Abstract
The dog serves as a large animal model for multiple neurologic diseases that may potentially benefit from neural progenitor cell (NPC) transplantation. In the adult brain, multipotent NPCs reside in the subventricular zone and its rostral and caudal extensions into the olfactory bulb and hippocampus. The olfactory bulb represents a surgically accessible site for obtaining cells for autologous NPC transplantation. To model conditions that would occur for ex vivo gene therapy in the postnatal brain, NPCs were isolated from the canine olfactory bulb, expanded ex vivo under different culture conditions, and compared quantitatively for growth and immunophenotype. Under standard growth conditions, canine olfactory bulb-derived NPCs (OB-cNPCs) could be expanded nearly 500-fold in the time evaluated. Canine OB-cNPCs grown on poly-D-lysine (PDL) or on PDL-fibronectin had similar growth rates, whereas supplementation with leukemia inhibitory factor (LIF) resulted in significantly slower growth. However, when OB-cNPC cultures were grown on PDL-fibronectin or PDL supplemented with LIF, a greater proportion of cells with neuronal markers were generated upon differentiation.
Keywords: Canine, olfactory bulb, neural progenitor cells, fibronectin, leukemia inhibitory factor, cell culture
Introduction
Neural stem cells (NSCs) have been isolated from the adult rodent brain by selective response to epidermal growth factor (EGF) and/or basic fibroblast growth factor (bFGF) (Reynolds and Weiss, 1992; Richards et al., 1992; Ray et al., 1993; Palmer et al., 1997). True NSCs are multipotential and self-renewing, whereas neural progenitor cells have more restricted renewal and differentiation ability (Seaberg and van der Kooy, 2003). The term ‘neural progenitor cell’ (NPC) will be used here to include both neural stem and progenitor cells. Neural progenitor cells are excellent candidates for cellular transplantation therapy because they have been shown to replace dead or dying neural tissue, elaborate trophic factors to rescue dysfunctional endogenous neurons, inhibit inflammation, and deliver therapeutic proteins in a widely disseminated manner (Snyder et al., 1995; Lacorazza et al., 1996; Ourednik et al., 2002; Einstein et al., 2003; Pluchino et al., 2003).
Olfactory bulb-derived NPCs are attractive as a source of NPCs for autologous transplantation after ex vivo gene correction because the olfactory bulbs are more easily accessed surgically than the subventricular zone (SVZ) or hippocampus. Cells with the stem cell properties of self-renewal and multipotency have been isolated from postnatal murine olfactory bulbs (Gritti et al., 2002; Liu and Martin, 2003). Similarly, neural stem cells isolated from adult human olfactory bulb biopsies contained self-renewing, multipotential neural stem cells as evidenced by clonal analysis (Pagano et al., 2000). Many neurologic diseases that may potentially benefit from NPC transplantation occur in the dog (Griffiths et al., 1981; Haskins et al., 1984; Alroy et al., 1985; Koppang, 1988; Fischer et al., 1998; Wenger et al., 1999). We recently found that defects in canine NPCs occur in a model of a lysosomal storage disease (Walton and Wolfe, 2007). With a brain that is much closer in size and architecture to the human brain, the dog represents a relevant model for NPC transplantation. Moreover, surgical approaches to the olfactory bulb of dogs have been developed, thus there is potential to perform ex vivo gene therapy experiments using autologous transplantation (Bagley et al., 1997; Glass et al., 2000).
Protocols used for human and rodent in vitro NPC cultures differ with respect to concentrations and combinations of growth factors and substrate (Reynolds and Weiss, 1992; Richards et al., 1992; Kilpatrick and Bartlett, 1993; Ray et al., 1993; Gage et al., 1995; Palmer et al., 1997; Moyer et al., 1997; Carpenter et al., 1999; Laywell et al., 2000). NPC response to growth factors can vary with developmental stage, the factors used, and their concentration (Whittemore et al., 1999; Zhu et al., 1999). For example, although NPCs grown in either EGF or bFGF with heparin are multipotential, studies show a preferential gliogenic effect from EGF-derived cultures and an increased capacity for neuronal differentiation in cultures derived from bFGF and heparin, (Whittemore et al., 1999; Zhu et al., 1999). Combining EGF with bFGF and heparin produces a synergistic effect on NPC cultures (Caldwell and Svendsen, 1998; Vescovi et al., 1999; Gritti et al., 1999; Caldwell et al., 2004). Supplementation of NPC medium with leukemia inhibitory factor (LIF) has been found to increase human embryonic NPC proliferation rate and longevity, and enhance ex vivo expansion (Carpenter et al., 1999; Wright et al., 2006). Culture conditions can also affect cell migration. Epidermal growth factor generated NPCs migrate further on a poly-D-lysine substrate (PDL) than bFGF generated NPCs (Zhu et al., 1999). Similarly, the substrate on which NPCs are grown can also affect cell growth and some functions; fibronectin and laminin enhance neurosphere motility and migration, whereas chondroitin sulfate proteoglycan inhibits sphere migration and velocity (Kearns et al., 2003). Lastly, ex vivo expansion of NPCs via epigenetic stimulation appears to differ between species. Human NPC cultures require both EGF and bFGF, and LIF enhances ex vivo expansion, whereas in rodents this is not the case (Reynolds and Weiss, 1992; Reynolds and Weiss, 1996; Carpenter et al., 1999; Tropepe et al., 1999; Vescovi et al., 1999).
In light of these findings, optimal growth conditions must be determined for NPCs according to developmental stage, species of origin, and intended use. Since treatment for most CNS diseases will be done postnatally, we were interested in modeling conditions that would occur in ex vivo gene therapy using autologous cells. To optimize culture conditions for ex vivo canine NPC expansion, we evaluated variables that have been shown to maintain neural progenitor cell qualities and/or increase NPC proliferation and expansion in vitro.
Materials and Methods
Experimental animals
Mixed breed dogs were raised in the Animal Models Core of the W. F. Goodman Center for Comparative Medical Genetics, University of Pennsylvania School of Veterinary Medicine. All dogs were treated in accordance with NIH and USDA guidelines for the use of animals in research and all experimental procedures involving dogs were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Isolation of canine olfactory bulb neural progenitor cells (OB-cNPCs)
Three dogs were humanely euthanized at three weeks of age (19–21 days), by intravenous injection of a barbituate solution. The brain was removed, placed into a balanced salt solution, and then dissected grossly. The olfactory bulbs were separated from the brain, minced and then digested in 0.25% trypsin (Worthington) in a 37°C water bath for 45 minutes to 1 hour. The enzymatic digestion was stopped with addition of fetal bovine serum (FBS; Hyclone). The tissue was then incubated with DNAse I (Sigma) for fifteen minutes in a 37°C water bath and triturated to a single cell suspension with successively smaller diameter pipettes, ending at a flame-polished Pasteur pipette. The cell suspension was centrifuged at 700 rpm at 4°C for 8 minutes, resuspended in 10% FBS plating medium (see below), and triturated. The total number of viable cells was determined by manual count on a hemacytometer; cell viability was assessed using trypan blue exclusion (0.4%; Sigma).
Canine OB-NPC culture and expansion
The OB-cNPCs were plated at a concentration of 4 × 104/cm2 in 10% serum-containing plating medium consisting of DMEM:F12 (1:1 ratio; GibcoBRL) supplemented with 10% FBS, 1% N2 supplement (GibcoBRL), 1% antibiotic-antimycotic (PSF)(100 U/mL penicillin; 100 μg/mL streptomycin; 0.25 μg/mL amphotericin B; GibcoBRL), and 1% L-glutamine (2mM; GibcoBRL). After 24–48 hours, the medium was changed to a serum-free feeding medium consisting of DMEM:F12 supplemented with 1% N2 supplement, 1% PSF, and 1% L-glutamine. The standard combination of growth factors consisted of 20 ng/mL epidermal growth factor (EGF) (recombinant murine; Roche), 20 ng/mL basic fibroblast growth factor (bFGF) (recombinant human; Promega), and heparin (5 μg/mL; Sigma). For a subset of cultures, 10 ng/mL leukemia inhibitory factor (LIF) (recombinant human; Chemicon) was added along with the standard growth factor combination. The cNPC cultures were maintained at 37°C in humidified 5% CO2 tissue culture incubators. Cultures were fed every 3–5 days by changing half of the medium and adding fresh growth factors. Canine NPCs were plated into 25 cm2 tissue culture flasks (Corning) coated with 10 μg/mL poly-D-lysine (Sigma) or poly-D-lysine with 5 μg/mL fibronectin (0.1% bovine; Sigma). Cultures were passaged at approximately 90% confluence by trypsinizing (0.05% trypsin-EDTA; GibcoBRL) and replating at a concentration of 4 × 104/cm2.
Neurosphere assay
For neurosphere assays, canine olfactory bulb-derived neurospheres were collected (passage 1), centrifuged at 700 rpm at 4°C for 5 minutes, trypsinized (0.05% trypsin-EDTA; GibcoBRL) at 37°C for 5 minutes, and triturated to a single cell suspension. The suspension was serially diluted to a final concentration of 0.04 cells/μl; 100 μl of OB-cNPC suspension was plated into each well of a 96-well plate (Corning) with 100 μl of filtered, conditioned OB-cNPC growth medium.
Immunocytochemistry
Cultures were differentiated by plating in the absence of growth factors in an 8-well chamber slide (Lab-Tek II; Nalge Nunc) at a concentration of 4 × 104 cells/well in feeding medium supplemented with 1% FBS. Undifferentiated OB-cNPCs were plated at a concentration of 2 × 104/well onto poly-D-lysine-coated 8-well glass slides (Cel-Line; Erie Scientific) and allowed to attach overnight in feeding medium containing growth factors. Differentiated and undifferentiated cNPC cultures were processed for immunocytochemistry after 10–12 days and 24 hours, respectively. In some cases, retinoic acid (1μM; Sigma) or platelet-derived growth factor (10 ng/mL; Sigma) was added to feeding medium in the absence of growth factors to promote differentiation.
The polyclonal primary antibodies used were rabbit polyclonal anti-nestin, 1:60 dilution (rabbit 130; kind gift of R. McKay, NIH) and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP), 1:100 dilution (Chemicon). Primary monoclonal antibodies consisted of rat anti-GFAP, 1:1 dilution (IgG; kind gift of V. Lee); mouse anti-β-tubulin III, 1:300 dilution (IgG; Chemicon); mouse anti-MAP2ab, 1:300 dilution (IgG; Chemicon); mouse anti-O4, 1:3 dilution (IgM); and mouse anti-galactocerebroside, 1:1 dilution (IgG3) (both oligodendrocyte markers were generous gifts of J. Grinspan). Secondary fluorescent antibodies used were goat anti-mouse IgG/IgM FITC, 1:300 dilution (Chemicon); goat anti-rabbit IgG Alexa fluor 594, 1:300 dilution (Molecular Probes); goat anti-rabbit IgG Alexa fluor 488, 1:300 dilution (Molecular Probes); and goat anti-mouse IgM Alexa fluor 488, 1:300 dilution (Molecular Probes).
For all intracellular markers, cells were rinsed in Tris-buffered saline (TBS) (50 mM Tris-base, 0.15M NaCl; pH 7.6), fixed for 10 minutes in 4% paraformaldehyde (Sigma), rinsed three times with TBS, blocked in 5% goat serum (GibcoBRL) with 0.1% Triton X-100 (Sigma) for 40 minutes, and then incubated with primary antibody in 1% goat serum with 0.02% Triton X-100 for 1 hour at room temperature or overnight at 4°C. After three TBS washes, the secondary antibody was applied for 1 hour at room temperature or overnight at 4°C. Cell surface marker staining was performed on live cells. The cells were rinsed in TBS, incubated with primary antibody diluted in TBS for 30 minutes, rinsed briefly with TBS, incubated with secondary antibody for 40 minutes, rinsed with TBS, and then fixed for 10 minutes in 4% paraformaldehyde. All slides were washed three times with TBS before mounting in Vectashield containing 4′,6 diamidino-2-phenylindole (DAPI; Vector Laboratories).
Quantitation of immunofluorescence
A minimum of 10 fields for each marker was photographed using a SPOT RT camera (Diagnostic Instruments). An effort was made to sample representatively; when differentiation was not uniformly distributed, similar numbers of fields without positive staining were analyzed. The DAPI-stained nucleus was used to count the total number of cells in all fields using a manual tag with Image-Pro Plus software (version 4.0; Media Cybernetics). The percentage of cells that stained positively for an immunofluorescent marker was obtained by averaging the percentage of positive cells for all fields.
Canine OB-NPC population parameters and statistics
For each cell culture, the number of cells plated at the beginning and harvested at the end of each passage was recorded. For comparison of growth on fibronectin and in the presence of leukemia inhibitory factor, OB-cNPCs from three dogs were evaluated in duplicate for each condition. The total potential cell number generated was calculated by multiplying the total number of viable cells harvested after each passage by the ratio of the total cells harvested to the total cells plated. The product for each passage was added to the subsequent passage to produce the total potential cells generated if all cells harvested were plated (Reynolds and Weiss, 1996). Cell doubling time was determined from the standard formula: doubling time = [(log P − log H)/log 2]/D; where P = the number of cells plated, H = the number of cells harvested, and D = the days between plating and harvest (Heuer et al., 2001).
Statistical analysis was performed to determine whether there was a difference in population doubling times and differentiation ability between OB-cNPCs grown under three separate culture conditions. Comparisons of variable culture conditions were performed using the Kruskal-Wallis non-parametric analysis of variance and, where appropriate, the Dunn’s multiple comparisons test.
Results
The canine olfactory bulb is a source of multipotential neural progenitor cells (NPCs) that can be expanded in vitro
We isolated a population of cells from postnatal canine olfactory bulbs that could be propagated in serum-free medium containing bFGF/heparin and EGF. Culture conditions selected for NPCs, as evidenced by both the morphology and immunophenotype of the proliferating cells. The vast majority of the population was immunophenotypically and morphologically immature; most cells were nestin-positive and GFAP-positive, with a bipolar, spindled morphology, as reported recently (Walton and Wolfe, 2007). The O4-negative, GalC-negative, GFAP-positive immunophenotype of proliferating canine olfactory bulb NPCs (OB-cNPCs) is inconsistent with the immunophenotype reported for rat and canine olfactory ensheathing cells (Franceschini and Barnett, 1996; Ito et al., 2006). Neurospheres could be generated from wells initially seeded with only one to four OB-cNPCs (Figure 1), and were commonly present in high-density cell cultures. When growth factors were removed and serum added to cell cultures, cells possessing phenotype-appropriate morphology and markers for either oligodendrocytes, astrocytes, or neurons appeared (Table 1; Figure 2).
Figure 1.

Neurosphere generation (96-well culture plate). (A) Single well, day 6 in vitro; 6 cells were detected. By day 18 in vitro, over 100 cells were present and neurospheres were noted in other wells (B). Bar = 50μm (A); 100μm (B).
Table 1.
Undifferentiated and Differentiated Immunophenotypes of Canine Olfactory Bulb NPCs
| Culture Conditionsa | Progenitor Cell | Intermediate | Mature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrocytic | Neuronal | Oligodendrocytic | ||||||||||
| Passage | PDL | PDL-fibro | PDL-LIF | Culture Type | Nestin | GFAP, small uni-/bipolar; high N:C ratio; diffuse stain | GFAP, large round/flat; low N:C ratio; diffuse stain | GFAP, large stellate; low N:C ratio; filamentous stain | β-Tubulin | Map2ab | O4 | GalC |
| 5 | +
+ |
−
− |
−
− |
Growthb
Diffc |
95.5 ± 1.3
84.6 ± 2.9 |
79.1 ± 4.7
48.0 ± 7.0 |
0.0
0.0 |
16.6 ± 4.3
31.0 ± 5.9 |
0.0
14.2 ± 3.1 |
1.6 ± 1.6
8.2 ± 3.0 |
0.0
2.6 ± 1.5 |
0.0
0.0 |
| +
+ |
+
+ |
−
− |
Growth
Diff |
98.1 ± 0.5
92.4 ± 1.3 |
86.3 ± 2.5
24.1 ± 5.6 |
0.0
0.0 |
6.6 ± 1.2
17.8 ± 3.0 |
3.9 ± 2.5
7.7 ± 1.8 |
0.3 ± 0.2
14.6 ± 2.4 |
0.0
3.4 ± 1.1 |
0.0
5.9 ± 3.2 |
|
| +
+ |
−
− |
+
+ |
Growth
Diff |
94.3 ± 1.4
83.2 ± 4.3 |
67.9 ± 4.7
41.8 ± 8.0 |
0.0
0.0 |
18.5 ± 2.7
40.6 ± 6.7 |
3.1 ± 0.7
13.2 ± 2.7 |
3.8 ± 1.1
16.5 ± 2.7 |
0.0
6.8 ± 2.3 |
0.0
0.8 ± 0.3 |
|
| 10 | +
+ |
−
− |
−
− |
Growth
Diff |
92.8 ± 1.5
91.6 ± 2.5 |
92.5 ± 2.3
48.4 ± 4.9 |
1.5 ± 0.7
43.5 ± 6.3 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
| +
+ |
+
+ |
−
− |
Growth
Diff |
97.7 ± 0.5
95.6 ± 1.3 |
99.0 ± 0.6
87.1 ± 2.1 |
0.6 ± 0.6
12.9 ± 2.1 |
0.0
0.0 |
0.0
0.0 |
0.0
0.1 ± 0.1 |
0.0
0.0 |
0.0
0.0 |
|
| +
+ |
−
− |
+
+ |
Growth
Diff |
96.5 ± 0.9
96.7 ± 1.0 |
93.7 ± 2.5
73.1 ± 6.0 |
0.0
28.4 ± 5.5 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
0.0
0.0 |
|
For each experimental variable, values represent the mean ± SEM for duplicate cultures from three isolations.
PDL, poly-D-lysine substrate with standard growth factors; PDL-fibro, poly-D-lysine plus fibronectin substrate with standard growth factors; PDL-LIF, poly-D-lysine substrate with standard growth factors and leukemia inhibitory factor (LIF).
Growth, proliferation culture;
Diff, differentiation culture.
Figure 2.

Immunophenotype of canine olfactory bulb NPCs (OB-cNPCs) after growth factor withdrawal and addition of 1% serum. (A) β-tubulin III + cells with neuronal morphology (arrowhead); (B) GFAP + cells with filamentous staining and mature astrocytic morphology; (C) O4 + cells with oligodendrocyte morphology. Bar = 20μm (A, C); 40μm (B).
Comparison of OB-cNPC growth parameters under three different culture conditions
To determine the effect of different culture conditions on cNPC cell growth, phenotype, and differentiation, cells from OB-cNPC isolations were subjected to three experimental variables. The OB-cNPCs were plated onto: 1) poly-D-lysine (PDL) and fed with the standard medium; 2) PDL with fibronectin (PDL-fibro) and fed with the standard medium; or 3) PDL and fed with standard medium supplemented with leukemia inhibitory factor (PDL-LIF). Olfactory bulb cNPCs were isolated from three dogs and plated in duplicate for each variable.
Population doubling times and cell expansion were determined over the course of ten passages. Cell expansion represents the calculated number of cells that would have been produced if all cells were plated after each passage (Reynolds and Weiss, 1996). The OB-cNPCs could be expanded at least 500-fold in the time evaluated, representing up to 28 generations (Figure 3). Population doubling times expressed as the mean ± SEM were: 2.8 ± 0.2 days (PDL); 4.4 ± 0.6 days (PDL-fibro); and 5.0 ± 0.8 days (PDL-LIF). There was no statistical difference between doubling times when standard medium (EGF with bFGF/heparin) was used with either substrate (p > 0.05). LIF supplementation significantly lengthened doubling time compared to standard medium on PDL (p = 0.0153); no significant difference was found between PDL-fibro and PDL-LIF doubling times. The OB-cNPC cultures supplemented with LIF produced fewer total cells (Figure 3) and the slower growth of LIF cultures was associated with greater difficulty in passaging; only two of the three isolations survived to passage (P) 10. We noted that maintenance and proliferation of OB-cNPC cultures was dependent upon cell density. Culture under low-density conditions could only be maintained by supplementation with conditioned medium. Thus, cultures that did not adequately proliferate shortly after passaging tended to die.
Figure 3.
Cell numbers represent the calculated number of total OB-cNPCs produced after nine passages (starting with 1 × 106 cells) if all the cells generated had been plated. After P8, the data for PDL-LIF was based on duplicate cultures from the surviving two isolations. PDL, poly-D-lysine substrate with standard growth factors; PDL-fibro, poly-D-lysine plus fibronectin substrate with standard growth factors; PDL-LIF, poly-D-lysine substrate with standard growth factors and leukemia inhibitory factor (LIF).
Immunophenotypic and morphologic comparisons of proliferating OB-cNPCs grown under three different culture conditions
In vivo studies of the rodent SVZ have shown that neural stem cells have a much longer doubling time than progenitor cells (Morshead et al., 1994). Thus, a slower doubling time may reflect a more undifferentiated progenitor. We therefore evaluated OB-cNPC phenotype and differentiation potential to determine whether substrate (fibronectin) or addition of LIF significantly affected either. Aliquots of cultures from each experimental variable at P5 and P10 were plated overnight in the presence of growth factors for analysis of the undifferentiated phenotype; immunophenotyping was performed at P5 and P10 to allow sufficient time for effect.
Regardless of experimental variable, the undifferentiated OB-cNPCs were primarily nestin-positive and GFAP-positive (Table 1). Glial fibrillary acidic protein (GFAP) is a marker of both differentiated astrocytes and neural stem cells (Doetsch et al., 1999; Laywell et al., 2000; Messing and Brenner, 2003; Morshead et al., 2003), whereas nestin is used as a marker of neural progenitor cells (Hockfield and McKay, 1985; Frederiksen and McKay, 1988; Lendahl et al., 1990). The neural stem cells of the SVZ are reported to be both nestin + and GFAP + (Doetsch et al., 1997). The GFAP + cells in proliferating (undifferentiated) OB-cNPC cultures were divided into two groups based on cellular morphology and staining. Cells with a small nucleus; unipolar, bipolar, or round shape; a high nuclear to cytoplasmic ratio (N:C ratio); and a non-filamentous, diffuse GFAP staining pattern were considered ‘immature’ or undifferentiated (Bonaguidi et al., 2005). In contrast, cells with a large nucleus and a stellate or flattened, fibroblast-like morphology, low N:C ratio, and filamentous staining pattern were considered ‘mature’ because these morphologies are consistent with mature type 1 and type 2 astrocytes (Raff et al., 1983; Bonaguidi et al., 2005) (Figure 4).
Figure 4.
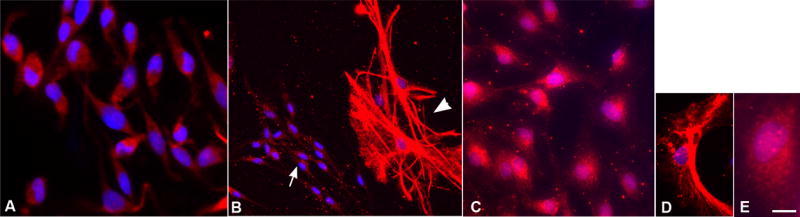
GFAP + cells showed three types of morphology and staining. (A) The majority of cells grown in the presence of growth factors were small, round or bipolar, and stained diffusely for GFAP. (B) Both immature (arrow) and mature (arrowhead) GFAP + cells were present in differentiation cultures. Mature GFAP + cells have a filamentous staining pattern and multiple processes. (C) At passage 10, after growth factor withdrawal the GFAP + cells had a diffuse, disorganized staining pattern (immature pattern), but a flattened, epithelioid morphology (mature morphology). (D, E) Examples of filamentous (D) and diffuse, disorganized (E) staining are shown. Bar = 25μm (A, C); 50μm (B); 12.5μm (D, E).
Since polyclonal anti-GFAP antibodies have been shown to cause spurious GFAP staining, likely due to vimentin or nestin cross-reactivity (Zhang, 2001), we co-stained cNPC cultures with a polyclonal rabbit GFAP antiserum and a rat monoclonal anti-GFAP antibody to verify that GFAP staining was specific. The polyclonal and monoclonal anti-GFAP antibodies stained the same cells. In addition, the same two types of staining patterns in immature and mature cells (diffuse and filamentous, respectively) were observed whether polyclonal or monoclonal antibodies were used (Figure 5). Rabbit anti-human nestin was previously shown to react with a population of multipotential canine NPCs derived from the striatum and ventral mesencephalon (Milward et al., 1997).
Figure 5.

Anti-GFAP staining of canine OB-NPCs using polyclonal and monoclonal antibodies. (A) Rabbit polyclonal anti-GFAP with a secondary Alexa fluor 594 antibody (red). (B) Rat monoclonal anti-GFAP with a FITC secondary antibody. (C) Merged image showing co-staining of GFAP + cells with both primary antibody types. Note the presence of two types of GFAP staining: diffuse and filamentous. Bar = 25μm.
While most of the cells in proliferating (undifferentiated) cultures were nestin + and GFAP + /immature, there was a small population that was morphologically and immunophenotypically of the mature type (Table 1). At P5, fewer than 4% of cells were β-tubulin III + or Map2ab + with neuronal morphology and < 20% of cells were GFAP + with mature astrocytic morphology. There also was a small population of Map2ab + cells with an immature, non-neuronal morphology (small unipolar or bipolar cells with a high N:C ratio; Table 2 and Figure 6). Of the three growth conditions, LIF-supplemented OB-cNPC cultures contained significantly more of these Map2ab + /immature cells at P5 (p < 0.01) than the other conditions, and only the LIF-supplemented cNPC cultures contained Map2ab + /immature cells at P10.
Table 2.
Morphology of Map2ab + Cells in Undifferentiated Canine Olfactory Bulb NPC Cultures
| Passage | Growth Condition | Map2ab Immunopositivity (%) | |
|---|---|---|---|
| Mature neuronal morphology | Immature small, uni-/bipolar; high N:C ratio | ||
| 5 | PDLa | 1.6 ± 1.6 | 5.0 ± 1.9 |
| PDL-fibrob | 0.3 ± 0.2 | 5.7 ± 1.8 | |
| PDL-LIFc | 3.8 ± 1.1 | 15.9 ± 3.0 | |
|
| |||
| 10 | PDL | 0.0 | 0.0 |
| PDL-fibro | 0.0 | 0.0 | |
| PDL-LIF | 0.0 | 11.6 ± 3.7† | |
For each experimental variable, values represent the mean ± SEM for duplicate cultures from three isolations.
PDL, poly-D-lysine substrate with standard growth factors;
PDL-fibro, poly-D-lysine plus fibronectin substrate with standard growth factors;
LIF, poly-D-lysine substrate with standard growth factors and leukemia inhibitory factor (LIF).
Value is the mean ± SEM from duplicate cultures of the two isolations that survived.
Figure 6.
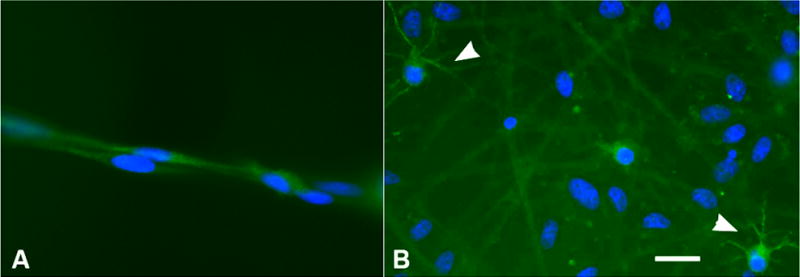
Morphology of Map2ab + cells in OB-cNPC cultures. (A) In the presence of growth factors, small Map2ab + cells are noted with bipolar or unipolar morphology. (B) Upon differentiation by withdrawal of growth factors and addition of serum, variably sized cells with multiple processes are observed (arrowheads). Bar = 20 μm.
Immunophenotypic comparison of differentiated OB-cNPCs grown under three different culture conditions
For analysis of the differentiated phenotype, aliquots were plated for 10–12 days in 1% serum-supplemented medium in the absence of growth factors. Removal of growth factors resulted in proportions of cells that stained positively for neuronal, astrocytic, and oligodendrocytic markers, and displayed a phenotype-appropriate morphology (Table 1 and Figure 2). In this passive differentiation assay, the greatest amount of differentiation was observed at P5. Immunologic and morphologic markers of differentiated neurons, astrocytes and oligodendrocytes were observed regardless of culture conditions. In addition, the proportion of cells that were double-positive for nestin and GFAP, with immature morphology, was generally lower than seen in the growth factor-maintained cultures at the same passage, but the difference did not reach statistical significance in these experiments. In contrast, there were significantly more mature Map2ab + cells present in PDL-fibro and PDL-LIF cultures than were present in PDL cultures (p < 0.001). Cultures supplemented with LIF also contained significantly more O4 + cells, a marker for the oligodendrocyte lineage, than PDL-fibro or PDL (p < 0.001).
By P10, spontaneous differentiation upon growth factor withdrawal was absent across all experimental variables. Although there were GFAP + cells with mature morphology, the staining pattern was immature, i.e., diffuse rather than filamentous (Figure 4). These GFAP + cells were classified as neither immature progenitor cells nor mature astrocytes, but as an ‘intermediate’ type of GFAP + cell (Table 1) (Walton and Wolfe, 2007). In order to determine whether the lack of differentiation at P10 was a function of the passive method of differentiation (withdrawal of growth factors), we attempted to potentiate differentiation with retinoic acid or platelet-derived growth factor (PDGF) (Takahashi et al., 1999; Mujtaba et al., 1999), but neither of these compounds increased differentiation in the P10 cultures. This appeared to be due to the inability of the cells to respond to the growth factors used, however, since neither growth factor increased the number of differentiated cells in cNPC cultures at P1 (data not shown data).
Discussion
The dog has a number of features that make it an excellent model for NPC transplantation: 1) the brain is much closer in size and more similar in physical organization to the human brain than the brains of rodents; 2) there are many well-defined neurological diseases, including genetic diseases, that are essentially the same as in human patients; and 3) relatively large numbers of offspring can be produced. Moreover, if neural progenitor cells (NPCs) could be obtained and adequately expanded from a surgically accessible site such as the olfactory bulb, the dog would serve as a valuable large animal model for autologous transplantation. However, such experiments may involve increased expense, specific requirements for USDA-covered species, and specialized facilities.
Leukemia inhibitory factor (LIF) has been shown to aid in the maintenance of neural stem cells, both in vivo and in vitro (Carpenter et al., 1999; Shimazaki et al., 2001; Pitman et al., 2004; Bonaguidi et al., 2005). In mice, LIF increases the proportion of GFAP + immature NPCs within days of administration (Bonaguidi et al., 2005), whereas the effects of LIF on human fetal NPC growth are not apparent until 50–60 days in culture (Carpenter et al., 1999). The canine olfactory bulb NPCs (OB-cNPCs) were maintained in LIF-supplemented medium for ten passages (79–80 days), which should be sufficient time even for a delayed positive effect to have been seen. Our data showed that the OB-cNPC cultures supplemented with LIF had a significantly slower doubling time and generated fewer cells than cNPCs grown without LIF. For autologous transplantation purposes, the growth delay and fewer total cells generated in LIF-supplemented cultures might not be considered desirable. On the other hand, if the slower doubling time reflects a less mature phenotype, having a higher proportion of progenitor cells in the transplanted population may be advantageous in some applications, particularly during development.
The LIF-supplemented OB-cNPC cultures contained significantly more Map2ab + /immature cells at passage 5 (P5) among the undifferentiated cultures in the three growth conditions. In addition, only the LIF-supplemented cNPC cultures contained Map2ab + immature cells at P10. While there was a small proportion of Map2ab + cells with mature neuronal morphology that represented differentiated cells, most of the Map2ab + cells had an immature morphology. It has previously been shown that subsequent to CNS injury, reactive astrocytes can express the neuronal antigens Tau, Map2, neuron-specific enolase, and GABA, in addition to GFAP and nestin (Lin and Matesic, 1994; Frisen et al., 1995; Schinstine and Iacovitti, 1996; Sahin Kaya et al., 1999). It has been hypothesized that reactive astrocytes represent either astrocytes that revert to bipotential progenitors (cells that show both neuronal and glial phenotypes) or that reactive astrocytes are recruited from neural stem cells (Lin et al., 1995; Schinstine and Iacovitti, 1996). Evidence to support the latter comes from both in vitro and in vivo studies. In vitro, primary astrocytes derived from mouse brain were nestin + and GFAP + , but showed no immunoreactivity to the neuronal antigens Map2 and Tau. However, astrocytes derived from neurospheres maintained in EGF, were positive for GFAP, nestin, Map2 and Tau (Schinstine and Iacovitti, 1996). In mice, cortical injury studies suggest that nestin + cells migrate from the subventricular zone to the site of injury (Frisen et al., 1995; Lin et al., 1995). Thus, the Map2ab + cells with immature morphology in the OB-cNPC cultures may represent a cell type analogous to reactive astrocytes. Supplementation with LIF appears to enhance this population.
When growth factors were removed in OB-cNPC cultures to induce differentiation, LIF-supplemented cultures did generate significantly more mature Map2ab + and O4 + cells compared to standard culture conditions. In human fetal cortical NPC cultures, LIF supplementation is reported to generate significantly more β-tubulin III + cells than in EGF-maintained cultures (Wright et al., 2006), and addition of LIF to adult human olfactory bulb cultures enhances neuronal potential (Pagano et al., 2000). In rodents, LIF and LIF-related cytokines signaling via the LIF receptor increase oligodendrocytic generation and survival (Mayer et al., 1994; Barres et al., 1996). In the dog cells, these results were observed only up to P5. By P10, LIF cultures, as all other cultures, did not generate mature neurons or glial cells. It seems unlikely that the decreased staining for mature markers is due to a lack of progenitors because one would expect that the undifferentiated cultures would then be composed largely of mature, differentiated cells, which is not the case. It is possible that after prolonged growth factor exposure, the withdrawal of growth factors and exposure to low serum concentration were insufficient stimuli for differentiation. However, the cells did not have an enhanced differentiation response to retinoic acid or PDGF at either early or late passage cultures. Although retinoic acid has been reliable for differentiation of primary mouse NSCs in our hands (Heuer et al., 2001), it is possible that other factors might prove more effective to induce differentiation in OB-cNPCs, for example neurotrophins or higher concentrations of fetal bovine serum. Similarly, it is possible that late passage cells might differentiate if they were transplanted into a neurogenic region in vivo, since more complex differentiation cues may be present.
Fibronectin is an important part of the extracellular matrix and is present in the ventricular and subventricular zones of postnatal rats and mice (Hynds and Snow, 2001; Campos et al., 2004). When used to facilitate NPC retroviral transduction, it increases NPC proliferation in the neonatal mouse (Rappa et al., 2004). In contrast, in the dog we found that fibronectin did not enhance growth rate or expansion of OB-cNPCs. Since the neonatal dog brain is developmentally more advanced than a neonatal rodent brain (Clancy et al., 2001), a better comparison may be to the adult rodent brain. The growth rate of adult rat SVZ-derived NPCs has been compared using various combinations of substrates and growth factors (Whittemore et al., 1999). Similar to our findings for canine OB-NPCs, adult rat NPCs grown on polyornithine-fibronectin with bFGF and heparin did not proliferate more than the other substrates used.
While LIF-supplemented OB-cNPC cultures demonstrated slower expansion potential, there was enhanced differentiation into neuronal and oligodendrocytic lineages. More cells with a neuronal phenotype were also produced when fibronectin was included as a substrate. These findings support the use of both substances in ex vivo propagation to promote neuronal potential in transplants. Transplantation studies into neurogenic regions of the dog brain will be necessary to determine if these results are recapitulated in vivo. Characterization of postnatal canine olfactory bulb NPCs should also aid in the development of an autologous transplantation model for neurodegenerative disease in a brain that is scaled to that of a human.
Acknowledgments
We gratefully acknowledge the expert assistance of P. O’Donnell and Dr. M. Haskins of the Animal Models Core (RR-02512), and A. Polesky and E. Cabacungan for expert technical assistance. This work was supported by NIH grants DK46637 and NS 56243. RMW was supported by a fellowship from the NCRR (RR07063).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alroy J, Orgad U, Ucci AA, Schelling SH, Schunk KL, Warren CD, Raghavan SS, Kolodny EH. Neurovisceral and skeletal GM1-gangliosidosis in dogs with beta-galactosidase deficiency. Science. 1985;229:470–2. doi: 10.1126/science.3925555. [DOI] [PubMed] [Google Scholar]
- Bagley RS, Harrington ML, Pluhar GE, Gavin PR, Moore MP. Acute, unilateral transverse sinus occlusion during craniectomy in seven dogs with space-occupying intracranial disease. Vet Surg. 1997;26:195–201. doi: 10.1111/j.1532-950x.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;8:146–56. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–14. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, Svendsen CN. Heparin, but not other proteoglycans potentiates the mitogenic effects of FGF-2 on mesencephalic precursor cells. Exp Neurol. 1998;152:1–10. doi: 10.1006/exnr.1998.6815. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, Garcion E, ter Borg MG, He X, Svendsen CN. Heparin stabilizes FGF-2 and modulates striatal precursor cell behavior in response to EGF. Exp Neurol. 2004;188:408–20. doi: 10.1016/j.expneurol.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–44. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158:265–78. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–82. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Fischer A, Carmichael KP, Munnell JF, Jhabvala P, Thompson JN, Matalon R, Jezyk PF, Wang P, Giger U. Sulfamidase deficiency in a family of Dachshunds: a canine model of mucopolysaccharidosis IIIA (Sanfilippo A) Pediatr Res. 1998;44:74–82. doi: 10.1203/00006450-199807000-00012. [DOI] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–43. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–51. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–64. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F, Ray J, Fisher L. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Glass EN, Kapatkin A, Vite C, Steinberg SA. A modified bilateral transfrontal sinus approach to the canine frontal lobe and olfactory bulb: surgical technique and five cases. J Am Anim Hosp Assoc. 2000;36:43–50. doi: 10.5326/15473317-36-1-43. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID, McCulloch M, Harvey MJ. Shaking pups: a disorder of central myelination in the Spaniel dog. Part 1. Clinical, genetic and light-microscopical observations. J Neurol Sci. 1981;50:423–33. doi: 10.1016/0022-510x(81)90154-4. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19:3287–97. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–45. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Heuer GG, Skorupa AF, Prasad Alur RK, Jiang K, Wolfe JH. Accumulation of abnormal amounts of glycosaminoglycans in murine mucopolysaccharidosis type VII neural progenitor cells does not alter the growth rate or efficiency of differentiation into neurons. Mol Cell Neurosci. 2001;17:167–78. doi: 10.1006/mcne.2000.0917. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–28. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynds DL, Snow DM. Fibronectin and laminin elicit differential behaviors from SH-SY5Y growth cones contacting inhibitory chondroitin sulfate proteoglycans. J Neurosci Res. 2001;66:630–42. doi: 10.1002/jnr.10020. [DOI] [PubMed] [Google Scholar]
- Ito D, Ibanez C, Ogawa H, Franklin RJ, Jeffery ND. Comparison of cell populations derived from canine olfactory bulb and olfactory mucosal cultures. Am J Vet Res. 2006;67:1050–6. doi: 10.2460/ajvr.67.6.1050. [DOI] [PubMed] [Google Scholar]
- Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp Neurol. 2003;182:240–4. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- Kilpatrick T, Bartlett P. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10:255–265. doi: 10.1016/0896-6273(93)90316-j. [DOI] [PubMed] [Google Scholar]
- Koppang N. The English setter with ceroid-lipofuscinosis: a suitable model for the juvenile type of ceroid-lipofuscinosis in humans. Am J Med Genet Suppl. 1988;5:117–25. doi: 10.1002/ajmg.1320310616. [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Flax JD, Snyder EY, Jendoubi M. Expression of human beta-hexosaminidase alpha-subunit gene (the gene defect of Tay-Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med. 1996;2:424–9. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–8. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lin RC, Matesic DF. Immunohistochemical demonstration of neuron-specific enolase and microtubule-associated protein 2 in reactive astrocytes after injury in the adult forebrain. Neuroscience. 1994;60:11–6. doi: 10.1016/0306-4522(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Lin RC, Matesic DF, Marvin M, McKay RD, Brustle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- Liu Z, Martin LJ. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J Comp Neurol. 2003;459:368–91. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–53. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Messing A, Brenner M. GFAP: functional implications gleaned from studies of genetically engineered mice. Glia. 2003;43:87–90. doi: 10.1002/glia.10219. [DOI] [PubMed] [Google Scholar]
- Milward EA, Lundberg CG, Ge B, Lipsitz D, Zhao M, Duncan ID. Isolation and transplantation of multipotential populations of epidermal growth factor-responsive, neural progenitor cells from the canine brain. J Neurosci Res. 1997;50:862–71. doi: 10.1002/(SICI)1097-4547(19971201)50:5<862::AID-JNR22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Moyer MP, Johnson RA, Zompa EA, Cain L, Morshed T, Hulsebosch CE. Culture, expansion, and transplantation of human fetal neural progenitor cells. Transplant Proc. 1997;29:2040–1. doi: 10.1016/s0041-1345(97)00221-2. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev Biol. 1999;214:113–27. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–10. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Pagano SF, Impagnatiello F, Girelli M, Cova L, Grioni E, Onofri M, Cavallaro M, Etteri S, Vitello F, Giombini S, Solero CL, Parati EA. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells. 2000;18:295–300. doi: 10.1634/stemcells.18-4-295. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci. 2004;27:255–66. doi: 10.1016/j.mcn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappa G, Kunke D, Holter J, Diep DB, Meyer J, Baum C, Fodstad O, Krauss S, Lorico A. Efficient expansion and gene transduction of mouse neural stem/progenitor cells on recombinant fibronectin. Neuroscience. 2004;124:823–30. doi: 10.1016/j.neuroscience.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90:3602–6. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89:8591–5. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin Kaya S, Mahmood A, Li Y, Yavuz E, Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain Res. 1999;840:153–7. doi: 10.1016/s0006-8993(99)01757-6. [DOI] [PubMed] [Google Scholar]
- Schinstine M, Iacovitti L. Expression of neuronal antigens by astrocytes derived from EGF-generated neuroprogenitor cells. Exp Neurol. 1996;141:67–78. doi: 10.1006/exnr.1996.0140. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–31. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–53. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Takahashi J, Palmer TD, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol. 1999;38:65–81. [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–88. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Walton RM, Wolfe JH. Abnormalities in neural progenitor cells in a dog model of lysosomal storage disease. J Neuropathol Exp Neurol. 2007;66:760–9. doi: 10.1097/nen.0b013e31812571c8. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Victoria T, Rafi MA, Luzi P, Vanier MT, Vite C, Patterson DF, Haskins MH. Globoid cell leukodystrophy in cairn and West Highland white terriers. J Hered. 1999;90:138–42. doi: 10.1093/jhered/90.1.138. [DOI] [PubMed] [Google Scholar]
- Whittemore SR, Morassutti DJ, Walters WM, Liu RH, Magnuson DS. Mitogen and substrate differentially affect the lineage restriction of adult rat subventricular zone neural precursor cell populations. Exp Cell Res. 1999;252:75–95. doi: 10.1006/excr.1999.4621. [DOI] [PubMed] [Google Scholar]
- Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–3. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- Zhu G, Mehler MF, Mabie PC, Kessler JA. Developmental changes in progenitor cell responsiveness to cytokines. J Neurosci Res. 1999;56:131–45. doi: 10.1002/(sici)1097-4547(19990415)56:2<131::aid-jnr3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]



