Abstract
Alcohol abuse is associated with sleep problems, which are often linked to circadian rhythm disturbances. However, there is no information on the direct effects of ethanol on the mammalian circadian clock. Acute ethanol inhibits glutamate signaling, which is the primary mechanism through which light resets the mammalian clock in the suprachiasmatic nucleus (SCN). Glutamate and light also inhibit circadian clock resetting induced by non-photic signals, including serotonin. Thus, we investigated the effects of acute ethanol on both glutamatergic and serotoninergic resetting of the SCN clock in vitro. We show that ethanol dose-dependently inhibits glutamate-induced phase shifts and enhances serotonergic phase shifts. The inhibition of glutamate-induced phase shifts is not affected by excess glutamate, glycine or D-serine, but is prevented by excess brain-derived neurotrophic factor (BDNF). BDNF is known to augment glutamate signaling in the SCN and to be necessary for glutamate/light-induced phase shifts. Thus, ethanol may inhibit glutamate-induced clock resetting at least in part by blocking BDNF enhancement of glutamate signaling. Ethanol enhancement of serotonergic phase shifts is mimicked by treatments that suppress glutamate signaling in the SCN, including antagonists of glutamate receptors, BDNF signaling and nitric oxide synthase. The combined effect of ethanol with these treatments is not additive, suggesting they act through a common pathway. Our data indicate further that the interaction between serotonin and glutamate in the SCN may occur downstream from nitric oxide synthase activation. Thus, acute ethanol disrupts normal circadian clock phase regulation, which could contribute to the physiological and psychological problems associated with alcohol abuse.
Keywords: suprachiasmatic nucleus, circadian rhythms, ethanol, glutamate, serotonin, brain-derived neurotrophic factor
The development of alcoholism and the likelihood of relapse drinking are associated with poor sleep quality and the deleterious effects of ethanol on sleep (Landolt and Gillin, 2001;Brower et al., 2001). Alcohol consumption reduces sleep quality (Kubota et al., 2002;Ehler and Slawecki, 2000;Landolt et al., 1996), which is closely linked to the circadian system. For example, the inability to fall asleep at the desired time can result from improper synchronization of the circadian clock to the environment (Reid and Zee, 2004). Recent studies show that chronic ethanol alters the circadian free-running period in rats (Rosenwasser et al., 2005a,b) and inhibits light- and triazolam-induced phase shifts in hamsters (Seggio et al., 2007). However, little work has focused on whether acute ethanol affects circadian rhythms, and whether such effects involve direct actions on the master circadian clock located in the suprachiasmatic nucleus (SCN).
The SCN circadian clock synchronizes with the external environment through direct retinal input to the SCN. This signaling primarily involves glutamate acting on SCN NMDA and AMPA receptors. Light (in vivo) and glutamate (in vitro) presented during the night robustly phase-shift the SCN clock; these effects are mimicked by NMDA (Ding et al., 1994;Mintz et al., 1999;Mintz and Albers, 1997;Gamble et al., 2004) and blocked by NMDA antagonists (Abe et al., 1992;Rea et al., 1993). NMDA receptors are one of the principal targets of ethanol in the brain (Lovinger et al., 1989;Abrous et al., 2005;Krystal et al., 2003;Chandler, 2003). Acute ethanol exposure both in vivo and in vitro suppresses NMDA receptor-induced ion currents, Ca2+ influx, and downstream cellular events (Wirkner et al., 2000;Roberto et al., 2004;Spanagel et al., 2002).
Non-photic stimuli also regulate SCN circadian clock phase. Arousal or behavioral activity during the daytime (for nocturnal rodents) induce robust phase advances (Mrosovsky, 1988;Reebs et al., 1989;Antle and Mistlberger, 2000) that may involve neuropeptide Y (Biello et al., 1994;Golombek et al., 1996;Marchant et al., 1997) and/or serotonin (5-HT) (Prosser, 2003;Tominaga et al., 1992;Horikawa and Shibata, 2004;Marchant et al., 1997;Glass et al., 2003) signaling in the SCN. Interestingly, acute ethanol alters serotonergic signaling (Hayashi et al., 2003;Thielen et al., 2002;Daws et al., 2006), raising the possibility that ethanol could also affect the circadian system through modulating serotonergic signaling. Moreover, there is a mutually antagonistic relationship between non-photic stimuli that induce daytime phase shifts and photic input mediating nighttime phase shifts. Light or glutamate stimulation during the day inhibits non-photic phase shifts (Mrosovsky, 1991;Grossman et al., 2000;Hall et al., 1999;Gamble et al., 2004;Biello et al., 1997;Prosser, 2001;Kallingal and Mintz, 2007), while nighttime presentation of non-photic stimuli suppresses light/glutamate-induced phase shifts (Ralph and Mrosovsky, 1992;Yannielli and Harrington, 2004;Smith et al., 2001;Gamble et al., 2004;Biello et al., 1997;Kallingal and Mintz, 2007). Conversely, suppressing glutamate signaling during the day can enhance phase shifts induced by non-photic stimuli (Fedorkova et al., 2002), while decreasing neuropeptide Y or 5-HT signaling at night can enhance photic phase shifts (Yannielli and Harrington, 2004;Lall and Harrington, 2006;Smart and Biello, 2001;Muscat et al., 2005). Based on these observations, we sought to determine how acute ethanol affects both glutamatergic and serotonergic resetting of the SCN clock in vitro, and to begin exploring mechanisms through which ethanol acts.
EXPERIMENTAL PROCEDURES
Brain slice preparation
Coronal brain slices (500 μm) containing the SCN were prepared during the daytime from adult, male C57BL/J6 mice, housed in 12:12 LD conditions, as reported previously (Prosser and Gillette, 1989;Prosser et al., 1993;Prosser, 1998). Slices were prepared between Zeitgeber time (ZT) 0–4 (where ZT 0 = lights-on and ZT 12 = lights-off in the donor animal colony). Slices were maintained at the interface of a Hatton-style brain slice chamber (Hatton et al., 1980), where they were perfused continuously with warm (37°C), oxygenated (95% O2/5% CO2), glucose/bicarbonate-supplemented Earle’s Balanced Salt Solution (EBSS; Sigma-Aldrich, St. Louis, MO), pH 7.4–7.5. Gentamicin (0.05%) was also added to the perfusion medium.
Experimental protocols
———All drugs were prepared in warm, oxygenated EBSS and were bath-applied to the brain slices. At the onset of the drug treatments, perfusion of the standard medium was stopped, the medium was completely removed from the chamber, and fresh medium containing the drugs was applied. Previous experiments have demonstrated that changing the perfusion medium by itself does not affect the phase of the circadian clock.
———Glutamate treatments
Glutamate (Sigma-Aldrich) was applied for 10 minutes beginning at either ZT 16, ZT 23, or ZT 6, alone or in combination with varying concentrations of ethanol (diluted from 95% ethanol, AAPER Alcohol). In some experiments glutamate treatments were preceded by a 15 min pre-application of glycine or D-serine (Sigma-Aldrich). In other experiments brain-derived neurotrophic factor (BDNF; Alomone Labs, Jerusalem, Israel) was applied beginning 30 min prior to co-application with glutamate. Previously we have shown that the effects of BDNF in the SCN require this duration of pre-treatment (Prosser et al., 2004).
Serotonergic treatments
At ZT6 brain slices were treated for 10 min with10 uM (+)8-hydroxy-2-(di-N-propylamino)tetralin (DPAT; a 5-HT agonist selective for 5HT1A/5/7 receptors), applied alone or in combination with ethanol, aminophosphovaneric acid (AP5), 1,2,3,4-tetrahydro-4-nitro-2,3-dixo-benzo(f) quinoxaline-7-sulfonamide (NBQX), K252a, NG -nitro-L-arginine methyl ester hydrochloride (L-NAME), or a combination of these compounds (all from Sigma-Aldrich).
Single-unit recordings and data analysis
Single-unit recordings commenced near the beginning of day 2 in vitro (between ZT 22 and ZT 1). The procedure for neuronal recordings has been described previously (Prosser et al., 1993;Prosser, 1998). Briefly, the spontaneous activity of single SCN neurons was recorded extracellularly using glass capillary microelectrodes filled with 3M NaCl. Each neuron was recorded for 5 min, and the data stored for later determination of firing rate using a DataWave system (Berthoud, CO). Typically, 4–7 cells were recorded during each hour. These individual firing rates were then used to calculate 2 h running averages, lagged by 1 h (± SEM), to obtain a measure of population neuronal activity. As in previous studies (Prosser et al., 1993;Prosser, 1998), the time of peak neuronal activity was assessed visually by estimating, to the nearest quarter hour, the time of symmetrically highest activity. For example, if the two highest 2 h means are equal, then the time of peak is estimated to be halfway between them. Phase shifts were calculated as the difference in time-of-peak of untreated slices vs. drug-treated slices. Using these methods, the consistency of the results obtained for each experimental manipulation is such that differences in phase of as little as one hour are often statistically significant with few (n=2 to 3) replicates (e.g., (Prosser, 2003;Prosser et al., 2006).
Statistical Analysis
Differences in the time of peak neuronal activity were assessed using Student’s t-calculationsc test or ANOVA. In all cases, the level of significance was set at p<0.05. ED50 were performed by non-linear regression analysis (Prism, San Diego, CA).
RESULTS
Ethanol dose-dependently blocks glutamate-induced phase delays
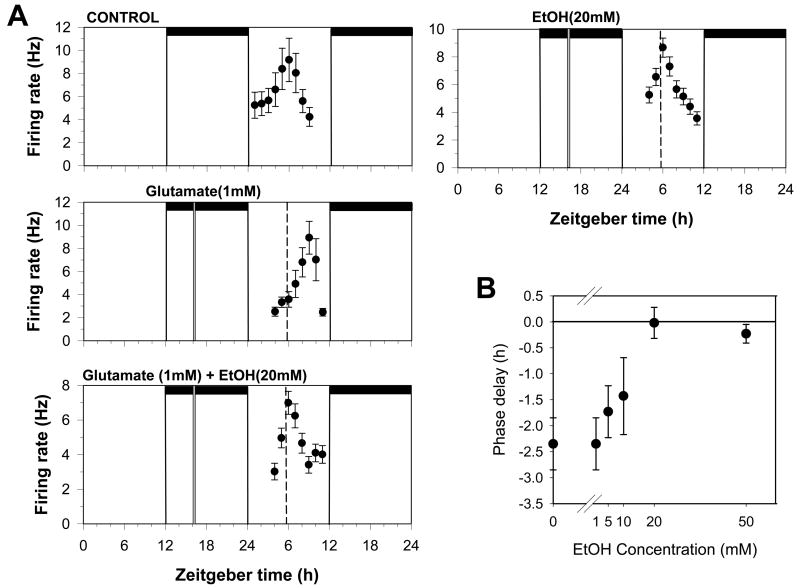
In untreated brain slices, neuronal activity peaks near the middle of the subjective day (mean time-of-peak of ZT5.9±0.3 h, n=5). As the experiment in Fig. 1A illustrates, applying 1mM glutamate for 10 minutes at ZT 16.0, shifts the time of peak neuronal activity to about ZT 9, corresponding to a mean (±SEM) phase shift of 2.35 ± 0.5 h (n=3; Fig. 1B). Although ethanol (20mM) applied alone at ZT 16 had little effect on the time of peak neuronal activity, co-application with glutamate at ZT 16 completely blocked the glutamate-induced phase delay (Fig. 1A; mean time-of-peak = ZT 5.9 ±0.3; n=3). This effect was of 10.26 mM (Fig. 1B). The results of these experiments dose-dependent, with an EC50 are summarized in Figs. 1 and 2.
Figure 1.
Glutamate-induced phase delays are inhibited by ethanol. A. Shown are the 2 h means ± SEM of SCN neuronal activity obtained in a control experiment and in experiments where slices were treated at ZT 16 on the first day in vitro with the compounds indicated. In control experiments, neuronal activity peaks near ZT 6 on the second day in vitro. Neuronal activity peaks approximately 3 h later after treatment with glutamate (1mM) applied alone at ZT 16, indicating the SCN clock had been phase-delayed by 3 h. Co-application of 20mM ethanol treatment with glutamate blocked the phase delay, while ethanol treatment alone had no effect. Horizontal bars: time of lights-off in the animal colony; vertical bars: time of drug treatment; dotted line: mean time-of-peak in control experiments. B. Dose response curve for ethanol inhibition of glutamate-induced phase shifts. Shown are the mean phase delays (±SEM) induced by glutamate applied alone or together with difference concentrations of ethanol.
Figure 2.
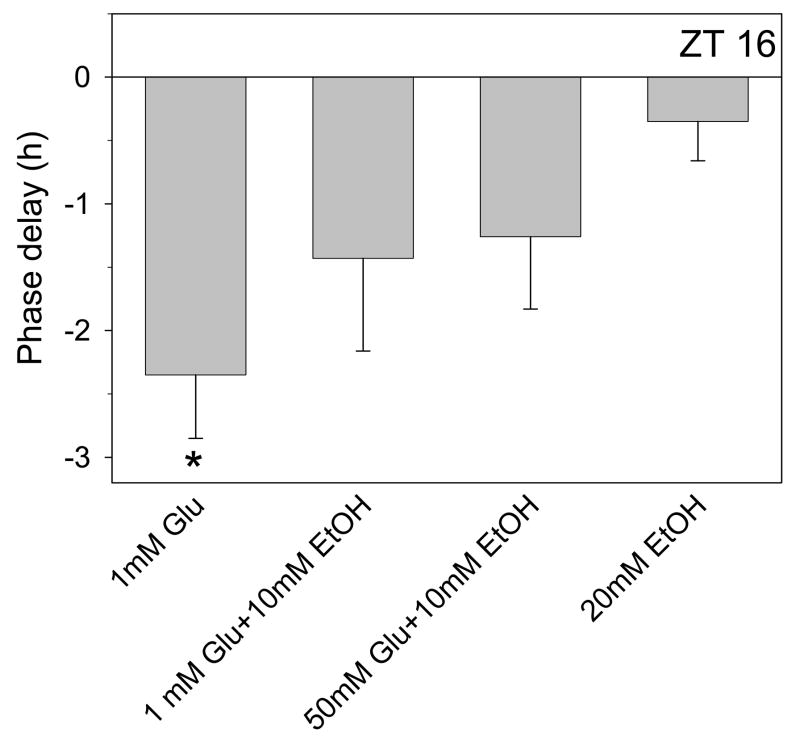
Summary of phase-shifting experiments with glutamate and ethanol. Shown are the mean (± SEM) phase delays induced by different treatments applied at ZT 16. Glutamate applied at ZT 16 induces phase delays of about 2.5 h that are partially inhibited by 10mM ethanol and fully inhibited by 20mM ethanol. A 10-fold increase in glutamate (50mM) does not prevent the partial inhibition by 10mM ethanol. Ethanol applied alone has no effect. *p<0.05 vs. control experiments
Glutamate, glycine, and D-serine do not prevent ethanol inhibition of phase delays
To investigate how ethanol inhibits glutamate signaling in the SCN, we first tested whether excess glutamate could overcome the inhibition induced by a half-maximal concentration of ethanol (10mM). The results (Fig. 2) show that a 50-fold increase in glutamate concentration (50 mM) was unable to overcome the inhibition induced by 10mM ethanol.
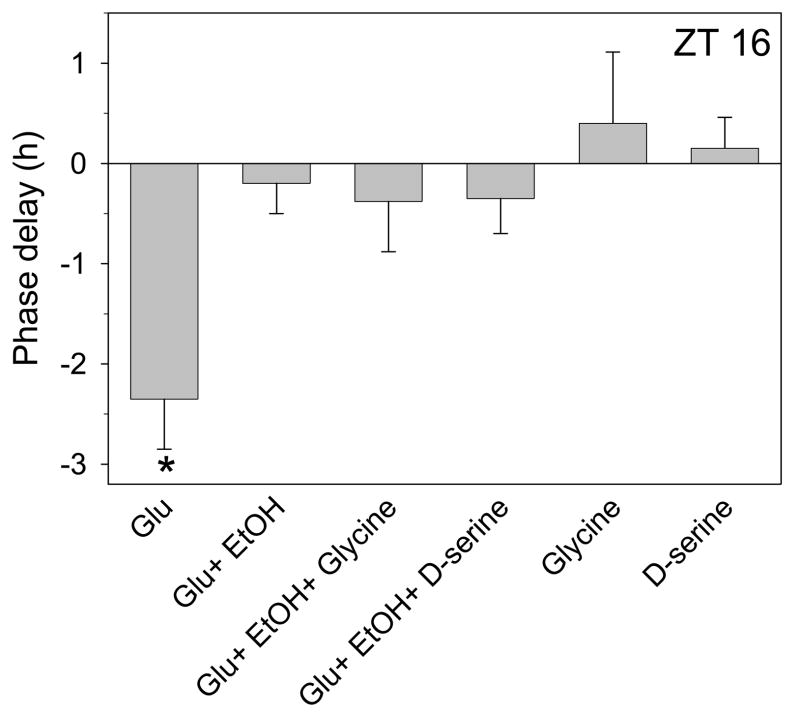
Next we investigated whether exogenous glycine or D-serine prevent ethanol inhibition of glutamate-induced phase delays. Both glycine and D-serine are considered co-agonists of NMDA receptors (Mothet et al., 2000). Some studies suggest that ethanol blocks glycine binding (Rabe and Tabakoff, 1990;Woodward and Gonzales, 1990), while others have obtained different results (Gonzales and Woodward, 1990;Wright et al., 1996). Most relevant to our studies, 1nM glycine increases NMDA activity in the SCN (Ito et al., 1991). Therefore we treated SCN brain slices with 10nM glycine together with ethanol (20mM) and glutamate (1mM). As shown in Fig. 3, glycine did not block ethanol inhibition of glutamate-induced phase delays. Also, 10nM glycine applied alone had no effect on the time of peak activity. Higher concentrations of glycine could not be tested for their ability to prevent ethanol inhibition, since they induced significant phase shifts on their own (data not shown), possibly through activation of glycine receptors (Ito et al., 1991).
Figure 3.
Summary of phase-shifting experiments with glutamate applied alone or with ethanol at ZT 16 with or without different co-agonists. Glutamate (1mM)-induced phase delays (mean ± SEM) are inhibited by 20mM ethanol and this effect is not reversed by either 10nM glycine or 100uM D-serine. The co-agonists had no effect when applied alone. *p<0.01 vs. control experiments
Similar results were obtained with D-serine. As shown in Fig. 3, application of100 uM D-serine had no effect on ethanol inhibition of glutamate-induced phase delays. D-serine also did not alter the phase of neuronal activity when applied alone. Together with the previous results, these data suggest that ethanol inhibition of glutamate-induced phase delays does not occur through blocking the binding of either glutamate or its co-agonist to its receptor.
BDNF prevents ethanol inhibition of glutamate-induced phase delays
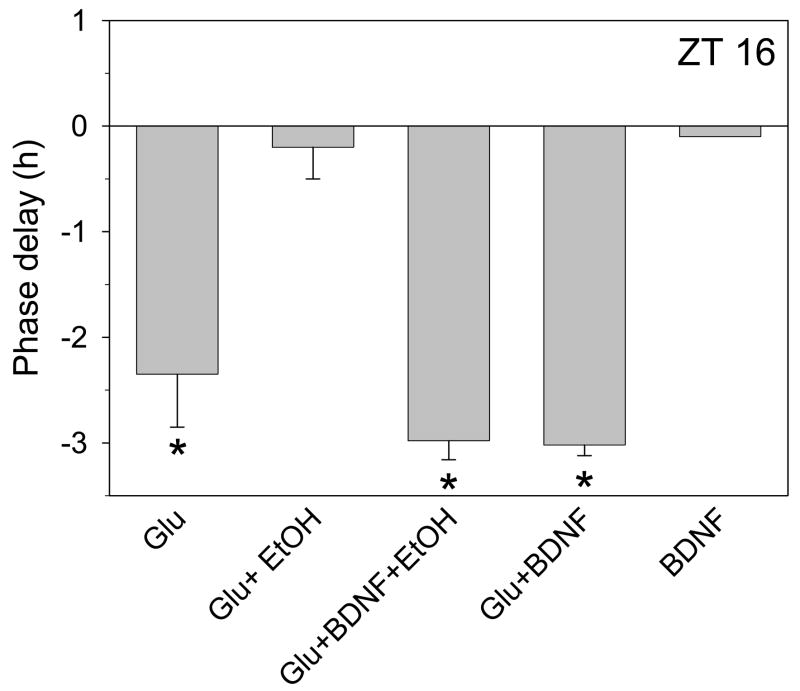
Ethanol blocks BDNF-induced enhancement of glutamate signaling in the hippocampus (Kolb et al., 2005), while increases in BDNF signaling can decrease ethanol consumption (McGough et al., 2004). BDNF is produced in the SCN (Liang et al., 1998b), and its signaling through TrkB receptors enhances glutamate signaling in the SCN (Michel et al., 2006). Thus, in these next experiments we investigated whether exogenous BDNF (100ng/ml) prevents ethanol inhibition of glutamate-induced phase delays. BDNF treatment by itself at ZT 16 did not affect the phase of in vitro neuronal activity, and the phase delay induced by co-application of BDNF with glutamate was the same as that induced by glutamate alone (Fig. 4). However, BDNF administration completely prevented ethanol inhibition of glutamate-induced phase delays (Fig. 4). Since BDNF enhancement of glutamate signaling in the SCN involves TrkB receptor activation (Liang et al., 1998a;Liang et al., 2000;Michel et al., 2006;Kim et al., 2006), these data support the conclusion that ethanol blocks glutamate-induced phase delays by inhibiting BDNF-TrkB signaling.
Figure 4.
Summary of phase-shifting experiments with glutamate applied alone or with ethanol at ZT 16 with or without BDNF. Glutamate(1mM)-induced phase delays (mean ± SEM) are inhibited by 20 mM ethanol and this effect is completely reversed by co-treatment with 100ng/ml BDNF. BDNF treatment did not alter glutamate-induced phase delays when the two are applied together, and did not induce a phase shift when applied alone. *p<0.01 vs. control experiments
Ethanol inhibition of glutamate-induced phase advances is inhibited by BDNF
Next we investigated the effects of acute ethanol treatment on glutamate-induced phase advances. Glutamate (1mM) applied for 10 min at ZT 23 induced robust phase advances of about 3 h (Fig. 5), consistent with previously reported results in rats (Ding et al., 1994;Ding et al., 1997;Ding et al., 1998). As seen with glutamate-induced phase delays, co-application of 20 mM ethanol completely blocked glutamate-induced phase advances, while having no effect on the phase of neuronal activity when applied alone. To explore whether the mechanisms of ethanol actions at ZT 23 are consistent with those at ZT 16, we investigated whether BDNF prevents ethanol inhibition of glutamate-induced phase advances. BDNF had no effect on the phase of neuronal activity when it was applied alone or in combination with glutamate at ZT 23. However, BDNF completely prevented ethanol inhibition of glutamate-induced phase advances (Fig. 5).
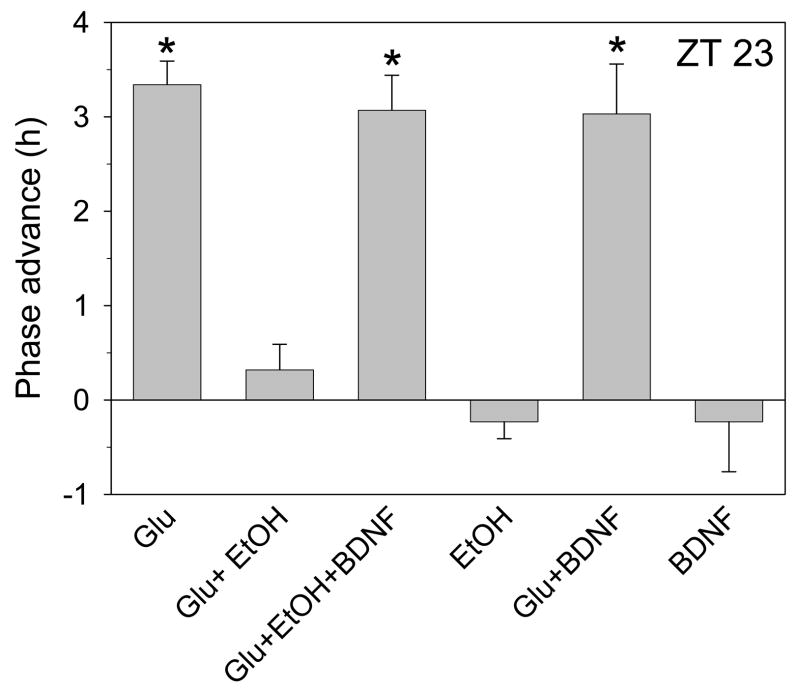
Figure 5.
Summary of phase-shifting experiments with glutamate applied alone or with ethanol at ZT 23 with or without BDNF. Glutamate (1mM) advances the neuronal activity rhythm by about 3.5 h when applied alone at ZT 23, and this is completely blocked by co-treatment with 20mM ethanol. Pre-treatment with 100ng/ml BDNF reverses the ethanol inhibition. Neither ethanol nor BDNF treatment affected the phase of neuronal activity when applied alone, and BDNF did not alter glutamate-induced phase advances when the two were applied together. *p<0.01 vs. control experiments.
Ethanol inhibition of daytime glutamate-induced phase advances
Light pulses normally do not shift the circadian clock when presented during the subjective day when BDNF levels are low. However, infusion of BDNF into the SCN allows expression of daytime light-induced phase advances (Liang et al., 2000). We therefore investigated whether similar daytime phase shifts can be generated by glutamate treatment in vitro and if they are inhibited by ethanol. As shown in Fig. 6, treatment of SCN brain slices at ZT 6 by glutamate alone has no effect, but glutamate co-applied with BDNF induces a mean phase shift of 3.32 ± 0.27 h, n=3 (p<0.01 vs control). This phase advance is prevented by co-application of 20 mM ethanol. Conversely, BDNF and ethanol applied alone or together had no effect, and glutamate applied with ethanol had no effect on the phase of neuronal activity. Together with the previous experiments, these data are consistent with ethanol inhibiting glutamate-induced phase shifts by inhibiting BDNF signaling in the SCN.
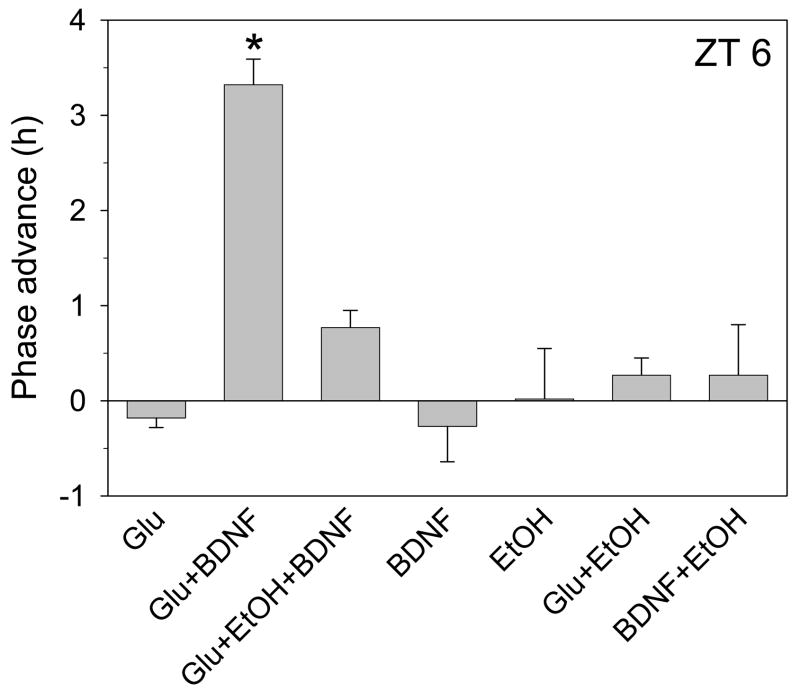
Figure 6.
Summary of phase-shifting experiments with glutamate, BDNF and/or ethanol applied at ZT 6. Glutamate (1mM) advances the neuronal activity rhythm by about 3.5 h when applied with 100 ng/ml BDNF at ZT 6, but not when applied alone. This effect is completely blocked by co-treatment with 20mM ethanol. Neither ethanol nor BDNF treatment affected the phase of neuronal activity when applied alone or together, and ethanol + glutamate treatment also did not induce a phase shift when applied together. *p<0.01 vs. control experiments.
Ethanol dose-dependently enhances daytime serotonergic phase shifting
Next we investigated whether ethanol affects daytime serotonergic phase shifting. We previously have shown that application of the 5-HT agonist, DPAT, at ZT 6 to mouse SCN brain slices dose-dependently phase-advances the circadian clock, with maximum phase shifts of about 3 h in response to 10 uM DPAT (Prosser, 2003;Prosser et al., 2006). Therefore, this dosage was used in the present experiments. While application of ethanol alone at ZT 6 had no effect on the phase of neuronal activity, co-application of ethanol (100mM) with DPAT increased the size of the DPAT-induced phase advances by about 50% (4.6 ±0.14 h, n=4 for DPAT + ethanol vs. 3.05 ±0.2h, n=5 for DPAT alone; p<0.01; of Fig. 7A). This effect of ethanol was dose-dependent, as shown in Fig 7B, with an ED50 19.84 mM.
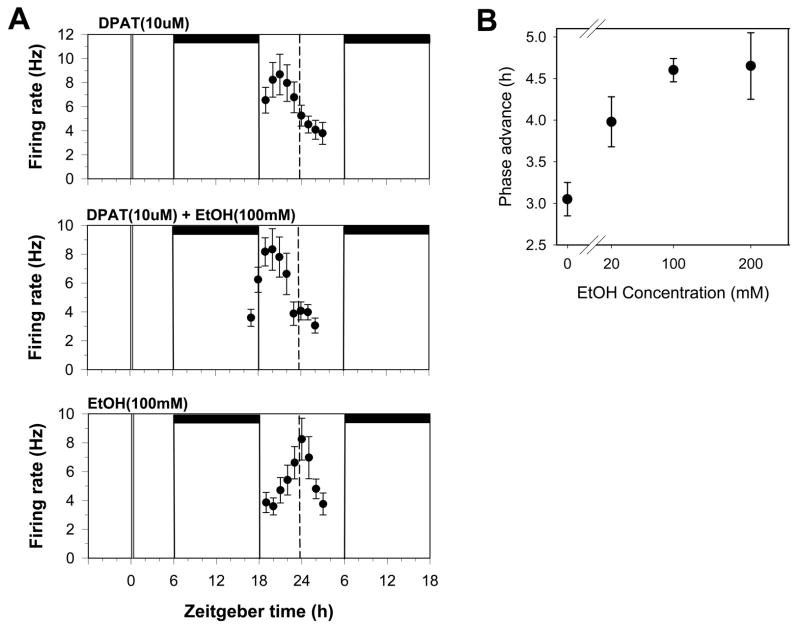
Figure 7.
DPAT-induced phase advances are enhanced by ethanol. A. Shown are the 2 h means ± SEM of SCN neuronal activity obtained in individual experiments. Neuronal activity peaks approximately 3 h early after treatment with DPAT (10uM) applied alone at ZT 6, indicating the SCN clock has been phase-advanced by 3 h. Treating the slices with DPAT + ethanol at ZT 6 induced a larger phase advance of about 4.5 h. Ethanol treatment alone had no effect. B. Dose response curve for ethanol enhancement of DPAT-induced phase shifts. A maximal enhancement of about 1.5 h is produced by 100mM ethanol, while 20mM ethanol induced a partial enhancement. See Fig. 1 for details.
Glutamatergic antagonists also enhance serotonergic phase shifts
To determine if the enhancing effect of ethanol on serotonergic phase shifts is due to blocking glutamate signaling, we tested whether glutamate receptor antagonists (AP5 and NBQX) have a similar effect to that of ethanol. As seen in Fig. 8A, co-application of AP5 with DPAT at ZT 6 enhanced the size of the phase advance to a similar degree to that seen with ethanol (mean phase shift = 4.4 ± 0.0 h, n=3; p<0.05). Again, this effect wasdose-dependent, with an ED50 of 0.75 uM (Fig. 8B). Co-application of NBQX also enhanced DPAT-induced phase advances (mean phase shift = 4.15 ±0.4, n=2; p<0.05; Fig. 8A). Neither AP5 nor NBQX had any effect when applied alone.
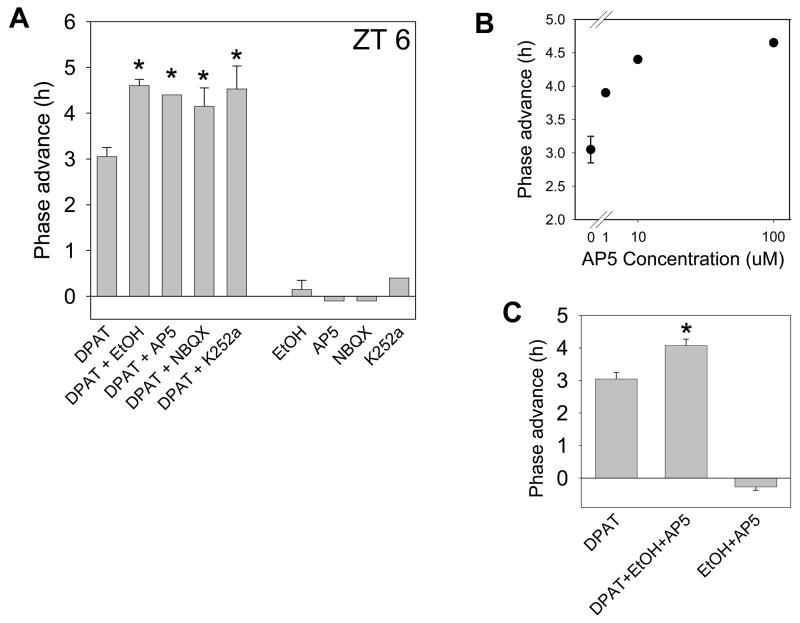
Figure 8.
Summary of phase-shifting experiments with DPAT, DPAT with various co-treatments, or the co-treatments applied alone. A. Mean (± SEM) phase advances induced by DPAT applied at ZT 6 are significantly enhanced to similar degrees by different co-treatments that all inhibit glutamate signaling, while the co-treatments alone had no effect. Drug concentrations: DPAT, 10uM; ethanol, 100mM; AP5, 10uM; NBQX, 10uM; K252a, 1uM. B. Dose response curve for AP5 enhancement of DPAT-induced phase shifts. AP5 induces a maximal enhancement of about 1.5 h. C. Enhancement of DPAT-induced phase advances by a combination of ethanol + AP5 is comparable to that seen when each treatment is applied alone with DPAT. Thus, there appears to be no additivity between these two treatments. *p<0.05 vs. DPAT alone.
Blocking TrkB receptor activation enhances serotonergic phase shifting
As the inhibitory effects of acute ethanol on glutamate-induced phase shifts involve blocking BDNF signaling, we next investigated whether blocking TrkB receptor activation with K252a mimics the effects on ethanol on DPAT-induced phase shifts. While K252a application alone at ZT 6 had no effect, co-application of K252a with DPAT enhanced serotonergic phase advances to a similar extent as seen with ethanol (mean phase shift = 4.53 ± 0.5 h, n=2; p<0.05; Fig. 8A). Thus, these data support the conclusion that acute ethanol enhances serotonergic phase shifts in vitro by blocking the actions of glutamate, and that this inhibition could involve inhibition of BDNF-TrkB signaling.
Ethanol and glutamate signaling blockers enhance serotonergic phase shifting in a non-additive manner
It is notable that ethanol and the other agents that inhibit glutamate signaling all enhanced DPAT-induced phase shifts to a similar degree. If they act through different mechanisms, then a combination of two such compounds should have an additive effect when applied together. Therefore, we co-applied 100mM ethanol and 10uM AP5 with DPAT. As seen in Fig. 8C, these treatments induced a mean phase advance of 4.07 ± 0.2h (n=3), similar to the enhancement induced by either AP5 or ethanol when individually co-applied with DPAT. Thus, ethanol and AP5 likely enhance DPAT-induced phase shifts through affecting the same signaling pathway, namely blocking glutamatergic signaling.
Blocking nitric oxide generation enhances serotonergic phase shifting
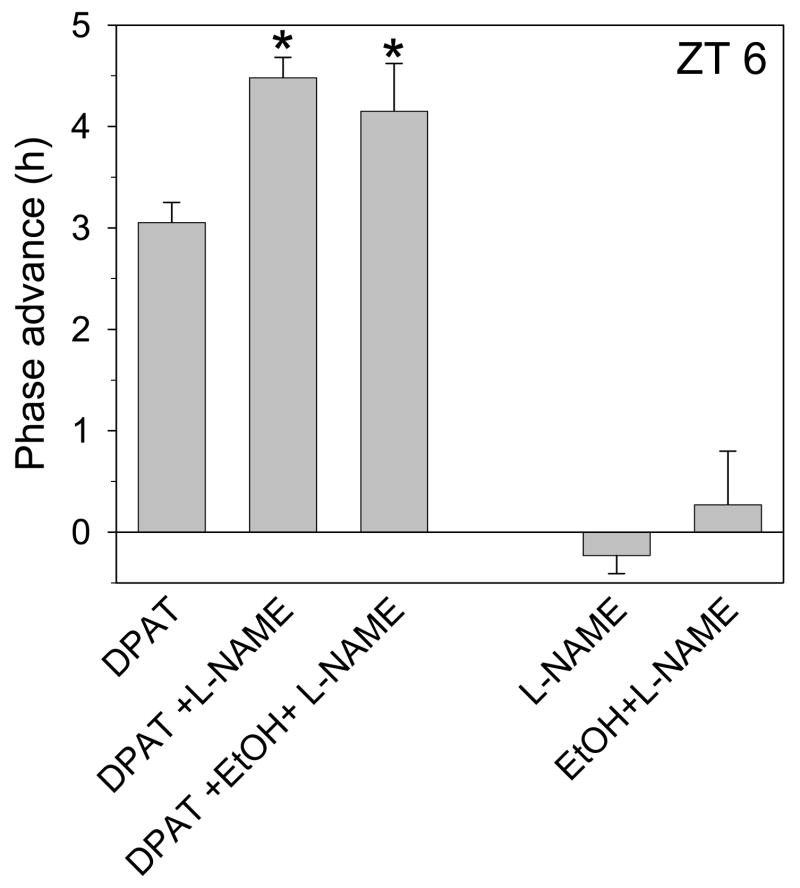
In these experiments we investigated whether inhibition of DPAT-induced phase shifts involves processes downstream from glutamate receptor activation. Previous work has shown that glutamate-induced phase shifts require generation of nitric oxide (NO) (Ding et al., 1994;Ding et al., 1997), and in vitro application of glutamate to SCN slices induces NO production that is inhibited by AP5, K252a and the nitric oxide synthase (NOS) antagonist, L-NAME (R.A. Prosser and Y. Lee, unpublished results). Here we tested whether DPAT-induced phase shifts are enhanced by blocking NOS activity with L-NAME. As shown in Fig. 9, co-application of L-NAME and DPAT induced phase shifts comparable to those induced by DPAT + ethanol (mean phase shift = 4.48 ± 0.2, n=2; p<0.05), while L-NAME application alone had no effect. Likewise, co-treating slices with DPAT, ethanol and L-NAME induced a phase advance of 4.15 ±0.47 (n=3; Fig 8), which is no larger than that induced by DPAT co-applied with either ethanol or L-NAME alone. Thus, the inhibitory actions of glutamate on serotonergic signaling in the SCN occur, at least in part, downstream from NO generation.
Figure 9.
Summary of phase-shifting experiments with 10uM DPAT, 100uM L-NAME and/or 100mM ethanol. DPAT-induced phase advances are enhanced by L-NAME, and this effect is not further enhanced by addition of ethanol. Neither L-NAME nor L-NAME+ethanol induced significant changes in phase when applied alone. *p<0.05 vs. DPAT alone.
DISCUSSION
Ethanol blocks glutamate-induced phase shifts
These experiments demonstrate for the first time that acute ethanol inhibits glutamate signaling in the SCN circadian clock, the primary mechanism through which circadian rhythms are synchronized to the external environment. Previous work has focused solely on the effects of chronic ethanol administration on circadian rhythms and has not addressed either the location or mechanism of ethanol action. Thus, these results significantly advance our understanding of how ethanol affects the circadian system. Importantly, the 20 mM ethanol used in these experiments corresponds to a blood alcohol content of 0.1%, suggesting that transient increases in ethanol produced by consuming just a few alcoholic beverages could lead to impaired clock synchronization. Still to be determined, however, is how blood alcohol content corresponds to ethanol concentration in the SCN.
Little is known about how ethanol affects circadian rhythms, and the results in the literature are somewhat inconsistent. For example, chronic ethanol in hamsters apparently does not alter circadian clock re-entrainment (Mistlberger and Nadeau, 1992), while chronic ethanol can inhibit light-induced phase advances and (daytime) triazolam-induced phase advances in hamsters, but not light-induced phase delays (Seggio et al., 2007). Rosenwasser, et al. (2005a,b) also showed that chronic ethanol affects circadian free-running periods in rats but not light-induced phase shifts. None of these studies investigated the acute effects of ethanol or whether ethanol acts directly in the SCN. Since glutamate signaling mechanisms show compensation in response to chronic ethanol (Krystal et al., 2003;Chandler, 2003), a lack of effect of chronic ethanol on photic resetting or entrainment could be due to compensatory changes occurring within the SCN or elsewhere.
On the other hand, the differences between our results and those of some previous studies could also be due to our use of an in vitro preparation. To test this possibility, we recently investigated the in vivo effects of acute ethanol on photic phase-shifting in hamsters. Our preliminary results indicate that acute late-night intra-peritoneal injections of ethanol dose-dependently inhibit light-induced phase advances in hamsters (Ruby et al., 2006). Thus, acute ethanol can inhibit photic phase shifts in vivo in hamsters, consistent with our in vitro results in mice.
Ethanol does not act by blocking glutamate or co-agonist binding
The dose dependent effects of ethanol could be mediated through competitive inhibition of glutamate or its co-agonist binding to its receptor. However, the fact that ethanol continues to fully block glutamate-induced phase shifts in the presence of excess glutamate suggests that ethanol does not inhibit glutamate binding to NMDA receptors, consistent with previous studies (Hoffman, 2003). Additionally, neither excess glycine nor excess D-serine were able to overcome the inhibitory effects of ethanol. Previous studies have provided mixed results with respect to whether ethanol interferes with glycine or D-serine binding to NMDA receptors (Gonzales and Woodward, 1990;Woodward and Gonzales, 1990;Rabe and Tabakoff, 1990;Wright et al., 1996). The results here suggest that ethanol does not affect co-agonist binding to NMDA receptors in the SCN.
Ethanol affects BDNF signaling in the SCN
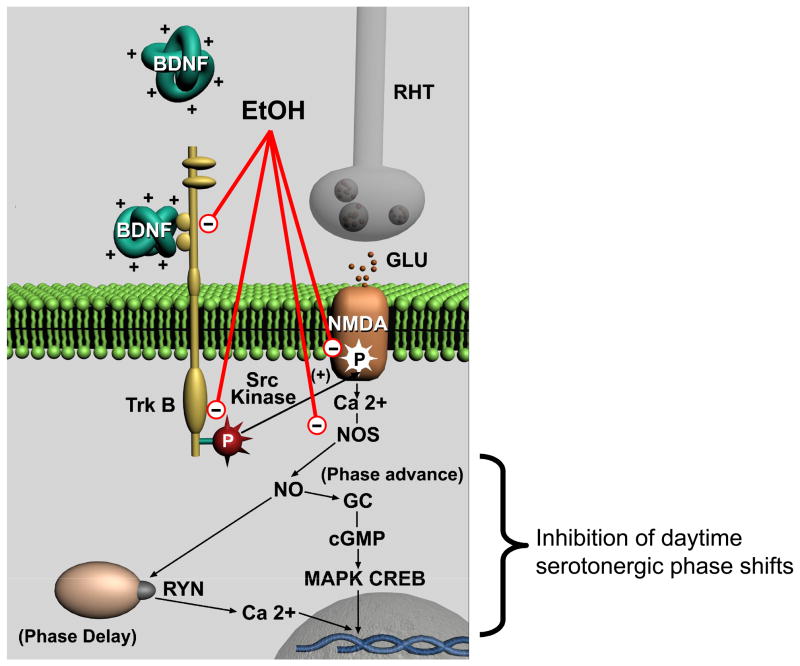
BDNF pre-treatment prevented ethanol inhibition of both glutamate-induced phase delays and advances, suggesting that ethanol inhibits BDNF signaling in the SCN. Such an action by ethanol would be consistent with previous work showing that acute ethanol blocks BDNF enhancement of NMDA currents in the hippocampus (Kolb et al., 2005). Interestingly, infusions of BDNF antisense oligonucleotides into the amygdala increased anxiety-like behaviors and alcohol consumption (Pandey et al., 2006), consistent with ethanol inhibiting BDNF signaling in the amygdala. Another line of research suggests that increases in BDNF transcription in the hippocampus are associated with increased ethanol consumption, and that inhibiting this increase in BDNF leads to decreased ethanol consumption (McGough et al., 2004). BDNF signaling via TrkB receptors enhances NMDA receptor-generated ion currents in brain regions including the hippocampus (Levine et al., 1998;Kovalchuk et al., 2002;Kolb et al., 2005) and the SCN (Kim et al., 2006;Michel et al., 2006). This enhancement may involve activation of src-like kinases, which phosphorylate residues on NMDA receptor subunits (Xu et al., 2006). BDNF production in the SCN exhibits a circadian rhythm with high levels at night, and is required for nocturnal light- and glutamate-induced phase shifts (Liang et al., 2000;Allen et al., 2005;Prosser et al., 2004;Michel et al., 2006). The importance of BDNF for glutamate signaling in the SCN is further supported by results showing that addition of exogenous BDNF allows light (Liang et al., 2000) and glutamate (present results) to induce phase advances in the subjective day. The fact that ethanol inhibits these daytime glutamate-induced phase shifts further supports our overall conclusion that ethanol inhibits glutamate actions in the SCN by blocking BDNF-TrkB signaling. A model illustrating the cellular mechanisms involved in glutamatergic phase shifts and how ethanol might act to inhibit these phase shifts is shown in Fig. 10.
Figure 10.
Model for BDNF enhancement of glutamate-induced phase shifting in the SCN, and potential locations in the signaling pathway where ethanol could inhibit this process. Glutamate released from the retinohypothalamic nerve terminals (RHT) activates post-synaptic NMDA receptors, induces an increase in intracellular Ca2+, and activates NOS to produce NO. Subsequent downstream processes lead to phase delays or phase advances, depending on the time of stimulation (early or late night, respectively) by altering transcription of circadian clock genes. Full activation of these processes requires phosphorylation of the NMDA receptors through BDNF/TrkB receptor activation of a Src family kinase. Ethanol could inhibit BDNF/TrkB enhancement of glutamate signaling through several different mechanisms. Glutamate inhibition of serotonergic phase shifting during the subjective day involves processes downstream from NO generation. GC: guanylate cyclase, cGMP: cyclic GMP; MAPK: mitogen-activated kinase; CREB: cyclic AMP-dependent response element binding protein; Ryn: ryanodine receptor.
Another possibility is that ethanol blocks glutamate-induced phase shifts by enhancing GABA activity in the SCN (Weiner and Valenzuela, 2006). GABA is known to block photic phase shifts (Ralph and Menaker, 1989;Mintz et al., 2002). However, ethanol was shown to not enhance GABA currents in SCN neurons (Kawahara et al., 1993), and it is not clear how excess BDNF would be able to reverse GABAergic inhibition of glutamate phase shifts, as seen here. Thus, the data are more consistent with ethanol acting on glutamate signaling.
Acute ethanol enhances non-photic phase shifts
In contrast to its inhibitory effects on glutamate-induced phase shifts, ethanol dose-dependently enhances daytime serotonergic phase advances. One potential mechanism through which ethanol could enhance serotonergic phase shifts would be to increase serotonin availability, an effect seen in other studies (Hayashi et al., 2003;Thielen et al., 2002;Daws et al., 2006). However, our previous data indicate that the concentration of DPAT used in these experiments is saturating with respect to inducing phase advances (Prosser, 2003). Importantly, DPAT activates all the serotonin receptor subtypes currently thought to participate in daytime serotonergic phase shifts (Lovenberg et al., 1993;Sprouse et al., 2004b;Sprouse et al., 2005;Sprouse et al., 2004a). Therefore, it is unlikely that the enhancing effect of ethanol is due to an increase in serotonergic tonus.
Ethanol enhancement of serotonergic phase shifts could occur through inhibiting glutamate signaling
It is well established that light and glutamate agonists inhibit non-photic phase shifts (Mrosovsky, 1991;Grossman et al., 2000;Hall et al., 1999;Gamble et al., 2004;Biello et al., 1997;Prosser, 2001). Further, we have shown that non-photic phase shifts, including in vitro serotonergic phase shifts, are enhanced by a treatment (enzymatic removal of polysialic acid) that inhibits glutamate signaling in the SCN (Fedorkova et al., 2002). Given the strong inhibitory effect of acute ethanol on glutamatergic signaling (Ron, 2004;Chandler, 2003;Woodward, 2000;Krystal et al., 2003) including in the SCN (Ruby et al., 2006; data presented here), we hypothesize that ethanol enhances serotonergic phase shifts through inhibiting glutamate signaling. To test this, we co-applied DPAT with a variety of agents that inhibit photic/glutamate signaling in the SCN, including AP5, NBQX, K252a and L-NAME (Rea et al., 1993;Ding et al., 1994;Mintz et al., 1999;Schurov et al., 1999;Michel et al., 2006;Kallingal and Mintz, 2006). In each case, the antagonist treatment enhanced DPAT-induced phase advances by about 50%. Notably, we found further that co-application of either AP5 or L-NAME with ethanol did not produce an additive stimulation of DPAT-induced phase shifts. Serotonergic treatments can generate significantly larger phase shifts than those seen here (Knoch et al., 2004;Medanic and Gillette, 1992), so it is therefore unlikely that the lack of additivity seen in these experiments is attributable to a ceiling effect. Thus, these data are consistent with the conclusion that ethanol inhibits glutamate signaling in the SCN, and this inhibition produces the enhancement of serotonergic phase shifts.
Glutamate inhibition of serotonergic phase shifts may occur downstream from nitric oxide generation
We also assessed the mechanism of interaction between glutamate and serotonergic phase resetting. The interaction could occur proximally or distally to glutamate receptor activation. For example, glutamate-induced changes in intracellular Ca2+ can suppress serotonin receptor responsiveness (Bockaert et al., 2006). Conversely, the interaction could occur further downstream from glutamate receptor activation. To explore this latter possibility, we determined whether blocking NOS activity enhances serotonergic phase shifts. Blocking NOS activity with L-NAME inhibits glutamate- and light-induced phase shifts (Ding et al., 1994;Ferreyra et al., 1998), while application of NOS donors at night induces photic-like phase shifts (Ding et al., 1994). Furthermore, we have shown that glutamate induces NO generation in the SCN in vitro, and this induction is inhibited by ethanol, AP5, K252a and L-NAME (Prosser and Lee, unpublished results). Our data showing that co-application of L-NAME with DPAT again enhanced DPAT-induced phase shifts by about 50% supports the theory that glutamate inhibits serotonergic phase shifts through process(es) downstream, rather than upstream, of NOS activation (see Fig. 10). Additional research directed at the specific mechanisms of the glutamate/serotonin interaction is needed in order to expand on this portion of the model.
Acute ethanol disruption of circadian clock synchronization
Although acute ethanol is widely known to disrupt sleep processes, its effects on circadian rhythms have received little attention. Our data show that low concentrations of ethanol can affect both daytime and nighttime phase shifting processes in mice in vitro, and suggest that both effects involve inhibiting glutamate signaling processes. Ethanol inhibition of in vitro glutamate-induced phase shifts is also consistent with our preliminary data demonstrating inhibition of photic phase shifts in hamsters in vivo, showing the potential for ethanol to suppress normal circadian rhythm entrainment to light-dark cycles. This disruption of normal rhythm synchronization is likely to be exacerbated by the enhanced daytime serotonergic phase shifting by ethanol. Non-photic phase shifts may normally be under tonic suppression through glutamatergic inhibition (Fedorkova et al., 2002), potentially an important mechanism reinforcing proper entrainment. For example, it could suppress inappropriate clock resetting in response to brief daytime increases in arousal or activity (in nocturnal animals). Combining ethanol-induced suppression of nocturnal phase-shifting signals with a potentiation of daytime resetting could lead to more erratic circadian entrainment. In humans this problem might manifest itself as irregular rhythmicity in sleep and other behaviors.
In summary, we show here that acute treatment with low levels of ethanol inhibits glutamate-induced phase shifts and enhances serotonergic phase shifts of the SCN circadian clock in vitro. The data support the conclusion that ethanol blocks BDNF-TrkB enhancement of glutamate signaling in the SCN, which in turn dis-inhibits serotonergic signaling in the SCN. Together, these results suggest that acute exposure to the amount of ethanol consumed in 3-4 alcoholic beverages could significantly impair the normal entrainment processes of the circadian clock, which could lead to poorly synchronized behavioral and physiological rhythms.
Acknowledgments
We gratefully acknowledge the advice of Dr. Kimberly Nixon. This research was supported by National Institute of Health grant AA015948 and the University of Tennessee.
Abbreviations
- AP5
aminophosphovaneric acid
- BDNF
brain-derived neurotrophic factor
- DPAT
(+)8-hydroxy-2-(di-N-propylamino)tetralin
- EBSS
Earle’s Balanced Salt Solution
- 5-HT
serotonin
- L-NAME
NG -nitro-L-arginine methyl ester hydrochloride
- NBQX
1,2,3,4-tetrahydro-4-nitro-2,3-dixo-benzo(f) quinoxaline-7-sulfonamide
- NO
nitric oxide
- NOS
nitric oxide synthase
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abe H, Rusak B, Robertson HA. NMDA and non-NMDA receptor antagonists inhibit photic induction of fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull. 1992;28:831–835. doi: 10.1016/0361-9230(92)90269-4. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Allen GC, Qu X, Earnest DJ. TrkB-deficient mice show diminished phase shifts of the circadian activity rhythm in response to light. Neurosci Lett. 2005;378:150–155. doi: 10.1016/j.neulet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other’s phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience. 1997;77:1049–1057. doi: 10.1016/s0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tiss Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcholism. Am J Psychiat. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmac Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Fedorkova L, Rutishauser U, Prosser RA, Shen H, Glass JD. Removal of polysialic acid from the SCN potentiates nonphotic circadian phase resetting. Physiol Behav. 2002;77:361–369. doi: 10.1016/s0031-9384(02)00880-6. [DOI] [PubMed] [Google Scholar]
- Ferreyra GA, Cammarota MP, Golombek DA. Photic control of nitric oxide synathase activity in the hamster suprachiasmatic nuclei. Brain Res. 1998 doi: 10.1016/s0006-8993(98)00376-x. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Novak CM, Albers HE. Neuropeptide Y and N-methyl-D-aspartic acid interact within the suprachiasmatic nuclei to alter circadian phase. Neuroscience. 2004;126:559–565. doi: 10.1016/j.neuroscience.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. NeuroReport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Woodward JJ. Ethanol inhibits N-methyl-D-aspartate-stimulated [H3]norepinephrine release from rat cortical slices. J Pharm Exp Ther. 1990;253:1138–1144. [PubMed] [Google Scholar]
- Grossman GH, Mistlberger RE, Antle MC, Ehlen JC, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport. 2000;11:1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- Hall AC, Earle-Cruikshanks G, Harrington ME. Role of membrane conductances and protein synthesis in subjective day phase advances of the hamster circadian clock by neuropeptide Y. Eur J Neurosci. 1999;11:3424–3432. doi: 10.1046/j.1460-9568.1999.00761.x. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Doran AD, Salm AK, Tweedle CD. Brain slice preparation: Hypothalamus. Brain Res Bull. 1980;5:405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nakai T, Bandoh T, Hoshi K. Acute effect of simultaneous administration of tryptophan and ethanol on serotonin metabolites in the locus coeruleus in rats. Eur J Pharmacol. 2003;462:61–66. doi: 10.1016/s0014-2999(03)01318-9. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. NMDA receptors in alcoholism. Int Rev Neurobiol. 2003;56:35–82. doi: 10.1016/s0074-7742(03)56002-0. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Shibata S. Phase-resetting response to (+)8-OH-DPAT, a serotonin 1A/7 receptor agonist, in the mouse in vivo. Neurosci Lett. 2004;368:130–134. doi: 10.1016/j.neulet.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Ito C, Wakamori M, Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991;260:C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Glutamatergic activity modulates the phase-shifting effects of gastrin-releasing peptide and light. Eur J Neurosci. 2006;24:2853–2858. doi: 10.1111/j.1460-9568.2006.05165.x. [DOI] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Gastrin releasing peptide and neuropeptide Y exert opposing actions on circadian phase. Neurosci Lett. 2007;422:59–63. doi: 10.1016/j.neulet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara F, Saito H, Katsuki H. Pharmacological characteristics of GABAA responses in postnatal suprachiasmatic neurons in culture. Neurosci Lett. 1993;160:45–48. doi: 10.1016/0304-3940(93)90913-6. [DOI] [PubMed] [Google Scholar]
- Kim YI, Choi H-J, Colwell CS. Brain-derived neurotrophic factor regulation of N-methyl-D-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons. J Neurosci Res. 2006;84:1512–1520. doi: 10.1002/jnr.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch ME, Gobes SMH, Pavlovska I, Su C, Mistlberger RE, Glass JD. Short-term exposure to constant light promotes strong circadian phase-resetting responses to nonphotic stimuli in Syrian hamsters. Eur J Neurosci. 2004;19:2779–2790. doi: 10.1111/j.0953-816X.2004.03371.x. [DOI] [PubMed] [Google Scholar]
- Kolb JE, Trettel J, Levine ES. BDNF enhancement of postsynaptic NMDA receptors is blocked by ethanol. Synapse. 2005;55:52–57. doi: 10.1002/syn.20090. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D-Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmac Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Expt Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Lall GS, Harrington ME. Potentiation of the resetting effects of light on circadian rhythms of hamsters using serotonin and neuropeptide Y receptor antagonists. Neuroscience. 2006;141:1545–1552. doi: 10.1016/j.neuroscience.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Landolt H-P, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Landolt H-P, Roth C, Dijk D-J, Borbely AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. J Clin Psychopharm. 1996;16:428–436. doi: 10.1097/00004714-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F-Q, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20:2978–2987. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F-Q, Sohrabji F, Miranda R, Earnest B, Earnest D. Expression of brain-derived neurotrophic factor and its cognate receptor, TrkB, in the rat suprachiasmatic nucleus. Exper Neurol. 1998a;151:184–193. doi: 10.1006/exnr.1998.6804. [DOI] [PubMed] [Google Scholar]
- Liang F-Q, Walline R, Earnest DJ. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998b;242:89–92. doi: 10.1016/s0304-3940(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylate cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J Neurosci. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NNH, He D-Y, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol. 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Clark JP, Ding JM, Colwell CS. Brain-derived neurotrophic factor and neurotrophin receptors modulate glutamate-induced phase shifts of the suprachiasmatic nucleus. Eur J Neurosci. 2006;24:1109–1116. doi: 10.1111/j.1460-9568.2006.04972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res. 1997;758:245–249. doi: 10.1016/s0006-8993(97)00022-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Jasnow AM, Gillespie CF, Huhman KL, Albers HE. GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience. 2002;109:773–778. doi: 10.1016/s0306-4522(01)00519-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Beh. 1992;43:159–165. doi: 10.1016/0091-3057(92)90652-v. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Phase response curves for social entrainment. J Comp Physiol. 1988;162:35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Double-pulse experiments with nonphotic and photic phase-shifting stimuli. J Biol Rhythms. 1991;6:167–179. doi: 10.1177/074873049100600207. [DOI] [PubMed] [Google Scholar]
- Muscat L, Tischler RC, Morin LP. Functional analysis of the role of the median raphe as a regulator of hamster circadian system sensitivity to light. Brain Res. 2005;1044:59–66. doi: 10.1016/j.brainres.2005.02.083. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 1998;808:31–41. doi: 10.1016/s0006-8993(98)00808-7. [DOI] [PubMed] [Google Scholar]
- Prosser RA. Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors. J Neurosci. 2001;21 :7815–7822. doi: 10.1523/JNEUROSCI.21-19-07815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Serotonin phase-shifts the mouse suprachiasmatic circadian clock in vitro. Brain Res. 2003;966:110–115. doi: 10.1016/s0006-8993(02)04206-3. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system: I. In vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Huang Z, Glass JD. Polysialylated neural cell adhesion molecule is necessary for BDNF-potentiated glutamatergic phase shifting of the SCN circadian clock during the day. Soc Neurosci. 2004 Abst #428.10. [Google Scholar]
- Prosser RA, Lee H-M, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience. 2006;142:547–555. doi: 10.1016/j.neuroscience.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Rabe CS, Tabakoff B. Glycine site-directed agonists reverse the actions of ethanol at the N-methyl-D-aspartate receptor. Molec Pharmacol. 1990;38:753–757. [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–359. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- Rea MA, Buckley B, Lutton LM. Local administration of EAA antagonists block light-induced phase shifts and c-fos expression in hamster SCN. Am J Physiol. 1993;265:R1191–R1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Lavery RJ, Mrosovsky N. Running activity mediates the phase-advancing effects of dark pulses on hamster circadian rhythms. J Comp Physiol. 1989;165:811–818. doi: 10.1007/BF00610879. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Zee PC. Circadian rhythm disorders. Sem Neurology. 2004;24:315–325. doi: 10.1055/s-2004-835063. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdata: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. The Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005a;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Int. 2005b;22:227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, Glass JD. Ethanol attenuates photic phase-resetting in Syrian hamsters in vivo. Soc Neurosci Abst. 2006:156.5. [Google Scholar]
- Schurov IL, McNulty S, Best JD, Sloper PJ, Hastings MH. Glutamatergic induction of CREB phosphorylation of fos expression in primary cultures of the suprachiasmatic hypothalamus in vitro is mediated by co-ordinate activity of NMDA and non-NMDA receptors. J Neuroendocrin. 1999;11:43–51. doi: 10.1046/j.1365-2826.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Beh. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CM, Biello SM. WAY-100635, a specific 5-HT1A antagonist, can increase the responsiveness of the mammalian circadian pacemaker to photic stimuli. Neurosci Lett. 2001;305:33–36. doi: 10.1016/s0304-3940(01)01797-9. [DOI] [PubMed] [Google Scholar]
- Smith BN, Sollars PJ, Dudek FE, Pickard GE. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J Biol Rhythms. 2001;16:25–38. doi: 10.1177/074873040101600104. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Siegmund S, Cowen M, Schroff K-C, Schumann G, Fiserova M, Sillaber I, Wellek S, Singer M, Putzke J. The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol. J Neurosci. 2002;22:8676–8683. doi: 10.1523/JNEUROSCI.22-19-08676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Li X, Stock J, McNeish J, Reynolds L. Circadian rhythm phenotype of 5-HT7 receptor knockout mice: 5-HT and 8-OH-DPAT-induced phase advances of SCN neuronal firing. J Biol Rhythms. 2005;20:122–131. doi: 10.1177/0748730404273432. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Braselton J, Schmidt A. Serotonin-induced phase advances of SCN neuronal firing in vitro: a possible role for 5-HT5A receptors? Synapse. 2004a;54:111–118. doi: 10.1002/syn.20070. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004b;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Bare DJ, McBride WJ, Lumeng L, Li T-K. Ethanol-stimulated serotonin release in the ventral hippocampus: an absence of rapid tolerance for the alcohol-preferring P rat and insensitivity in the alcohol-nonpreferring NP rat. Pharmacol Biochem Beh. 2002;71:111–117. doi: 10.1016/s0091-3057(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Shibata S, Ueki S, Watanabe S. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Eur J Pharmacol. 1992;214:79–84. doi: 10.1016/0014-2999(92)90099-p. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmac Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn-Schmiedeberg’s Arch Pharm. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Gonzales RA. Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: reveral by glycine. J Neurochem. 1990;54:712–715. doi: 10.1111/j.1471-4159.1990.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Weight FF. Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res. 1996;738:249–256. doi: 10.1016/s0006-8993(96)00780-9. [DOI] [PubMed] [Google Scholar]
- Xu F, Plummer MR, Len G-W, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- Yannielli P, Harrington ME. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog Neurobiol. 2004;74:59–76. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]