattern for mortality over time. There was no evidence that the effect of treatment on mortality interacted with sex, ethnic group, a history of child death in the family, or baseline mid-upper-arm circumference (table 3), or maternal literacy or education (data not shown). In the group for which birthweight data were available (8324 [39%] and 7871 [40%] of the placebo and zinc groups, respectively), there was evidence of an interaction, with infants born weighing under 2000 g showing a non-significant 44% reduction in mortality versus no effect for those over 2000 g (table 3; test for interaction, p=0·06).
Table 3.
Mortality by treatment group
|
Placebo |
Zinc |
HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Person-years | Deaths | Rate/1000 person-years | Person-years | Deaths | Rate/1000 person-years | ||
| Sex | |||||||
| Male | 15161·4 | 141 | 9·30 | 15909·7 | 137 | 8·61 | 0·93 (0·71−1·21) |
| Female | 14683·2 | 192 | 13·08 | 14882·0 | 179 | 12·03 | 0·92 (0·72−1·17) |
| Age (months) | |||||||
| 1−11 | 9237·1 | 168 | 18·19 | 9582·1 | 181 | 18·89 | 1·04 (0·83−1·31) |
| 12−35 | 20607·2 | 165 | 8·01 | 21209·7 | 135 | 6·37 | 0·80 (0·60−1·06) |
| Age of boys (months) | |||||||
| 1−11 | 4709·4 | 81 | 17·20 | 4943·0 | 79 | 15·98 | 0·93 (0·68−1·28) |
| 12−35 | 10451·8 | 60 | 5·74 | 10966·8 | 58 | 5·29 | 0·92 (0·60−1·41) |
| Age of girls (months) | |||||||
| 1−11 | 4527·6 | 87 | 19·22 | 4639·1 | 102 | 21·99 | 1·14 (0·85−1·54) |
| 12−35 | 10155·4 | 105 | 10·34 | 10242·9 | 77 | 7·52 | 0·73 (0·52−1·02) |
| Ethnic group (NR=227) | |||||||
| Pahadi | 9087·8 | 57 | 6·27 | 9097·3 | 60 | 6·60 | 1·03 (0·71−1·48) |
| Madeshi | 20640·5 | 273 | 13·23 | 21570·0 | 254 | 11·78 | 0·90 (0·72−1·12) |
| History of child death (NR=149) | |||||||
| No | 22987·6 | 216 | 9·40 | 23512·8 | 216 | 9·19 | 0·98 (0·79−1·21) |
| Yes | 6768·1 | 113 | 16·70 | 7163·8 | 99 | 13·82 | 0·83 (0·61−1·13) |
| Mid-upper-arm circumference (cm) (NR=848) | |||||||
| <11·5 | 19288·8 | 261 | 13·53 | 19849·4 | 247 | 12·44 | 0·92 (0·74−1·13) |
| 11·5−12·4 | 3525·5 | 30 | 8·51 | 3769·0 | 31 | 8·22 | 0·98 (0·59−1·61) |
| 12·5−13·4 | 3470·5 | 19 | 5·47 | 3687·4 | 18 | 4·88 | 0·90 (0·45−1·81) |
| ≥13·5 | 2889·4 | 13 | 4·50 | 2997·2 | 10 | 3·34 | 0·74 (0·32−1·75) |
| Birthweight within 72 h of delivery (g) (NR=25081) | |||||||
| <2000 | 457·5 | 26 | 56·83 | 471·5 | 15 | 31·81 | 0·56 (0·30−1·04) |
| 2000−2499 | 2929·1 | 53 | 18·09 | 3039·8 | 54 | 17·76 | 0·97 (0·66−1·44) |
| ≥2500 | 8291·7 | 76 | 9·17 | 8842·2 | 87 | 9·84 | 1·08 (0·79−1·46) |
| Total | 29 844·6 | 333 | 11·16 | 30791·7 | 316 | 10·26 | 0·92 (0·75−1·12) |
HR=hazard ratio. NR=not reported.
Figure 2.
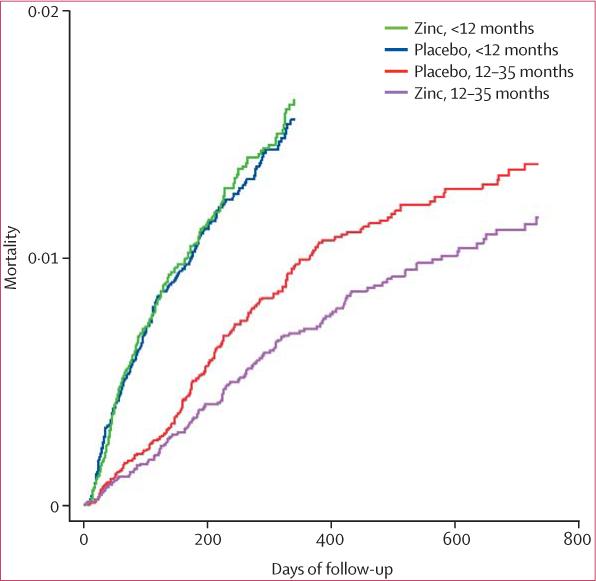
Mortality curves by treatment group and age
The most common causes of death were diarrhoea, dysentery, and acute lower respiratory illness (table 4). Mortality rates did not differ by treatment group for any cause. As might be expected for classification based on information from verbal autopsies, about a fifth of deaths could not be reliably classified by either the algorithm or the physician reviewers. The rates of diarrhoea, persistent diarrhoea, dysentery, and acute lower respiratory illness did not differ between the zinc and placebo groups (tables 5 and 6). This lack of treatment effect was seen for all definitions of diarrhoea that we used. Similarly, there was no difference between the treatment groups in the rate of more than one, more than two, more than three, or more than four episodes per child for any of the morbidities.
Table 4.
Cause-specific mortality by treatment group
|
Placebo (29 844·6 person-years) |
Zinc (30 791·7 person-years) |
HR (95% CI) | |||
|---|---|---|---|---|---|
| Deaths | Rate/1000 person-years | Deaths | Rate/1000 person-years | ||
| Congenital defects | 4 | 0·13 | 5 | 0·17 | 1·21 (0·33−4·44) |
| Injury | 16 | 0·52 | 9 | 0·30 | 0·55 (0·24−1·26) |
| Diarrhoea or dysentery | 130 | 4·22 | 108 | 3·62 | 0·81 (0·61−1·07) |
| ALRI | 83 | 2·70 | 77 | 2·58 | 0·90 (0·64−1·26) |
| Measles | 1 | 0·03 | 6 | 0·20 | 5·81 (0·64−52·7) |
| Malnutrition | 9 | 0·29 | 12 | 0·40 | 1·29 (0·52−3·22) |
| Sepsis or infection | 4 | 0·13 | 4 | 0·13 | 0·97 (0·25−3·73) |
| SIDS | 24 | 0·78 | 20 | 0·67 | 0·81 (0·43−1·52) |
| Uncertain or missing* | 62 | 2·01 | 75 | 2·51 | 1·17 (0·79−1·74) |
HR=hazard ratio. ALRI=acute lower respiratory illness. SIDS=sudden infant death syndrome. Verbal autopsy information was not available for 17 deaths in the placebo group and 15 deaths in the zinc group.
Cause of death could not be classified because of uncertainty or missing information on some items in the verbal autopsy.
Table 5.
Morbidity incidence rates by treatment group
|
Placebo (n=2032) |
Zinc (n=2038) |
RR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Episodes (n) | Person-years | Episodes/child/year | Episodes (n) | Person-years | Episodes/child/year | ||
| Diarrhoea (stools/day) | |||||||
| ≥3 | 5074 | 1331·4 | 3·81 | 5002 | 1375·0 | 3·64 | 0·96 (0·91−1·02) |
| ≥4 | 4147 | 1340·6 | 3·12 | 4142 | 1384·6 | 2·99 | 0·96 (0·90−1·02) |
| ≥5 | 2802 | 1350·8 | 2·07 | 2822 | 1394·5 | 2·02 | 0·96 (0·90−1·03) |
| ≥6 | 1814 | 1356·4 | 1·34 | 1865 | 1400·4 | 1·33 | 0·96 (0·88−1·05) |
| ≥7 | 1047 | 1359·9 | 0·77 | 1057 | 1404·3 | 0·75 | 0·96 (0·87−1·07) |
| Persistent diarrhoea | 85 | 1359·7 | 0·06 | 93 | 1404·0 | 0·07 | 1·06 (0·77−1·46) |
| Dysentery | 414 | 1359·5 | 0·30 | 427 | 1404·2 | 0·30 | 1·06 (0·87−1·28) |
| ALRI | 1887 | 1351·1 | 1·40 | 1999 | 1395·0 | 1·43 | 1·01 (0·92−1·10) |
RR=relative risk. ALRI=acute lower respiratory illness.
Table 6.
Proportion of children with multiple episodes per year by treatment group
| Placebo (n=2032) | Zinc (n=2038) | RR (95% CI) | |
|---|---|---|---|
| Diarrhoea | |||
| >1 | 953 (47%) | 997 (49%) | 1·03 (0·94−1·14) |
| >2 | 661 (33%) | 641 (31%) | 0·97 (0·85−1·10) |
| >3 | 422 (21%) | 397 (19%) | 0·93 (0·79−1·10) |
| >4 | 269 (13%) | 269 (13%) | 0·98 (0·80−1·20) |
| Persistent diarrhoea | |||
| >1 | 8 (<1%) | 7 (<1%) | 0·87 (0·32−2·36) |
| Dysentery | |||
| >1 | 73 (4%) | 88 (4%) | 1·27 (0·85−1·89) |
| >2 | 20 (1%) | 27 (1%) | 1·33 (0·70−2·51) |
| ALRI | |||
| >1 | 459 (23%) | 480 (24%) | 1·04 (0·89−1·22) |
| >2 | 226 (11%) | 257 (13%) | 1·14 (0·91−1·43) |
| >3 | 102 (5%) | 131 (6%) | 1·26 (0·91−1·74) |
Data are n (%). RR=relative risk. ALRI=acute lower respiratory illness.
Discussion
Total mortality of children receiving zinc supplementation was not significantly different from that of children in the placebo group. There was suggestive evidence of an interaction with age: there was no difference in mortality in children under 12 months of age, but in older children there was a non-significant difference of lower mortality in the zinc group than in the control group. The serum zinc concentrations in the placebo group showed high levels of zinc deficiency, which is consistent with previous data and suggests that this population was appropriate for testing of this intervention. The zinc provided in the supplements was bioavailable, as shown by the serum zinc concentrations after 12 months of supplementation, although the effect on serum zinc was not as large as has been shown in other studies of zinc supplementation.7
Selection bias is unlikely to explain our results because participation rates exceeded 96% at enrolment for both treatment groups. Similar proportions (about 8%) of children in the zinc and placebo groups moved out of the study area or were lost to follow-up after enrolment. All these children were alive when they were censored. Any differences in the mortality rates of these children by treatment group could have biased our estimates of the treatment effects. Although these children might have had a different overall risk of mortality from those who were followed-up, we think that this selection is unlikely to have differed between the treatment groups.
Compliance, whether measured as the proportion of enrolled children who continued to participate until discharge or as the proportion of days for which the supplement was taken, was lower in the zinc group, suggesting that some children could detect an unacceptable taste in the active supplements. Similar to the issue of migration, our estimates of treatment effects would be biased only if mortality risk differed within the treatment groups between children who refused and those who continued to participate. This bias cannot be ruled out, but it is likely to be small, if present at all, because most children who discontinued did so soon after enrolment. This difference in compliance might explain the slightly lower change in serum zinc status in this study as compared with previous trials of shorter duration. Imbalance in risk factors for child mortality is an unlikely explanation for our results, because the treatment groups had similar baseline characteristics.
A companion trial in a malaria-endemic area of Zanzibar22 reported no effect of zinc supplementation on overall mortality, but a beneficial effect in children of 12 months or older. This is similar to our findings, even though the characteristics of malaria in the two study populations differ (there is intense Plasmodium falciparum transmission in Zanzibar, by contrast with infrequent Plasmodium vivax transmission in Nepal). Although the results of these two large, community-based trials are consistent with each other, they reported a much smaller effect of zinc supplementation on mortality than did trials from Bangladesh.12,13 In view of the large sample sizes used in Nepal and Zanzibar, the true effect of zinc supplementation on mortality is probably closer to that reported in these two studies than that in the studies from Bangladesh. The non-significant 44% reduction in mortality (table 3) in the small group of children in NNIPS-4 who were known to have been born weighing less than 2000 g, together with a 68% reduction in mortality in infants who were small for gestational age in a trial in India,10 suggests that this high-risk group might benefit from zinc supplementation.
In a meta-analysis of two reports from south Asia (our study and one from Bangladesh13) and two reports from sub-Saharan Africa (those from Zanzibar22 and Burkina Faso23), the pooled estimated relative risk of mortality across all ages was 0·91 (95% CI 0·82−1·02) (figure 3). There was no evidence that this relative risk varied between the Asian and African studies. The pooled estimate for children younger than 12 months showed no effect of zinc supplementation on mortality (1·04, 0·90−1·21) and this was consistent by region. By contrast, zinc supplementation reduced mortality in children 12 months or older in Nepal and Zanzibar (0·82, 0·70−0·96) (figure 3).
Figure 3.
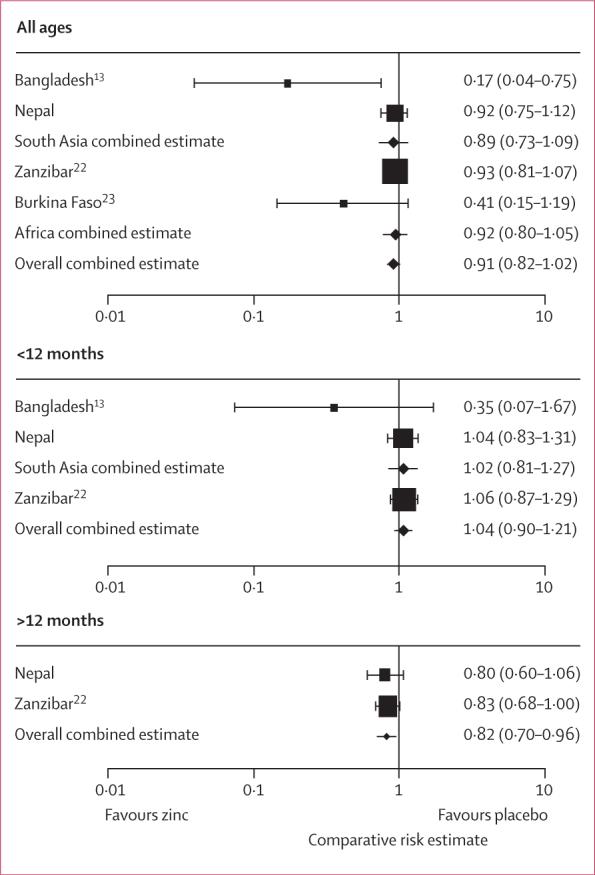
Meta-analysis of the effect of zinc supplementation on mortality in children aged up to 48 months
The absence of a preventive effect on the number of episodes and duration of diarrhoea, dysentery, and acute respiratory infections in our study contrasts with the meta-analysis published by the Zinc Investigators Collaborative Group8 in 1999. However, most of the studies cited in that review were of selected subsets of children at high risk, including children with, or just recovering from, an episode of acute or persistent diarrhoea,24–29 and underweight or stunted children.30,31 Of the three studies that included unselected children, the largest, from Papua New Guinea,32 showed no difference in days with diarrhoea, fever, or cough between 138 children assigned to placebo and 136 assigned to zinc supplementation. In Guatemala, there were 22% fewer episodes of diarrhoea in 45 children given zinc (8·1 episodes per 100 person-days) than in 44 children given placebo (6·3 episodes per 100 person-days); the definition of diarrhoea was left to the mother, which might explain the unusually high rates.33 In the Mexican study,34 there was a lower diarrhoea attack rate in 109 children given zinc than in 108 given no zinc (0·8 vs 1·3 episodes per person-year). Since the meta-analysis in 1999, ten other studies have reported on the effect of zinc supplementation on morbidity in unselected populations. Of those trials, five showed no effect of zinc supplementation on the incidence of diarrhoea.35–39 Four studies showed reductions in diarrhoea incidence that ranged from 6% to 23% in the zinc versus placebo comparisons,13,40–42 and in one study children in the zinc group had on average one fewer day with diarrhoea than did children in the placebo group.23 Eight of these trials report data on acute lower respiratory illness, five of which showed no difference in rates between zinc and placebo groups.35,36,38,39,42 The three studies that showed differences between the zinc and placebo groups had relative risks that range from 0·72 to 1·84 for severe definitions of acute lower respiratory illness.13,40,43 Our non-specific definition might explain the absence of an effect in our results. However, the absence of an effect on either diarrhoea or acute lower respiratory illness is consistent with the heterogeneity of results from other studies, and suggests that the effects of zinc supplementation on attack rates of diarrhoea and respiratory disease vary depending on how children are selected for study. Children with specific histories of morbidity or malnutrition might benefit from zinc supplementation.
The effect of zinc supplementation on serum zinc status in this trial was lower than expected on the basis of data from shorter-term supplementation trials. There are a few potential explanations for this finding. First, the dose used in this study might have been lower than necessary to improve zinc status, which might explain the smaller effect on morbidity and mortality than in studies that used higher doses. However, morbidity results vary substantially in previous trials, even those that used higher doses than did this trial. The recommended daily allowance (RDA), as calculated by the US Institute of Medicine, for zinc in children aged 1−35 months is 2·5−3·0 mg.44 Our dose was over three times the RDA for children aged 12 months or older and about 1·5 times the RDA for those younger than 12 months. The recommended nutrient intake (RNI) from the Food and Agriculture Organization of the UN and WHO for zinc in this age-group is 1·1−8·4 mg daily, with the exact RNI depending on age and dietary bioavailability.45 The classification of bioavailability of a dietary supplement is unclear, but the doses used in this trial are 1·2−4·0 times the RNI. Second, the diet in rural southern Nepal is high in phytate, which might have restricted the absorption of the zinc supplement. The diet in our study area is similar to that in many other areas on the Indian subcontinent, so any difficulty with bioavailability would probably not be unique to our study population. However, the combination of a low dose with a diet high in phytate might have limited the absorption of zinc below that necessary for optimum effect on morbidity and mortality. Third, serum zinc concentrations must be interpreted with caution because they are reduced by infection and inflammation, which were common in our study population.
The results from this study show that zinc supplementation has no effect on the incidence of diarrhoea or respiratory infections in children of 1−3 years of age. There was a slight, non-significant reduction in mortality with zinc supplementation beyond that achieved with vitamin A supplementation. The meta-analysis supports this finding, and shows a protective effect in children of 12 months or older when estimates are pooled across studies. However, whether improvements in population zinc status via universal routine supplementation are feasible remains to be shown. Further research on the optimum dose for reduction in morbid outcomes in various settings is needed before large-scale implementation of universal supplementation pro grammes is justified.
Acknowledgments
We thank the data and safety monitoring board members Michael Hambidge, William Blackwelder, and Anil Mishra. We also thank Olivier Fontaine of the Department of Child and Adolescent Health at WHO for his role in procuring supplements for this trial and for coordinating the efforts of the data and safety monitoring board, and Abdullah Brooks and Sunil Sazawal for making data from their studies available for the meta-analysis. This study was funded by grants from the National Institutes of Health, Bethesda, MD, USA (HD 38753), the Bill & Melinda Gates Foundation, Seattle, WA, USA (810-2054), and a Cooperative Agreement between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development, Washington, DC, USA (HRN-A-00-97-00015-00).
Footnotes
Contributors JMT, RJS, JK, and RB contributed to the design of the trial. JMT, SKK, RJS, JK, SCL, RA, LCM, and SS participated in the conduct of the trial, and JMT, SKK, JK, SCL, LCM, and SS contributed to quality control. JMT, RJS, JK, and LCM participated in data analysis, JMT and JK participated in data management, and all authors contributed to data interpretation. RA participated in review of the verbal autopsies. JMT, RJS, and JK participated in writing this report. All authors have read and approved the final version.
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.Fishman S, Caulfield LE, de Onis M, et al. Childhood and maternal underweight. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. pp. 39–162. [Google Scholar]
- 3.Rice AL, West KP, Black RE. Vitamin A deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. pp. 211–56. [Google Scholar]
- 4.Caulfield L, Black RE. Zinc deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. pp. 257–80. [Google Scholar]
- 5.Sempertegui F, Estrella B, Correa E, et al. Effects of short-term zinc supplementation on cellular immunity, respiratory symptoms, and growth of Equadorian children. Eur J Clin Nutr. 1996;50:42–46. [PubMed] [Google Scholar]
- 6.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(suppl):447S–63S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 7.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–71. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Black RE, Brown KH, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 9.Bhutta ZA, Bird SM, Black RE, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr. 2000;72:1516–22. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 10.Sazawal S, Black RE, Menon VP, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108:1280–06. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 11.Lira PIC, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function, and growth of low-birth-weight, full-term infants in northeast Brazil. Am J Clin Nutr. 1998;86(suppl):418S–24S. doi: 10.1093/ajcn/68.2.418S. [DOI] [PubMed] [Google Scholar]
- 12.Baqui AH, Black RE, El Arifeen S, et al. Effect of zinc supplementation started during diarrhea on morbidity and mortality in Bangladeshi children: community randomized trial. BMJ. 2002;325:1059. doi: 10.1136/bmj.325.7372.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks WA, Santosham M, Naheed A, et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhea in children younger than 2 years in an urban, low-income population in Bangladesh: randomized controlled trial. Lancet. 2005;366:999–1004. doi: 10.1016/S0140-6736(05)67109-7. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Administrative Coordinating Committee, Subcommittee on Nutrition . Fourth report on the world nutrition situation. UNACC/SCN; Geneva: 2000. [Google Scholar]
- 15.Jiang T, Christian P, Khatry SK, WE L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135:1106–12. doi: 10.1093/jn/135.5.1106. [DOI] [PubMed] [Google Scholar]
- 16.Tielsch JM, Khatry S, Stoltzfus RJ, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367:144–52. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Central Bureau of Statistics . Nepal living standards survey 2003/04, statistical report volume two. National Planning Commission Secretariat, His Majesty's Government of Nepal; Kathmandu: 2004. [Google Scholar]
- 18.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical cord prevent neonatal omphalitis and reduce mortality in southern Nepal: a community-based, cluster-randomized trial. Lancet. 2006;367:910–18. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tielsch JM, Darmstadt GL, Mullany LC, et al. Impact of newborn skin cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–40. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 22.Sazawal S, Black RE, Ramsan M, et al. Effects of zinc supplementation on mortality in children 1–48 months of age: a community-based randomized placebo-controlled trial. Lancet. 2007;369:927–34. doi: 10.1016/S0140-6736(07)60452-8. [DOI] [PubMed] [Google Scholar]
- 23.Müller O, Becher H, Van Zweeden AB. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomized double blind placebo controlled trial. BMJ. 2001;322:1–6. doi: 10.1136/bmj.322.7302.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy SK, Tomkins AM, Akramuzzaman SM, et al. Randomized controlled trial of zinc supplementation in malnourished Bangladeshi children with acute diarrhea. Arch Dis Child. 1997;77:196–200. doi: 10.1136/adc.77.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy SK, Tomkins AM, Mahalanabis D, et al. Impact of zinc supplementation on persistent diarrhea in malnourished Bangladeshi children. Acta Paediatr. 1998;87:1235–39. doi: 10.1080/080352598750030898. [DOI] [PubMed] [Google Scholar]
- 26.Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics. 1998;102:1–5. doi: 10.1542/peds.102.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Sazawal S, Black RE, Bhan MK, Jalla S, Sinha A, Bhandari N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea—a community-based, double-blind, controlled trial. Am J Clin Nutr. 1997;66:413–18. doi: 10.1093/ajcn/66.2.413. [DOI] [PubMed] [Google Scholar]
- 28.Penny ME, Peerson JM, Marin RM, et al. Randomized, community-based trial of the effect of zinc supplementation, with and without other micronutrients, on the duration of persistent childhood diarrhea in Lima, Peru. J Pediatr. 1999;135:208–17. doi: 10.1016/s0022-3476(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 29.Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics. 1999;103:e42. doi: 10.1542/peds.103.4.e42. [DOI] [PubMed] [Google Scholar]
- 30.Meeks Gardner JM, Witter MM, Ramdath DD. Zinc supplementation: effects on the growth and morbidity of undernourished Jamaican children. Eur J Clin Nutr. 1998;52:34–39. doi: 10.1038/sj.ejcn.1600509. [DOI] [PubMed] [Google Scholar]
- 31.Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin-like growth factor 1 (IGF-1) in growth-retarded Vietnamese children. Am J Clin Nutr. 1996;63:514–19. doi: 10.1093/ajcn/63.4.514. [DOI] [PubMed] [Google Scholar]
- 32.Shankar AH, Genton B, Baisor M, et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000;62:663–69. doi: 10.4269/ajtmh.2000.62.663. [DOI] [PubMed] [Google Scholar]
- 33.Ruel MT, Rivera JA, Santizo MC, Lonnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics. 1997;99:808–13. doi: 10.1542/peds.99.6.808. [DOI] [PubMed] [Google Scholar]
- 34.Rosado JL, Lopez P, Munoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am J Clin Nutr. 1997;65:13–19. doi: 10.1093/ajcn/65.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Baqui AH, Zaman K, Persson LA. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. J Nutr. 2003;133:4150–07. doi: 10.1093/jn/133.12.4150. [DOI] [PubMed] [Google Scholar]
- 36.Osendarp SJM, Santosham M, Black RE, Wahed MA, van Raaij JMA, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am J Clin Nutr. 2002;76:1401–08. doi: 10.1093/ajcn/76.6.1401. [DOI] [PubMed] [Google Scholar]
- 37.Gupta DN, Mondal SK, Ghosh S, Rajendran K, Sur D, Manna B. Impact of zinc supplementation on diarrhoeal morbidity in rural children of West Bengal. Acta Paediatr. 2003;92:531–36. [PubMed] [Google Scholar]
- 38.Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. Am J Clin Nutr. 2006;83:693–700. doi: 10.1093/ajcn.83.3.693. [DOI] [PubMed] [Google Scholar]
- 39.Berger J, Ninh NX, Khan NC, et al. Efficacy of combined iron and zinc supplementation on micronutrient status and growth in Vietnamese infants. Eur J Clin Nutr. 2006;60:443–54. doi: 10.1038/sj.ejcn.1602336. [DOI] [PubMed] [Google Scholar]
- 40.Rahman MM, Vernumd SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Simuntaneous zinc and vitamin A supplementation in Bangladeshi children: randomized double blind controlled trial. BMJ. 2001;323:314–18. doi: 10.1136/bmj.323.7308.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhandari N, Bahl R, Taneja S, et al. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics. 2002;109:e86. doi: 10.1542/peds.109.6.e86. [DOI] [PubMed] [Google Scholar]
- 42.Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:126–31. doi: 10.4269/ajtmh.2006.75.1.0750126. [DOI] [PubMed] [Google Scholar]
- 43.Bhandari N, Bahl R, Taneja S, et al. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomized controlled trial in an urban slum. BMJ. 2002;324:1358–62. doi: 10.1136/bmj.324.7350.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otten JJ, Hellwib JP, Meyers LD, editors. Dietary reference intakes: the essential guide to nutrient requirements. Institute of Medicine, National Academies Press; Washington DC: 2006. [Google Scholar]
- 45.FAO/WHO Expert Consultation . Report of a Joint FAO/WHO Expert Consultation. World Health Organization and Food and Agriculture Organization of the United Nations; Rome: 2002. Human vitamin and mineral requirements. [Google Scholar]