Abstract
Recall of fear extinction, which is thought to aid in recovery from a psychologically traumatic event, is hypothesized to be deficient in post-traumatic stress disorder (PTSD), but this has not yet been demonstrated in the laboratory, nor has its origin been investigated. To address these two issues, 14 pairs of monozygotic twins discordant for combat exposure, in 7 of which the combat-exposed twin had PTSD, underwent a two-day fear conditioning and extinction procedure. On Day 1, subjects viewed colored light conditioned stimuli, some of which were paired with mild electric shock, followed by extinction of the conditioned responses. On Day 2, recall of Day 1 extinction learning (i.e., extinction retention) was assessed. Skin conductance response (SCR) was the dependent measure. There were no group differences during acquisition or extinction learning. However, a significant PTSD Diagnosis (in the exposed twin) x combat Exposure interaction emerged during extinction recall, with the PTSD combat veterans having larger SCRs than their own co-twins, and than the non-PTSD combat veterans and their co-twins. These results indicate that retention of extinction of conditioned fear is deficient in PTSD. Furthermore, they support the conclusion that this deficit is acquired as a result of combat trauma leading to PTSD, rather than being a predisposing factor to developing PTSD upon the stress of combat.
Keywords: Stress disorders, post-traumatic; Fear; Conditioning, classical; Galvanic skin response; Memory; Twins, monozygotic
Introduction
Extinction is the reduction in conditioned responses (CRs) that occurs when the conditioned stimulus (CS) no longer predicts the unconditioned stimulus (US). Posttraumatic stress disorder (PTSD) involves learned fear (Rothbaum and Davis, 2003). Abnormally high psychophysiological conditioned responses to reminders of traumatic events can persist as long as 50 years following its cessation (Orr et al., 1993). These data suggest that a deficit in either extinction learning or retention of that learning may underlie failure to recover from the effects of the traumatic stressor (Rauch et al., 2006; Milad et al., 2006b; Davis et al., 2006; Sotres-Bayon et al., 2004; Maren and Quirk, 2004). Consistent with this view, slower extinction of corrugator electromyogram responses were found to represent a pre-trauma risk factor for PTSD-related symptoms following a traumatic event (Guthrie and Bryant, 2006). Although studies have supported impaired extinction learning in PTSD (Blechert et al., 2007; Orr et al., 2000; Peri et al., 2000), no previous studies have reported deficits in extinction retention.
If extinction retention is deficient in PTSD, it could represent either an acquired PTSD sign, e.g., result from the traumatic stress that caused the PTSD and/or the stress of having PTSD, or a pre-existing vulnerability factor for developing PTSD upon traumatic exposure. We have been studying monozygotic twin pairs discordant for combat exposure to address the pre-existing vs. acquired origin of biological abnormalities found in PTSD (Pitman et al., 2006). If an abnormality is genetic or due to environmental influences shared by twins during their rearing, i.e., is a “familial” vulnerability factor, then it should be present in the non-trauma-exposed co-twins of trauma-exposed twins with PTSD. Alternatively, if the abnormality results from the traumatic event, then their combat-unexposed co-twins should not share it.
To test the presence and origin of deficient extinction retention in PTSD, we used a two-day fear conditioning and extinction protocol that has been successfully employed in persons without mental disorders (Milad et al., 2005a; Milad et al., 2006a). On the first day, subjects underwent fear conditioning in one virtual context followed by extinction learning in another virtual context. On the second day, extinction recall was tested in the previous extinction context. The conditioned stimuli (CSs) were colored lights that were presented within both contexts. This protocol differed from other studies that examined conditioning and extinction learning in PTSD (for example, Orr et al., 2000) in two ways: 1) conditioning and extinction learning were conducted in two different virtual contexts, and 2) an extinction retention test was conducted 24 hours after extinction learning.
Materials and Methods
Subjects
Subjects were drawn from a pool of identical twins who had participated in a previous study of physiological responses to loud tones. A description of the recruitment strategy, and characteristics of the participant population has been reported elsewhere (Orr et al., 2003). Fourteen pairs of male monozygotic twins participated. One “exposed” (Ex) twin had served in the Vietnam combat theater, whereas his “unexposed” (Ux) co-twin had not. Of the Ex twins, 7 developed combat-related PTSD (P+), and 7 did not (P-), as determined by the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995; Weathers et al., 2001) using DSM-IV criteria. Thus, there were four cells of 7 subjects each as follows: ExP+: combat-exposed veteran with current, combat-related PTSD, and UxP+: his combat-unexposed co-twin; as well as ExP-: combat-exposed veteran who never had combat-related PTSD, and UxP-: his combat-unexposed co-twin.
Demographics and psychometrics
Demographic and psychometric means (SDs) were as follows: Age (years): ExP+/UxP+ 58.1 (2.8), ExP-/UxP- 59.1 (2.5), t(12)=0.7, p=0.50; Combat severity score (Janes et al., 1991) (range 0-18): ExP+ 7.7 (2.4), ExP- 3.4 (2.4), t(12)=3.4, p=0.005; Total CAPS score (range 0-136): ExP+ 59.0 (24.1), ExP- 5.1 (9.4), t(12)=5.5, p<0.001. All subjects were also administered the CAPS with regard to their most severe non-combat related event, as well as the Structured Clinical Interview for DSM-IV (SCID) for non-PTSD Axis I mental disorders (First et al., 2002). Current comorbid disorders included one ExP+ subject with both major depressive disorder and non-combat-related PTSD, two ExP+ subjects with dysthymia, and one UxP- subject with dysthymia. No subjects in the ExP-, UxP+, or UxP- groups had non-combat-related PTSD.
Conditioning Procedure
The procedures used in the present study were previously described (Milad et al., 2005b; Milad et al., 2005a; Rauch et al., 2005). Digital photographs of two different rooms constituted the visual contexts. Each room contained a lamp, and two different colors (i.e. blue and red) of the lighted lampshade constituted the CSs. The selection of the CS+ and CS- colors was randomly determined and counterbalanced across participants. Contexts and CSs were displayed on a computer monitor three feet in front of the participants. The US was a 500 ms electric shock delivered through electrodes attached to the second and third fingers of the dominant hand. The intensity of the shock was previously selected by each participant so as to be “highly annoying but not painful” (Orr et al., 2000).
The experimental protocol was administered over two separate days. On Day 1, the Habituation phase consisted of 8 trials, in which the to-be CS+ and to-be CS- (4 of each) were presented in a counterbalanced manner within either the to-be conditioning context (CX+) or the to-be extinction context (CX-). The Acquisition phase consisted of 5 CS+ and 5 CS- trials, all presented within CX+. The shock US occurred immediately following each CS+ offset without delay. The Extinction Learning phase was divided into two sub-phases: early and late, which were separated by an approximately 1-minute rest period. Each sub-phase consisted of 5 CS+ and 5 CS- trials, all presented within the CX-. On Day 2, the Extinction Recall phase was identical to an Extinction sub-phase from the previous day. Subjects were instructed that at all times (except for the Habituation phase), they may or may not receive the electric shock US. However, although the shock electrodes remained attached to the participant’s fingers during the Exinction Learning and Extinction Recall phases, no shocks were delivered.
For each trial during the experiment, the virtual context was presented for 18 seconds: 6 seconds alone followed by 12 seconds in combination with the CS+ or CS-. The mean inter-trial interval was 16 seconds (range: 12-21 seconds). Skin conductance response (SCR) was scored as previously described (Milad et al., 2005a; Orr et al., 2000; Orr and Lanzetta, 1980; Pitman and Orr, 1986). Specifically, SCR was calculated for each CS trial by subtracting the mean skin conductance level during the 2 seconds immediately prior to CS onset (during which the context alone was being presented) from the highest skin conductance level recorded during the 12-second CS duration. Thus, all SCRs to the CS+ and CS- reported herein reflect changes in skin conductance level above and beyond any changes in skin conductance level produced by the context. We have used this method in previous, published human psychophysiological research, which has supported its validity (Orr et al., 2000; Milad et al., 2006a; Milad et al., 2005a; Milad et al., 2007). Each skin conductance response was square-root transformed prior to analysis. Unless specified, all data are presented as means ± standard error of the mean.
Extinction Indices
To facilitate comparison of the results with those in prior publications (Milad et al., 2005a; Milad et al., 2007), we also calculated Extinction Indices, in which the average SCR to the last two CS+ trials during the late Extinction Learning Phase and again during the Extinction Recall Phase were divided by the maximum SCR to a CS+ during the Acquisition Phase and then multiplied by 100. This yielded % of fear expressed, which was then subtracted from 100% to yield an Extinction Learning Index (ELI) and an Extinction Retention (recall) Index (ERI) respectively.
Statistical analysis
SCR data were analyzed separately for the Acquisition, late Extinction Learning, and Extinction Recall Phases by a three-factor mixed model that treated Stimulus (CS+ vs. CS-) as a within-subjects repeated measure, combat Exposure (Ex vs. Ux) as a within-pairs repeated measure, Diagnosis (P+ vs. P-) as a between-pairs measure, and twin pairs as a random effect. Extinction Indices were analyzed in a similar manner, except that 1.) there was no Stimulus effect; and 2.) data from the late Extinction Learning and Extinction Recall Phases were first analyzed within the same model with Phase (Learning vs. Recall) as a within-subjects effect, followed by separate analyses for the two phases. All hypotheses were directional, viz., 1.) For SCR, CS+ >CS-, Ex>Ux; and P+>P-; 2.) for extinction indices (which had inverse relationships with SCR), P->P+ and Ux>Ex; and 3.) for extinction index phases, Retention > Learning. Therefore, p values from the above analyses were halved.
Results
Acquisition (Day 1)
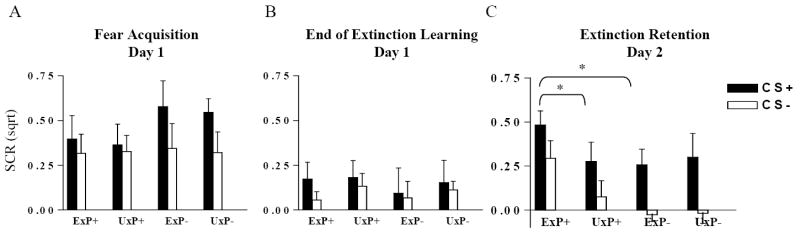
We examined the maximum SCRs to the CS+ and CS- during the last 4 of the 5 acquisition trials (figure 1a). There was significant a Stimulus effect, with SCRs to the CS+ greater than to the CS-: F1,11.3=8.6, p=0.007.
Figure 1.
Intact fear acquisition and extinction learning but impaired extinction recall (retention) in PTSD. A. Largest skin conductance response (SCR) to the conditioned stimulus paired with the shock (CS+) and to the conditioned stimulus not paired with the shock (CS-) exhibited during the Acquisition phase. B. SCR to the CS+ and CS- averaged across the last two trials of the late Extinction Learning phase. C. SCR to the CS+ and CS- averaged across the first two trials of the Extinction Recall phase. *p<0.05 ExP+: PTSD twins, UxP+: their combat-unexposed co-twins; ExP-: combat-exposed non-PTSD twins, UxP-: their combat-unexposed co-twins.
Extinction Learning (Day 1)
We examined the average SCRs to the last two late Extinction Learning Phase trials (figure 1b). Subjects in all groups extinguished approximately 70% of the acquired conditioned responses. There were no significant differences in the levels of extinction across all groups.
Extinction Recall (Day 2)
We examined the average SCRs to the first two Recall Phase trials (figure 1c). There was a significant Stimulus main effect, with SCRs to the CS+ greater than to the CS-: F1,11.0=16.9, p<0.001. There was also a significant Diagnosis × Exposure interaction: F1,12.1=4.3, p=0.03, with ExP+ subjects showing larger SCRs than ExP- subjects: F1,12=7.1, p=0.01, and larger SCRs than UxP+ subjects: F1,7.2=5.2, p=0.03.
Extinction Indices
We examined these indices as defined above (Figure 2). There was a significant Diagnosis × Exposure × Phase interaction: F1,11.1=4.3, p=0.03, with overall greater extinction retention during the Learning than the Recall Phase, reflecting some return of the SCRs to the CS+ on Day 2. Analyzing the two phases separately, for ELI (figure 2-left), there were no significant results. However, for ERI (figure 2-right) there was a significant Diagnosis × Exposure interaction: F1,11.1=4.2, p=0.03, with ExP+ subjects showing (nearly significantly) less extinction retention than ExP- subjects: F1,12=3.0, p=0.06, and less extinction retention than UxP+ subjects: F1,5.7=4.9, p=0.04. These results paralleled the results obtained with the SCR analyses.
Figure 2.
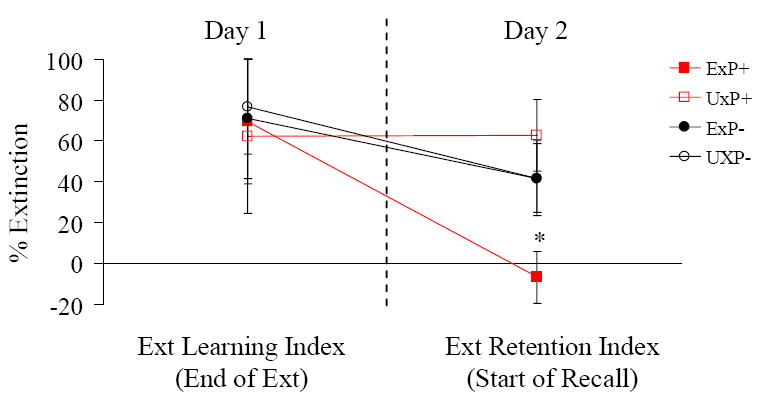
Extinction Learning and Extinction Retention Indices (see text for definition) demonstrate intact extinction learning across all groups but impaired extinction retention in PTSD twins only. *p<0.05. ExP+: PTSD twins, UxP+: their combat-unexposed co-twins; ExP-: combat-exposed non-PTSD twins, UxP-: their combat-unexposed co-twins.
Analyses Addressing Potentially Confounding Factors
The significant Diagnosis × Exposure interaction for SCRs during Extinction Recall was not explained by: 1.) SCRs during Acquisition; 2.) SCRs during Extinction Learning; 3.) Age; 4.) Combat Severity (Ex subjects only); 5.) number of Potentially Traumatic, Lifetime, Non-Combat Events; 6.) reported use of one or more potentially confounding Medications or Substances (including antihistamines, sympathomimetics, sympatholytics, parasympathomimetics, parasympatholytics, skeletal muscle relaxants, hypotensive agents, vasodilating agents, pressor agents, β-blockers, antiarrhythmics, calcium channel blockers, narcotics, anticonvulsants, antidepressants, neuroleptics, benzodiazepines, other psychotherapeutic agents, cerebral stimulants, sedatives, and hypnotics) during the month prior to testing, or a “dirty” urine specimen (i.e., containing amphetamines, barbiturates, cocaine, opiates, benzodiazepines, methaquolone, propoxyphene, phencyclidine, methadone, or cannabinoids; and number of 7.) Alcoholic Beverages, 8.) Caffeinated Beverages, or 9.) Cigarettes consumed used during the 24 hours preceding testing. The significant Diagnosis × Exposure interaction for ERI was similarly robust. However both interactions were no longer significant after adjusting for the presence of a current, comorbid mental disorder. The reason for this was that the three ExP+ subjects with a current, comorbid mental disorder showed larger SCRs during Recall (mean 0.48 μS collapsed across Stimulus type) than the four comorbidity free ExP+ subjects (mean 0.32 μS).
Analyses Addressing Type II Error
For each negative result, we calculated the 90% upper confidence limit (UCL) of the estimate. In every case, the UCL was greater than trivial, i.e., >0.05 μS (or >20% for ELI and ERI). Therefore we were unable to conclude that any of the negative results were significant.
Discussion
Although the acquisition and extinction of conditioned fear were intact across all groups on Day 1, the retention of this extinction measured on Day 2 was deficient in the PTSD combat veterans. Moreover, this deficit was not present in their co-twins, suggesting that deficient extinction retention represents an acquired PTSD sign rather than a familial vulnerability factor. The extinction retention index we observed in the present study was lower than what we have previously reported (Milad et al., 2005a; Milad et al., 2007). Nonetheless, extinction retention was significantly lower in the PTSD combat veterans relative to all other groups. However, the absence of a significant Stimulus × Diagnosis × Exposure interaction during the Recall phase indicates that the combat-exposed PTSD subjects’ produced larger SCRs to both the CS+ and CS- than the other groups, suggesting either sensitization or CR generalization.
In a recent study, Orr et al. (2006) found no extinction retention deficit in PTSD. The likely explanation for the discrepancy between those results and the results reported herein is methodological differences. In the present study, all subjects had the shock electrodes reattached during the extinction retention test 24 hours after extinction learning and were instructed that they may or may not receive the electric shock. In contrast, in the Orr et al. study, the shock electrodes were not reattached when the extinction retention was tested one week after extinction learning, thereby making the reappearance of the CR less likely.
Recent functional neuroimaging studies in healthy humans have reported activation of the ventromedial prefrontal cortex (vmPFC) during extinction recall (Phelps et al., 2004; Kalisch et al., 2006; Milad et al., 2007). The impaired extinction retention in the PTSD subjects in the present study is consistent with studies reporting deficient activation of this brain region in PTSD (Shin et al., 2004; Bremner et al., 2005; Liberzon et al., 2003; Phan et al., 2006; Britton et al., 2005; Shin et al., 2001). Moreover, a recent twin study reported acquired gray matter reduction in an area of vmPFC (rostral anterior cingulate cortex) in PTSD (Kasai et al., 2007). This is consistent with our present data showing that the extinction retention deficiency appears to be acquired.
We cannot rule out the possibility that the poorer extinction retention observed in the PTSD combat veterans was due to comorbid depression. The three comorbid subjects carried most of the effect, in that the extinction retention shown by the four remaining PTSD combat veterans was not very different from that of the other groups. However, none of the three comorbid PTSD veterans’ unexposed twins shared the comorbidity, suggesting that comorbid depression was a different facet of the same acquired posttraumatic psychopathology. Although research with an animal model suggests that extinction retention may be impaired in depression (Wellman et al., 2007), we are unaware of any human clinical research. Investigation of extinction retention in depression without PTSD appears indicated.
Acknowledgments
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007 doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related posttraumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon Miriam, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Patient. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for Acquired Pregenual Anterior Cingulate Gray Matter Loss from a Twin Study of Combat-Related Posttraumatic Stress Disorder. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Britton JC, Phan KL. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6:151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006a;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005a;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005b;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006b doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lanzetta JT. Facial expressions of emotion as conditioned stimuli for human autonomic responses. J Pers Soc Psychol. 1980;38:278–282. doi: 10.1037//0022-3514.38.2.278. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Orr SP, Milad MR, Metzger LJ, Lasko NB, Gilbertson MW, Pitman RK. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biol Psychol. 2006;73:262–271. doi: 10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. J Abnorm Psychol. 1986;95:208–213. doi: 10.1037//0021-843x.95.3.208. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, Ledoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]