Abstract
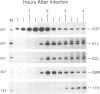
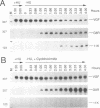
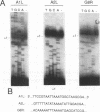
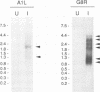
The steady-state levels of mRNAs encoded by three intermediate-stage genes of vaccinia virus, A1L, A2L, and G8R, were compared with those encoded by well-characterized early- and late-stage genes. After synchronous infection of HeLa cells, the early mRNA was detected within 20 min and peaked at about 100 min; all three intermediate mRNAs were detected at 100 min and peaked at about 120 min; and the late mRNA was detected at 140 min and increased thereafter. Upon reaching maximum levels, the early and intermediate mRNAs declined at rates consistent with half-lives of about 30 min, providing the basis for rapid changes in gene expression. Intermediate mRNA was not detected when viral DNA synthesis was prevented, whereas its accumulation was enhanced by blocking translation after removal of the replication inhibitor. The 5' ends of the mRNAs initiated within a TAAAT or TAAAAT sequence in the coding DNA strand but contained a poly(A) leader of up to 30 additional bases. Diffuse bands of A1L and G8R RNA, equal to and longer than the coding region, were resolved by agarose gel electrophoresis, suggesting preferred sites of 3'-end formation that did not correlate with early gene termination signals. The cis-regulatory sequences were investigated by constructing recombinant viruses containing mutated intermediate promoters preceding the beta-galactosidase reporter gene. The effects of mutations on expression were similar to those previously obtained by transfection studies (C.J. Baldick, Jr., J.G. Keck, and B. Moss, J. Virol. 66:4710-4719, 1992), providing further evidence for functional core, spacer, and initiator regions. In addition, an up-regulated bifunctional early/intermediate promoter was created by making four single-base substitutions in the G8R promoter.
Full text
PDF



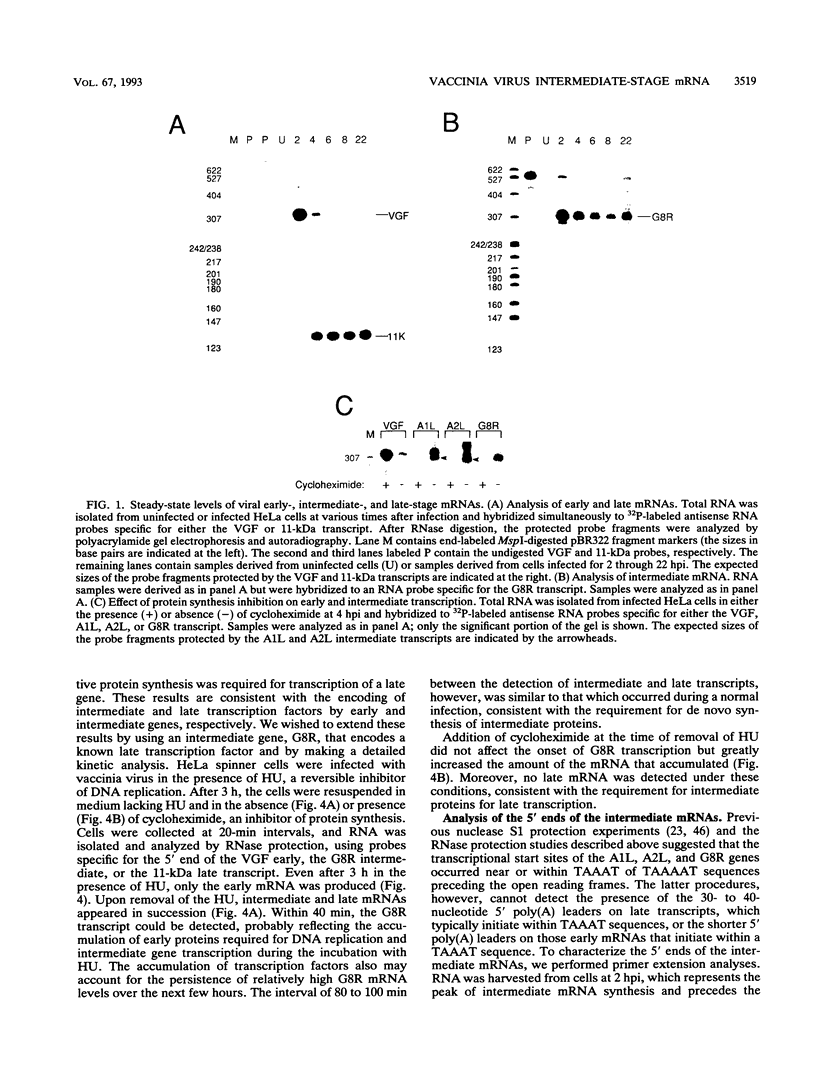
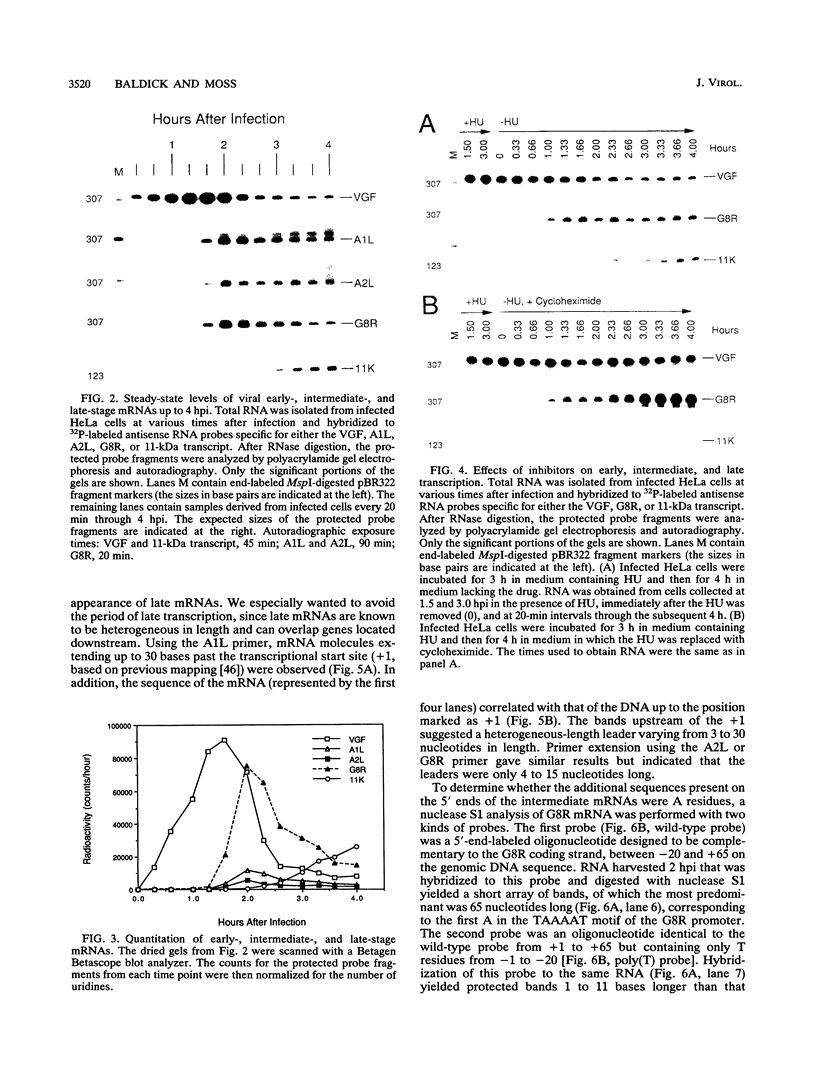
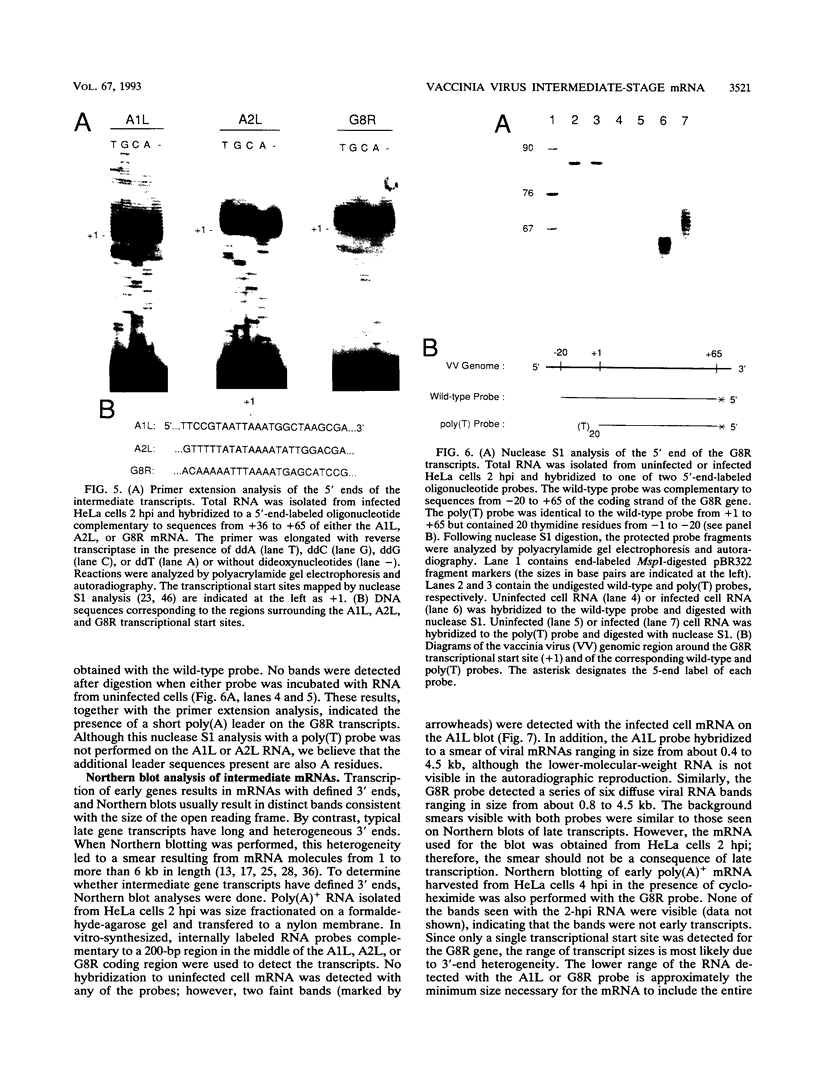
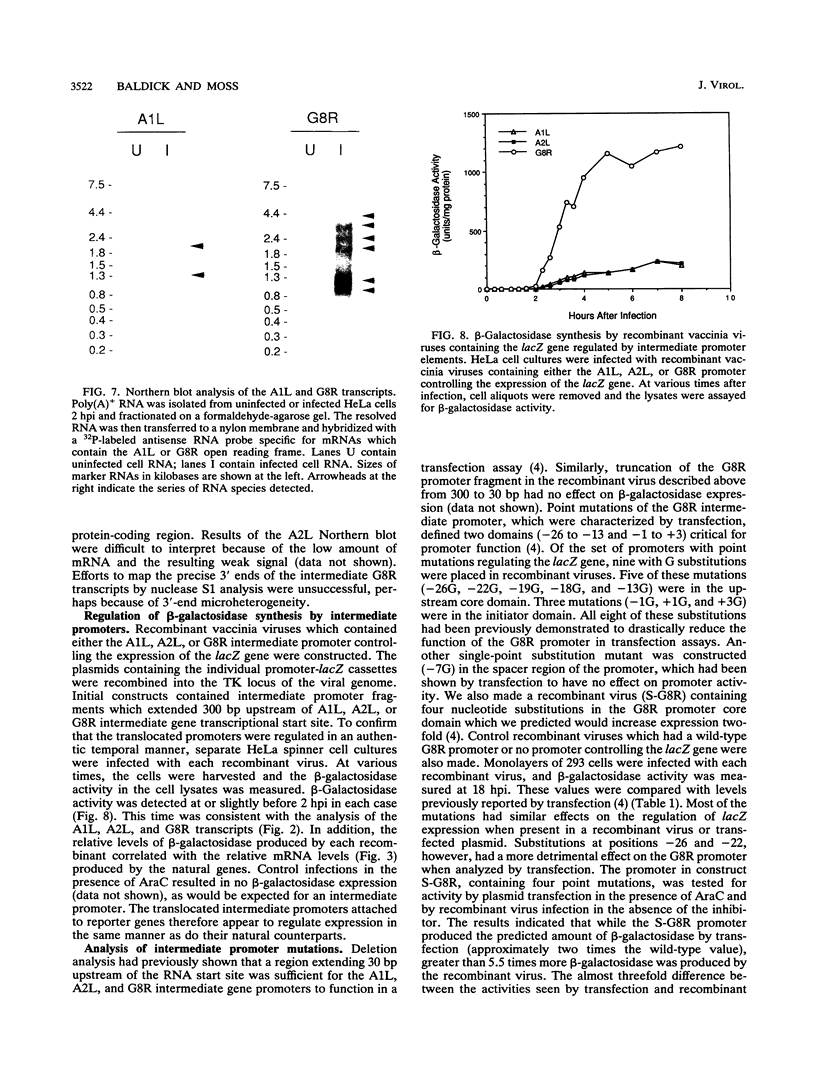
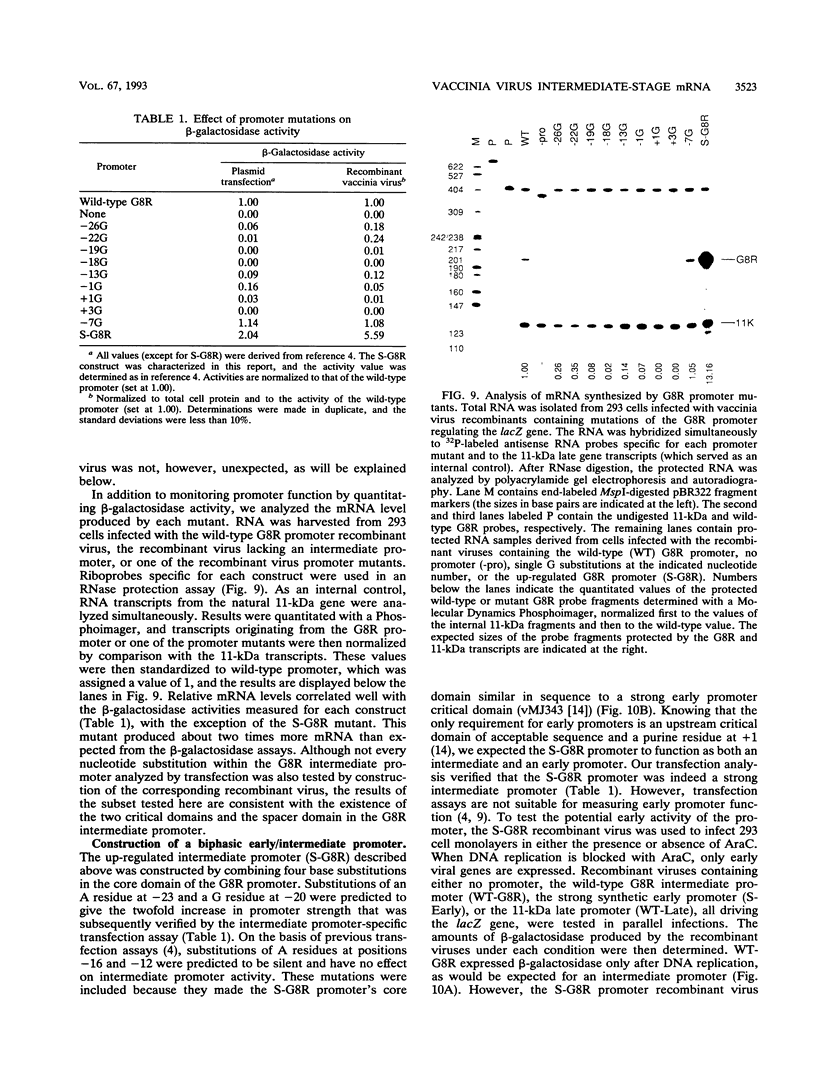
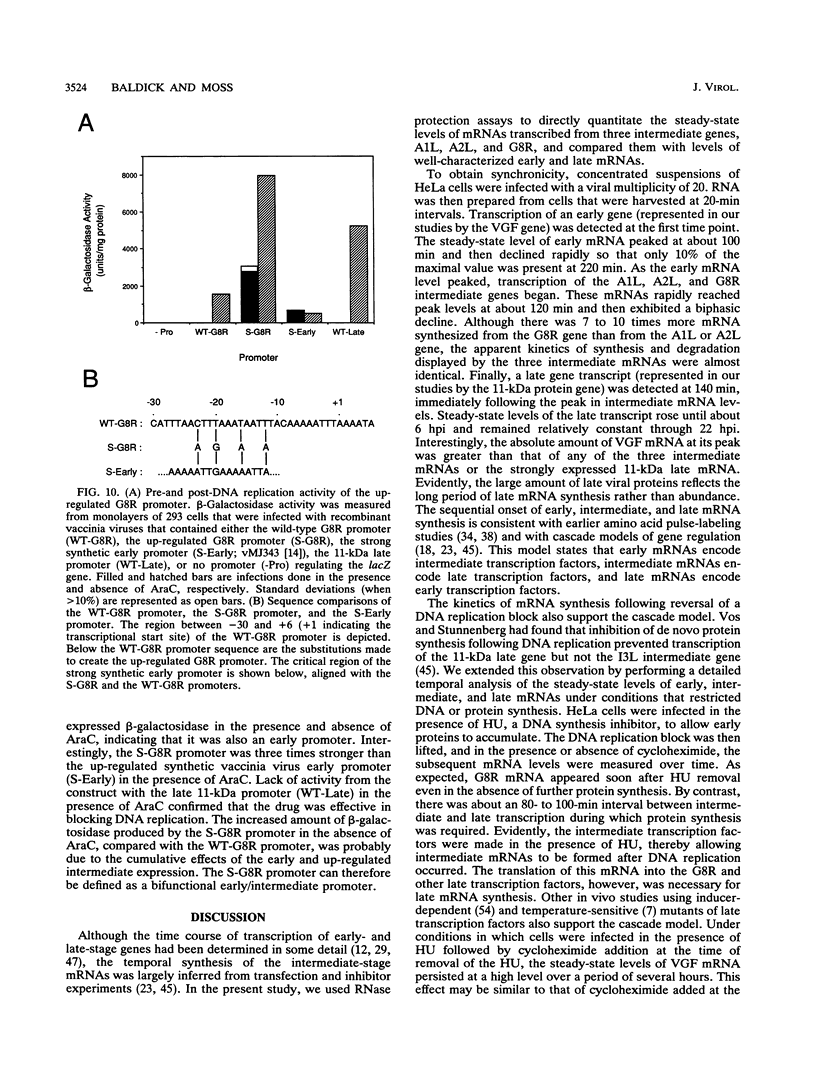



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Jones E. V., Moss B. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5' poly(A) leader on its early transcript. J Virol. 1990 Jun;64(6):3019–3024. doi: 10.1128/jvi.64.6.3019-3024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Moss B. Capped poly(A) leaders of variable lengths at the 5' ends of vaccinia virus late mRNAs. J Virol. 1989 Jan;63(1):226–232. doi: 10.1128/jvi.63.1.226-232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Rosel J., Cole N. B., Moss B. Identification and expression of rpo19, a vaccinia virus gene encoding a 19-kilodalton DNA-dependent RNA polymerase subunit. J Virol. 1992 Feb;66(2):971–982. doi: 10.1128/jvi.66.2.971-982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak J. B., Patel D. D., Ray C. A., Ink B. S., Pickup D. J. Site-specific RNA cleavage generates the 3' end of a poxvirus late mRNA. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12033–12037. doi: 10.1073/pnas.89.24.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick C. J., Jr, Keck J. G., Moss B. Mutational analysis of the core, spacer, and initiator regions of vaccinia virus intermediate-class promoters. J Virol. 1992 Aug;66(8):4710–4719. doi: 10.1128/jvi.66.8.4710-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Stocco P., Van Meir E., Wittek R. Functional analysis of the 5' flanking sequence of a vaccinia virus late gene. EMBO J. 1986 Aug;5(8):1951–1957. doi: 10.1002/j.1460-2075.1986.tb04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell. 1987 Jul 17;50(2):153–162. doi: 10.1016/0092-8674(87)90211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. S., DeLange A. M. Identification of a temperature-sensitive mutant of vaccinia virus defective in late but not intermediate gene expression. Virology. 1992 May;188(1):233–244. doi: 10.1016/0042-6822(92)90753-c. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Extension of the transcriptional and translational map of the left end of the vaccinia virus genome to 21 kilobase pairs. J Virol. 1981 Sep;39(3):733–745. doi: 10.1128/jvi.39.3.733-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Fathi Z., Condit R. C. Genetic and molecular biological characterization of a vaccinia virus temperature-sensitive complementation group affecting a virion component. Virology. 1991 Mar;181(1):258–272. doi: 10.1016/0042-6822(91)90491-s. [DOI] [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4401–4405. doi: 10.1073/pnas.87.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Hirschmann P., Vos J. C., Stunnenberg H. G. Mutational analysis of a vaccinia virus intermediate promoter in vivo and in vitro. J Virol. 1990 Dec;64(12):6063–6069. doi: 10.1128/jvi.64.12.6063-6069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J. Vaccinia virus directs the synthesis of early mRNAs containing 5' poly(A) sequences. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1536–1540. doi: 10.1073/pnas.87.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Kumar S., Boyle D. B. A poxvirus bidirectional promoter element with early/late and late functions. Virology. 1990 Nov;179(1):151–158. doi: 10.1016/0042-6822(90)90284-x. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Niles E. G. Map positions of the 5' ends of eight mRNAs synthesized from the late genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):80–92. doi: 10.1016/0042-6822(88)90235-8. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Niles E. G. Transcription and translation mapping of the 13 genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):52–63. doi: 10.1016/0042-6822(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Arrangement of late RNAs transcribed from a 7.1-kilobase EcoRI vaccinia virus DNA fragment. J Virol. 1984 Feb;49(2):510–520. doi: 10.1128/jvi.49.2.510-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. F., Crozel-Goudot V., Traktman P. Transient expression of the vaccinia virus DNA polymerase is an intrinsic feature of the early phase of infection and is unlinked to DNA replication and late gene expression. J Virol. 1992 Jan;66(1):534–547. doi: 10.1128/jvi.66.1.534-547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Pacha R. F., Meis R. J., Condit R. C. Structure and expression of the vaccinia virus gene which prevents virus-induced breakdown of RNA. J Virol. 1990 Aug;64(8):3853–3863. doi: 10.1128/jvi.64.8.3853-3863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J. Messenger RNAs of a strongly-expressed late gene of cowpox virus contain 5'-terminal poly(A) sequences. EMBO J. 1987 Dec 1;6(12):3787–3794. doi: 10.1002/j.1460-2075.1987.tb02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Salzman N. P. Metabolic properties of early and late vaccinia virus messenger ribonucleic acid. J Virol. 1967 Jun;1(3):550–558. doi: 10.1128/jvi.1.3.550-558.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Bromouridine triphosphate inhibits transcription termination and mRNA release by vaccinia virions. J Biol Chem. 1989 Dec 15;264(35):21356–21360. [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell. 1991 Apr 5;65(1):105–113. doi: 10.1016/0092-8674(91)90412-r. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 1991 Sep;10(9):2553–2558. doi: 10.1002/j.1460-2075.1991.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., Stunnenberg H. G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988 Nov;7(11):3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. A tandemly-oriented late gene cluster within the vaccinia virus genome. Nucleic Acids Res. 1986 Apr 11;14(7):3003–3016. doi: 10.1093/nar/14.7.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. Noncoordinate regulation of a vaccinia virus late gene cluster. J Virol. 1987 Mar;61(3):639–645. doi: 10.1128/jvi.61.3.639-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Determination of the promoter region of an early vaccinia virus gene encoding thymidine kinase. Virology. 1987 May;158(1):206–210. doi: 10.1016/0042-6822(87)90254-6. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Determination of the transcriptional regulatory region of a vaccinia virus late gene. J Virol. 1987 Jan;61(1):75–80. doi: 10.1128/jvi.61.1.75-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson B. Vaccinia mRNA synthesis under conditions which prevent uncoating. Biochem Biophys Res Commun. 1967 Apr 20;27(2):169–175. doi: 10.1016/s0006-291x(67)80057-3. [DOI] [PubMed] [Google Scholar]
- Wright C. F., Keck J. G., Tsai M. M., Moss B. A transcription factor for expression of vaccinia virus late genes is encoded by an intermediate gene. J Virol. 1991 Jul;65(7):3715–3720. doi: 10.1128/jvi.65.7.3715-3720.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Moss B. Identification of factors specific for transcription of the late class of vaccinia virus genes. J Virol. 1989 Oct;63(10):4224–4233. doi: 10.1128/jvi.63.10.4224-4233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Keck J. G., Moss B. Transcription of viral late genes is dependent on expression of the viral intermediate gene G8R in cells infected with an inducible conditional-lethal mutant vaccinia virus. J Virol. 1992 Nov;66(11):6470–6479. doi: 10.1128/jvi.66.11.6470-6479.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magistris L., Stunnenberg H. G. Cis-acting sequences affecting the length of the poly(A) head of vaccinia virus late transcripts. Nucleic Acids Res. 1988 Apr 25;16(8):3141–3156. doi: 10.1093/nar/16.8.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]