Abstract
Previous studies have shown that melatonin treatment increases the susceptibility of retinal photoreceptors to light-induced cell death. The purpose of this study was to evaluate under various conditions the potential toxicity of dietary melatonin on retinal photoreceptors. Male and female Fischer 344 (non-pigmented) and Long-Evans (pigmented) rats were treated with daily single doses of melatonin by gavage for a period of 14 days early in the light period or early in the dark period. In another group, rats were treated 3 times per week with melatonin early in the light period, and then exposed to high intensity illumination (1,000 −1,500 lux; HII) for two hours, and then returned to the normal cyclic lighting regime. At the end of the treatment periods, morphometric measurements of outer nuclear layer thickness (ONL; the layer containing the photoreceptors cell nuclei) were made at specific loci throughout the retinas. In male and female non-pigmented Fischer rats, melatonin administration increased the degree of photoreceptor cell death when administered during the nighttime and during the day when followed by exposure to HII. There were some modest effects of melatonin on photoreceptor cell death when administered to Fisher rats during the day or night without exposure to HII. Melatonin treatment caused increases in the degree of photoreceptor cell death when administered in the night to male pigmented Long-Evans rats, but melatonin administration during the day, either with or without exposure to HII, had little if any effect on photoreceptor cell survival. In pigmented female Long Evans rats, melatonin administration did not appear to have significant effects on photoreceptor cell death in any treatment group. The results of this study confirm and extend previous reports that melatonin increases the susceptibility of photoreceptors to light-induced cell death in non-pigmented rats. It further suggests that during the dark period, melatonin administration alone (i.e., no HII exposure) to pigmented male rats may have a toxic effect on retinal cells. These results suggest that dietary melatonin, in combination with a brief exposure to high intensity illumination, induces cellular disruption in a small number of photoreceptors. Chronic exposure to natural or artificial light and simultaneous intake of melatonin may potentially contribute to a significant loss of photoreceptor cells in the aging retina.
Keywords: Melatonin, Photoreceptor, Retina, Toxicity, Circadian
1. Introduction
The major hormone of the pineal gland, melatonin (N-acetyl-5-methoxytryptamine) is also synthesized by retinal photoreceptors (Cahill and Besharse, 1992; Green, et al., 1995; Guerlotte et al., 1996; Wiechmann, 1996; Wiechmann and Craft, 1993) under a cyclic rhythm with peak levels occurring during the dark period (Pang et al., 1980; Wiechmann et al., 1986). Melatonin may play an important role in dark adaptation; it alters the sensitivity of the central visual system to light (Reuss and Kiefer, 1989; Semm and Vollrath, 1982), and increases the sensitivity of horizontal cells to light in the salamander retina (Wiechmann et al., 1988). Melatonin, synthesized at night, may bind to receptors in the retina to increase the sensitivity of the visual system when photic input is low, and thus facilitate retinal dark adaptation by increasing horizontal cell coupling through inhibition of dopamine release (Krizaj and Witkovsky, 1993) and perhaps by stimulating the photoreceptors directly (Cosci et al., 1997, Wiechmann et al., 2003).
Melatonin treatment given immediately prior to continuous light exposure increases the degree of light-induced photoreceptor cell death in albino rats (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992), and a melatonin receptor antagonist protects photoreceptors from light-induced damage (Sugawara et al., 1998), thus demonstrating that the effect of melatonin is mediated through a retinal melatonin receptor. Because one function of melatonin may be to enhance the sensitivity of the retina to light as part of a dark-adaptation mechanism, an undesirable consequence of this may be an increased sensitivity to the damaging effects of light.
The recent discovery that the retinal photoreceptor cells express melatonin receptors (Scher et al., 2002; Wiechmann and Wirsig-Wiechmann, 2001) is intriguing in light of the overwhelming evidence that photoreceptors are the sites of retinal melatonin synthesis (Cahill and Besharse, 1992; Guerlotte et al., 1996; Wiechmann, 1996; Wiechmann and Craft, 1993; Niki et al., 1998). Although signals from the inner retina undoubtedly play a major role in the circadian activities of retinal photoreceptors (Boatright et al., 1994; Dubocovich, 1983; Dubocovich and Takahashi, 1987), intracrine (autocrine) melatonin signaling in photoreceptors likely contributes substantially to circadian regulation in the retina.
Inappropriate (i.e.; daytime) exposure of retinal cells to melatonin may be detrimental to photoreceptor cell survival, as supported by reports that melatonin increases the degree of light-induced photoreceptor cell death (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992). The many Americans that self-administer dietary melatonin may therefore be at a higher risk for developing retinal degenerations that ensue from the death of photoreceptors. Chronic exposure of the retina to melatonin at inappropriate times of day and lighting conditions may increase the risk of susceptibility of photoreceptors to damage by environmental illumination. There has been a dramatic increase in self-prescribed melatonin in recent years, and melatonin is widely available in grocery, drug, and health food stores, and is currently unregulated in the United States. The self medication may exceed recommended maximum levels (3 mg/person) and represents a large uncontrolled human experiment. Based on a previous study (Wiechmann and O'Steen, 1992), systematic animal studies were undertaken to test the hypotheses that melatonin exposure during exposure to light may cause damage and death of retinal photoreceptors. The major ocular toxicity results of a 14-day melatonin toxicity study are reported.
2. Materials and Methods
2.1 Animal Treatments
Two-month-old male and female non-pigmented Fischer 344 (Taconic, Germantown, NY) and Long-Evans (pigmented) rats (Charles River laboratories, Raleigh, NC), which were born and reared under a 12 hours light (480 lux at 3.5 feet above cage level)/12 hours dark lighting cycle, were randomized into three groups and maintained for 10−14 days on a full spectrum cyclic lighting regime of 12 hours light/12 hours dark (12L/12D: 6 AM lights on/ 6 PM lights off). The illumination intensity during the light period ranged from 10 to 40 lux. During the 14-day treatment period, ten animals per sex per strain received melatonin by gavage in 0.5% methylcellulose containing 0.25% ethanol (Table 1). The melatonin dosages were 5, 50, 5,000, 50,000, and 200,000 μg/kg of body weight. This research adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the Laboratory Animal welfare Act of 1966 and adhered to the principles enunciated in the “Guide for the care and Use of laboratory Animals” NRC, 1996, and the 1964 Declaration of Helsinki and “Principles of laboratory animal care”, (NIH publication no. 86−23, revised 1985).
Table 1.
Treatment schedule for 14-day melatonin gavage toxicity study.
| Lighting Conditions Day Treatment (Normal light cycle) | |
| Night Treatment (Light cycle reversed) | |
| High Intensity Illumination(HII) (Melatonin treatment and HII exposure at about midday) |  |
In the Day Treatment Groups, melatonin was administered between 9 and 11 AM (i.e., 3−5 hours after lights on) during the light phase (10−40 lux at cage level), and maintained on the cyclic lighting regime. In the Night Treatment Group, the animals were entrained to a reverse lighting cycle (12D/12L: 6 AM lights off/ 6 PM lights on; 10−40 lux). Melatonin was administered between 8 and 10 AM (i.e., 2−4 hours after lights off) under dim red light (0.1 lux) and maintained on the reverse cyclic lighting regime for the remainder of the study. The treatment schedule was twelve days of melatonin doses, not including weekend days.
In the High Intensity Illumination (HII) Treatment Groups, melatonin was administered between 9 and 11 AM (i.e., 3−5 hours after lights on) three times per week (M, W, F) during the light phase (10−40 lux), then were immediately exposed to high intensity illumination (HII; 1000 lux for the Fischer rats, 1500 lux for the Long-Evans rats) for two hours at room temperature, then returned to the normal cyclic lighting regime until the next treatment. The pupils were not dilated for HII exposure, since one goal of the study was to approximate normal lighting conditions. The higher light intensity for pigmented rats was used because it is well known that a higher intensity illumination is required to induce retinal damage in pigmented rats than in albino rats. In humans, 2500 lux is sufficient to shift circadian rhythms while 500 lux does not influence the endogenous production of melatonin (Lewy et al., 1980). The illuminances and exposure times were chosen to mimic the conditions of the human eye in the presence of high and low dosages of melatonin and exposed to intermittent bight sunlight. The low concentrations of melatonin were used to mimic normal dosages for taken as a sleep aid and high melatonin concentrations were used to mimic the dosages that are used in the treatment of cancer patients.
2.2 Tissue Preparation
At the end of the treatment period, animals were maintained on cyclic lighting for 12 days to allow the retinal tissue to recover and eliminate any cellular debris from apoptotic photoreceptor cells that may have accumulated during the melatonin and HII treatments. The right eye from each animal was enucleated from euthanized animals, placed into Davidson's fixative (31% ethanol, 22% formalin, and 12% glacial acetic acid, pH 3.5−4.0) for several hours, and then further fixed in 10% neutralized formalin. The tissues were embedded in paraffin wax, sectioned on the anterior-posterior axis at 7 μm thickness, mounted onto glass sides, stained with hematoxylin and eosin, and cover-slipped.
2.3 Morphometric Measurements
Retinas from a total of 720 animals were analyzed in the 14-day study. Each group was composed of 10 rats (10 rats × 2 sexes × 2 strains × 3 groups × 6 dosages = 720 eyes). Three mid-sagittal eye sections (containing the center of the optic nerve) from the right eye of each rat were analyzed. Morphometric measurements of outer nuclear layer thickness (ONL; the layer containing the photoreceptor cell nuclei) (Fig. 1) were made at 10 specific sites spanning the entire circumference of the retina (loci 1−5=superior quadrant; loci 6−10=inferior quadrant) on sections of the mid-sagittal region of the retina similar to the method described previously (Wiechmann and O'Steen, 1992). The relative thickness of the ONL is a direct measurement of the degree of photoreceptor cell death (Michon et al., 1991), and has been used previously to measure the effect of melatonin and its receptor antagonist on HII-induced photoreceptor cell death (Wiechmann and O'Steen, 1992; Sugawara et al., 1998). We have previously validated ONL measurement as a direct reflection of number photoreceptor cell nuclei in (unpublished observations), in which ONL thickness measurements were performed by counting columns of photoreceptor nuclei, then converting the value to ONL thickness based on the average nuclear diameter. It was determined that the number of photoreceptor cell nuclei and the ONL thickness were reduced by melatonin to the same degree. The average values of each set of 3 measurements (one measurement at each locus per section) were recorded as the ONL thickness for the particular animal at the specific locus (Fig. 1). Therefore, a total of 21,600 separate measurements were performed on the specimens analyzed in this study (720 slides × 3 sections × 10 loci = 21,600 measurements).
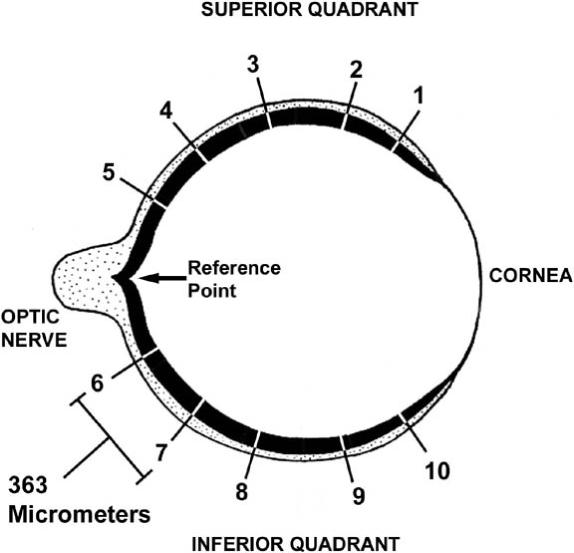
To measure the ONL thickness, the center of the optic nerve head was selected as the beginning reference point (Fig. 1). The field of view in the microscope (Nikon Optiphot; Tokyo, Japan) using a 20 × objective lens was 363 micrometers (Fig. 1), as measured by a calibrated ocular grid micrometer. The reference point in the center of the optic nerve head was placed at the edge of the field of view, then the center of the field of view was viewed using a 40 × objective lens, and the ONL thickness was measured using a calibrated ocular grid micrometer. The measurement was then entered onto a Microsoft Exel spreadsheet as the measurement at retinal locus number 5 (Fig. 1). For the next measurement, a landmark site at the opposite edge of the optic nerve head (reference point) was identified at 20 × magnification, and the slide was moved to place that landmark at the opposite edge of the field of view (i.e., where the starting reference point used to be). This effectively shifted the center of the field of view 363 micrometers (Fig. 1). The center of the field of view was viewed using a 40 × objective lens, and the ONL thickness was measured and recorded as before. This process was repeated three more times until the entire expanse of the superior quadrant was measured, then the entire process described for the superior quadrant was repeated for the inferior quadrant. This method of analysis provided a measurement of ONL thickness at uniform loci from superior peripheral (loci 1−5) to central (optic nerve) to inferior peripheral (loci 6−10). Measurement of many specific loci throughout the retina was crucial for accurate analysis of photoreceptor cell death (thinning of the ONL), since the ONL thickness is significantly greater in the more central regions of the retina than in the peripheral regions. Additionally, subjective estimates were made on the relative degrees of photoreceptor cell death by assigning the following symbols: − = no effect, + = small effect, ++ = moderate effect, +++ = severe effect. These subjective estimates took into account the number of melatonin dosages that caused an effect, the number of retinal loci that were affected, and the percent reduction of ONL thickness in the affected loci.
2.4 Statistical Analysis
Statistical analysis was performed separately for each sex and strain. Averages of the three measurements of ONL thickness for each animal at each specific locus were the endpoints of this analysis. Descriptive statistics for each effective melatonin dosage group are reported at each locus for each lighting treatment group.
A repeated measures analysis of variance (ANOVA) model was fitted to the ONL thickness measurements in order to evaluate the effects of melatonin and lighting condition in the study rats. The mixed effects model included main effects for melatonin dosage, locus, lighting treatment, two-way interactions between melatonin dosage and lighting treatment, melatonin dosage and locus, and lighting treatment and locus, and a three-way interaction among melatonin dosage, lighting treatment, and locus as fixed effects, and animal as a random effect. The errors from the same animal at different loci were assumed to be correlated, and an autoregressive AR(1) covariance structure was assumed. The degrees of freedom for the tests of fixed effects were computed using Kenward and Roger's method (Kenward and Roger, 1997). Fixed effects were tested by overall F-tests at the 0.05 significance level.
If the effects for melatonin dosage or lighting treatment were found to be significant, then additional tests were performed to investigate these effects. Initially, a statistical contrast was used to test for any differences in ONL thickness among the three lighting treatments in the absence of melatonin. This contrast compared the mean ONL thickness over all loci at the control (0 μg/kg melatonin) dosage among the three lighting treatments. If significant differences were found in this comparison, additional contrasts were used to compare mean ONL thickness over all loci at the control dosage for each pair of lighting treatments. A Bonferroni adjustment was used to maintain a 0.05 level of significance over these comparisons (that is, the significance level used for each pair-wise comparison was 0.05/3=0.0167). Significant differences between lighting treatments at the control dosage implies that lighting treatment alone had an effect on ONL thickness and precluded testing for general melatonin effects over all treatments. When this occurred, melatonin effects were investigated within each lighting treatment.
If there were no effects due to lighting treatment alone and no significant interactions between melatonin dosage and lighting treatment, then the following tests were performed to examine the main effects of melatonin dosage and lighting treatment. If the main effect for melatonin dosage was significant, Dunnett's test was used to compare the mean at each melatonin dosage over all lighting treatment groups and loci to the overall control mean. Dunnett's test is a multiple comparisons procedure that maintains a 0.05 significance level over all comparisons. If the main effect for lighting treatment was significant, then t-tests were used to compare the means of each pair of lighting treatments over all melatonin dosages and loci. A Bonferroni adjustment was used to maintain a 0.05 level of significance over these comparisons (that is, the significance level used for each pair-wise comparison was 0.05/3=0.0167). Similar tests were not performed to examine the effect of locus, as this effect was expected to be significant because the ONL is thinner at positions 1 and 10.
To further investigate the effects of melatonin on photoreceptor cell death (ONL thickness), three additional sets of tests were performed to examine 1) the effect of melatonin within each lighting treatment; 2) the effect of melatonin at each locus (if there were no effects due to lighting treatment alone); and 3) the effect of melatonin at each locus within each lighting treatment. The following approach was used for these tests. First, F-tests were conducted to determine whether a melatonin effect was evident for a specified lighting treatment, locus, or combination of interest. The significance level for each set of tests was adjusted using Hochberg and Benjamini's method for multiple testing at an overall 0.05 level. If a significant melatonin effect was found, then pair-wise comparisons were performed to determine which (if any) of the five melatonin dosage group means differed significantly from the control mean within the given treatment/locus of interest, at a Bonferroni-adjusted 0.05 significance level (that is, the significance level for each pair-wise comparison to the control was 0.05/5=0.01). Other differences among the melatonin dosage group means could contribute to a significant F-test, but were not of interest in establishing the effects of melatonin.
The ANOVA models and comparisons were performed using PROC MIXED in SAS version 8 (SAS Institute; 1999a). The Hochberg and Benjamini adjustment for multiple comparisons was conducted using PROC MULTTEST in SAS version 8 (SAS Institute; 1999b).
3. Results
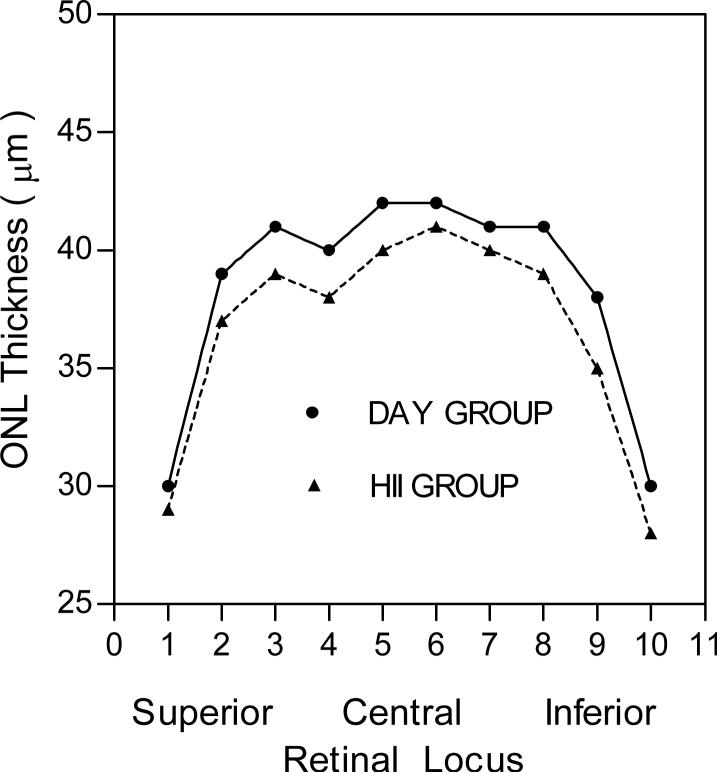
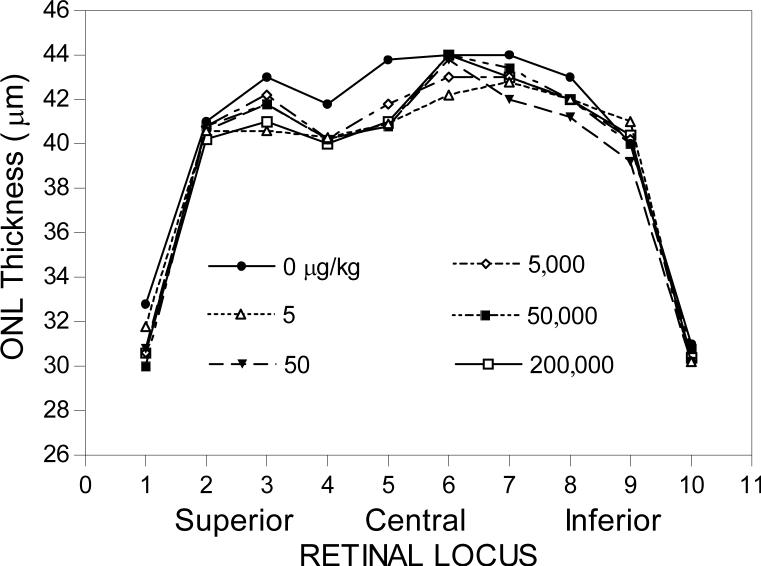
Long-Evans and Fischer 344 rats given 5−200,000 μg/kg of melatonin for 14 day treatment period followed by a 12-day recovery period showed no treatment effects on terminal body weights, organ weights, gross pathology or histopathology. In non-HII-exposed rats, the ONL thicknesses were normal (Fig. 2), indicating that the photoreceptors were undamaged, whereas HII-exposed animals showed a small decrease (approximately 5%) in ONL thickness, indicating that a moderate level of photoreceptor cell death occurred in this lighting regime (Fig. 3).
3.1 14-Day Study of Male Fischer 344 Rats
Tests of fixed effects indicated that there were significant melatonin dosage and locus effects the 14-day-treated male Fischer 344 rats, but that lighting treatment had only a marginal effect on ONL thickness (p=0.0811). All two-way interactions were statistically significant, indicating that the observed effects were not consistent over all levels of the other variables.
3.1.1. Day Treatment Group
Melatonin administration during the early daytime increased photoreceptor cell death at dosages of 5,000 and 50,000 μg/kg in 14-day-treated male Fischer 344 rats (Table 2). The approximate percentage decrease of photoreceptor cells (ONL thickness) in the two affected loci (7 and 9) in both of the effective melatonin dosages was about 3−7%. Statistical significance was based upon comparison of melatonin-affected loci compared to the identical set of loci in the control (0 μg/kg) group (Table 2). Evaluation of the effect of lighting treatment in the absence of melatonin indicated that there were significant differences in mean ONL thickness due to lighting treatment alone.
Table 2.
Summary of retinal morphometric analysis.
| Strain/Sex Treatment Group |
Effective Dosages (μg/kg; p<0.05) |
Retinal Loci Affected |
% Reduction in ONL Thickness |
Relative Degree of PH Cell Death (+/−) |
|---|---|---|---|---|
| Fischer/Male | 5,000 | 7, 9 | 7.0, 2.6 | |
| Day |
50,000 |
7, 9 |
4.6, 2.6 |
+ |
| Fischer/Male | ||||
| Night |
200,000 |
6 |
0* |
− |
| 5 | ||||
| Fischer/Male | 50 | |||
| High Intensity Light | 5,000 | 5 | 6.8 (all) | ++ |
| |
200,000 |
|
|
|
| Fischer/Female | ||||
| Day |
200,000 |
8 |
4.8 |
− |
| Fischer/Female | 5 | 6,7,8 | 4.5, 4.5, 7.0 | |
| Night |
50,000 |
2 |
10.2 |
+ |
| 5 | 7, 8 | 4.5, 4.7 | ||
| Fischer/Female | 50 | 6, 7, 8 | 6.8, 6.8, 7.0 | |
| High Intensity Light | 5,000 | 6, 7, 8 | 4.5, 6.8, 7.0 | +++ |
| |
50,000 |
6, 7, 8 |
4.5, 4.5, 7.0 |
|
| Long Evans/Male | ||||
| Day |
− |
− |
− |
− |
| 50 | 6, 7, 8 | 8.9, 6.7, 9.1 | ||
| Long Evans/Male | 50,000 | 6, 7, 8, 9 | 6.7, 6.7, 6.8, 5.0 | +++ |
| Night |
200,000 |
7, 8 |
6.7, 6.8 |
|
| Long Evans/Male | ||||
| High Intensity Light |
200,000 |
5 |
7.5 |
− |
| Long Evans/Female | ||||
| Day/ Night/ High | − | − | − | − |
| Intensity Light |
Effect of various dosages of melatonin on photoreceptor cell death under different lighting regimes, sexes, and strains of rats. In the non-pigmented Fischer male rats, melatonin causes an increase (+) in photoreceptor (PH) cell death in the Day (+) and High Intensity Illumination (HII, ++) treatment groups, and in females in the Night (+) and HII treatment (+++) groups. In pigmented Long Evans rats, melatonin causes an increase in photoreceptor cell death in males in the Night (+++) group. The scoring of the increased photoreceptor cell death (−, +, ++, +++) are subjective estimates. The statistically significant effective dosages of melatonin (compared to nonmelatonin treated control group in each treatment group, are shown in column two. Loci 1−5 are on the superior quadrant, and Loci 6−10 are on the inferior quadrant. The affected loci of each effective melatonin dosage in each treatment group are shown in the third column, and their respective % reductions in ONL thickness are shown in the fourth column. F tests were used to test for significant melatonin effects over all loci within lighting treatments and melatonin effect at each locus within lighting treatment where the Benjamini and Hochberg multiple comparison adjustment method was used to control the overall error rate at the 0.05 level. When significant melatonin effects were present, pair-wise comparisons were made between each of the five melatonin dosage groups and the control group within the lighting treatment group. Each pair-wise comparison was performed using Bonferroni-adjustment method, to ensure that the overall error rate associated with the five comparisons was no greater than 0.05. The effective melatonin dosages at which the ONL thickness differed significantly from the control at the Bonferroni-adjusted 0.05 level are in the second column.
3.1.2. Night Treatment Group
Melatonin administration during the early nighttime in 14-day-treated male Fischer 344 rats had no significant effect on photoreceptor cell death. However, at a melatonin dosage of 200,000 μg/kg, the ONL thickness was significantly higher compared to the untreated group (0 μg/kg; p<0.05; Table 2) at retinal locus 6 (Table 2). This is suggestive of a protective effect of melatonin on photoreceptor cells, but since there was no exposure to high intensity illumination, it is not a plausible outcome, since it would imply that new photoreceptors were generated during the treatment period. Since photoreceptor cells are post-mitotic, new photoreceptor cells cannot be generated. These results therefore provide an illustration of the degree of error to expect in these studies.
3.1.3. High Intensity Illumination (HII) Treatment Group
Melatonin administration (three times per week on alternating days) during the early daytime followed by two hours of high intensity illumination (HII; 1000 lux) in 14-day-treated male Fischer 344 rats increased photoreceptor cell death by 6.8% at all melatonin dosage groups except the 5,000 μg/kg dosage group at retinal locus 5 when compared to the HII controls (Table 2 and Fig. 4).
3.2 14-Day Study of Female Fischer 344 Rats
Evaluation of the effect of treatment in the absence of melatonin indicated that there were no significant differences in mean ONL thickness due to lighting treatment alone in 14-day-treated female Fischer 344 rats.
3.2.1. Day Treatment Group
Melatonin administration during the early daytime had a minimal significant effect on the enhancement of photoreceptor cell death at the 200,000 μg/kg dosage group in 14-day-treated female Fischer 344 rats (Table 2). This contrasts with the increased photoreceptor cell death at dosages of 5,000 and 50,000 μg/kg at two loci in the Fischer male rats, which is suggestive of a small gender difference in responsiveness to melatonin administration in the daytime.
3.2.2. Night Treatment Group
Melatonin administration during the early nighttime increased the level of photoreceptor cell death by about 5−7% in the 50,000 μg/kg dosage group compared to the control group at retinal loci 6, 7, and 8 in 14-day-treated female Fischer 344 rats (Table 2). The mean of the ONL thickness in the 5,000 μg/kg dosage group at retinal locus 2 was also significantly less (10.2%) than those of the control groups at retinal locus 2 (Table 2).
3.2.3. High Intensity Illumination (HII) Treatment Group
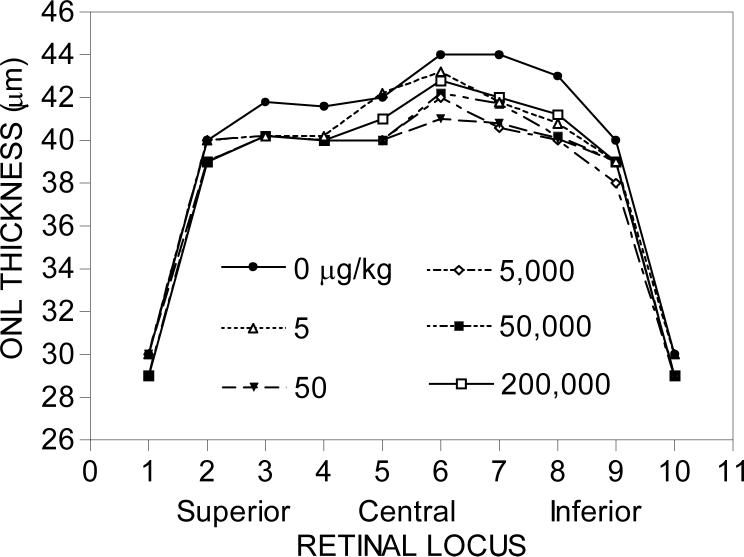
Melatonin administration (3x/week) during the early daytime followed by two hours of HII (1000 lux) in 14-day-treated female Fischer 344 rats increased photoreceptor cell death at all dosages except at the highest dosage of 200,000 μg/kg (Fig. 5). The approximate percentage decrease of photoreceptor cells in the affected loci (6, 7, and 8) was about 5−7%. The degree of melatonin-mediated photoreceptor cell loss in the female Fischer rats groups was therefore highest in the HII-treated group.
3.3 14-Day Study of Male Long-Evans Rats
Tests of fixed effects indicated that there were significant melatonin dosage and locus effects in 14-day-treated male Long-Evans rats, but that lighting treatment had only a marginal effect on ONL thickness (p=0.0811). All two-way interactions were statistically significant, indicating that the observed effects were not consistent over all levels of the other variables. Evaluation of the effect of lighting treatment in the absence of melatonin indicated that there were significant differences in mean ONL thickness due to lighting treatment alone.
3.3.1. Day Treatment Group
Melatonin administration during the early daytime had no significant effect on photoreceptor cell death at any dosage in 14-day-treated male Long-Evans rats (Table 2). This contrasts with the increased photoreceptor cell death at dosages of 5,000 and 50,000 μg/kg at two loci in the Fischer male rats, which is suggestive of mild strain or pigmentation differences in responsiveness to melatonin administration.
3.3.2. Night Treatment Group
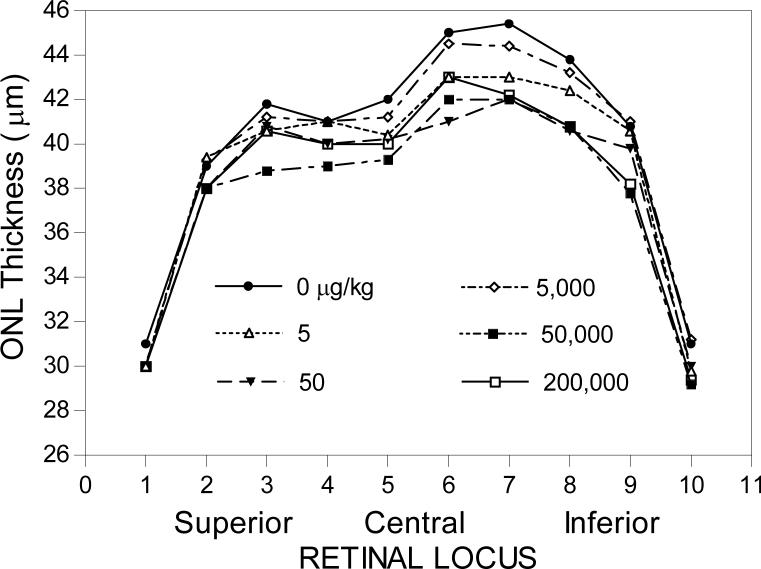
Melatonin administration during the early nighttime increased photoreceptor cell death at the 50, 5,000, and 200,000 μg/kg dosage groups in 14-day-treated male Long-Evans rats (Table 2 and Fig. 6). The approximate percentage decrease of photoreceptor cells in retinal loci 6−9 of all of the effective melatonin dosages in the affected loci was about 5−9% (Table 2). The approximate level and distribution of photoreceptor cell death in the affected groups were quite consistent among the affected groups (Fig. 6).
3.3.3. Light (HII) Treatment Group
Melatonin administration (3x/week) during the early daytime followed by two hours of HII (1500 lux) increased photoreceptor cell death at a dosage of 200,000 μg/kg in 14-day-treated male Long-Evans rats (Table 2). The percentage decrease of photoreceptor cells in the affected locus 5 was 7.5%. Surprisingly, melatonin treatment early in the dark period and without exposure to HII in male Long-Evans (non-pigmented) rats caused the most photoreceptor cell death as compared to the Day and HII exposed groups.
3.4 14-Day Study Long Evans Females
Tests of fixed effects indicated that there were significant lighting treatment and locus effects in 14-day-treated female Long-Evans rats, but that melatonin dosage did not have a statistically significant effect on ONL thickness. The two-way interaction between locus and lighting treatment was statistically significant. The two-way interaction between lighting treatment and melatonin dosage and the three-way interaction were marginally significant. Evaluation of the effect of lighting treatment in the absence of melatonin indicated that there were significant differences in mean ONL thickness due to lighting treatment alone. Further analysis indicated that the mean ONL thickness in the Day Treatment Group was significantly greater than that of the HII Treatment Group, for control animals.
No significant melatonin effects were observed within any of the three lighting treatments. Within lighting treatment and locus, significant melatonin effects were observed at some loci for the Day and Night Treatment Groups, but none of the five melatonin dosage groups were significantly different from the control groups at these loci.
Melatonin administration during the early daytime, early nighttime, and during the early daytime followed by two hours of HII (1500 lux) had no significant effect on photoreceptor cell death at any dosage in any of the female Long-Evans treatment groups.
In summary, while there were statistically significant differences in the mean ONL thickness of control animals between lighting conditions within sexes and strains, these differences indicated different responses for each sex and strain combination. No ONL mean of any control group varied more than 10 percent from any of the other control mean regardless of sex, strain or lighting condition.
Not only did the ONL means of control animals vary less than 10 percent between control groups but any ONL mean at any one locus for any melatonin dosage did not vary more than 10 percent from their respective control. While there were occasional statistically significant differences between ONL means, no melatonin-related trends were similar between sexes within a strain or within sexes between strains.
4. Discussion
The purpose of this study was to evaluate the potential toxicity of dietary melatonin on retinal photoreceptors. Previous studies (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992; Sugawara et al., 1998) have suggested a potential detrimental effect of melatonin on photoreceptor cell survival in albino rats, and many reports suggest that melatonin may be beneficial for combating cancer, (Aldeghi et al., 1994; Lissoni et al., 1994), alleviating jet lag (Claustrat et al., 1992; Petrie et al., 1989) and entraining circadian rhythms (Petrie et al., 1989; Armstrong, 1989) such as sleep patterns (Reiter, 1980; Dollins et al., 1994). These perceived benefits have motivated many Americans to self-administer melatonin. The access to melatonin is presently unregulated in the United States, and there is some concern in the scientific community that a potential health risk may exist for some individuals that self-administer melatonin at inappropriate dosages and under inappropriate environmental lighting conditions, and that an ocular melatonin toxicity study was needed to assess these possibilities. In the present study, melatonin was administered orally to rats in a range of dosages from low dosages of 5 and 50 μg/kg, in which 50 μg/kg is approximately equivalent to the recommended 3 mg human dose for treatment of jet lag and insomnia, and higher dosages (50,000 and 200,000 μg/kg), which are similar to what cancer patients may receive (Lissoni et al., 1994).
In this 14-day study, melatonin caused an increase in the degree of photoreceptor cell death when administered during the daytime to male Fischer 344 rats (non-pigmented), either with or without exposure to HII (high intensity illumination; Day and HII groups), but melatonin administration at night essentially had no effect, since the one effective melatonin dosage at one affected retinal locus showed an increase in ONL thickness, which is implausible since photoreceptors cannot replicate. In the female Fischer rats, there was a negligible effect of one or two dosages of melatonin on photoreceptor cell death when administered during the day or night, but melatonin did cause a significant increase in photoreceptor cell death in female rats treated at daytime with melatonin then followed by two hours of HII (HII group). The female Fischer rats appeared to have more photoreceptor cell death than the males when melatonin was administered alone during the night (Night group). When treated with melatonin in daytime followed by two hours of HII (HII group), female Fischer rats also had a statistically higher degree of photoreceptor cell death than did the male rats, as indicated by the number of retinal loci affected. The results of this component of the study confirm previous reports that melatonin increases the susceptibility of photoreceptor cells to HII-induced cell death in albino rats (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992; Sugawara et al., 1998). The degree of HII-induced cell death was lower in this study compared to previous studies (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992). This was predicted, since the light exposure regime was quite different; our previous study (Wiechmann and O'Steen, 1992) used 1600 lux continuously for 24 hrs at 30°C, whereas the present study used only 1000 lux for 2 hrs (3x/week for two weeks) at room temperature (RT). The HII conditions in this toxicity study were used in an attempt to more closely mimic the intensity and duration of natural and artificial light to which humans may be routinely exposed.
In male Long-Evans (pigmented) rats, melatonin administration alone had no effect on the degree of photoreceptor cell death when administered during the day (Day group), but it did increase photoreceptor cell death when administered alone at night. We speculate that perhaps the increased photoreceptor cell death that occurs when melatonin is administered alone at night is because the high levels of melatonin resulting from the gavage administration initiates a down-regulation of the melatonin receptors, which would make the receptors unavailable for stimulation by the endogenous nocturnal levels of retinal melatonin. This would make the photoreceptor cells unresponsive to the beneficial role of melatonin at night, such as preparing the retina for the assault of light that occurs in the morning. Another possibility is that high endogenous melatonin levels at night are necessary for proper function of the photoreceptors, but if melatonin levels are greatly increased above the normal endogenous melatonin levels, this could be toxic to the photoreceptors.
The major conclusions that may be derived from this study are that: 1) melatonin administered during the day followed by immediate exposure to moderate levels and duration of HII increases the degree of photoreceptor cell death in a non-pigmented rat strain, and 2) melatonin administered at night to pigmented male rats appears to be toxic to photoreceptors. The results of this study also confirm previous reports that melatonin increases the susceptibility of photoreceptor cells to HII-induced cell death in albino rats (Bubenik and Purtill, 1980; Wiechmann and O'Steen, 1992; Sugawara et al., 1998).
When different groups of animals such as males vs. females and Long Evans vs. Fischer rats were compared, the results suggested some possible gender and/or strain differences in sensitivity to some dosages of melatonin administration. Male Long-Evans (pigmented) appeared to be more sensitive to melatonin treatment at night than were the Long-Evans females. Also, Fischer male and female rats appeared to be more sensitive to the toxic effects of melatonin treatment flowed by HII than were the Long-Evans male and female rats. This is consistent with previous reports that pigmented strains of rats tend to be more resistant to HII (Rapp and Williams, 1980).
Throughout this study, the differences in sensitivity to melatonin and/or light treatments were quite small. By using a large number of animals in this study, we were we able to detect statistically significant differences between some treatment groups. One noteworthy feature of this study is that the relatively modest intensity and duration of light exposure was capable of inducing small but measurable differences between treatment groups. Although the observations that melatonin administration alone has some statistically significant toxic effects in some groups, some caution in interpreting these results is warranted. For example, there were some statistically significant differences between two groups that indicated that melatonin promoted some minor photoreceptor proliferation, which is implausible. So, although some small differences may be detected statistically, they may not necessarily reflect a biological significance. The evidence that melatonin increases the degree of HII-induced photoreceptor cell death is quite strong, whereas the evidence suggesting that melatonin administration alone is toxic to photoreceptor cells is rather weak, except for the toxic effect at night on pigmented males.
Melatonin administration increases the degree of photoreceptor cell death in response to environmental light exposure, but is also involved in the regulation of necessary circadian events that occur in the retina, such as photoreceptor outer segment disk shedding and phagocytosis (Besharse and Dunis, 1983; White and Fisher, 1989), modulation of dopamine release (Dubocovich, 1983; Dubocovich and Takahashi, 1987), and increased sensitivity of the retina to light (Wiechmann et al., 1988, 2003). Melatonin also delays photoreceptor degeneration in a dystrophic animal model (Liang et al., 2001). The dichotomy of beneficial versus detrimental effects of melatonin may be the result of a cyclic rhythm of intracellular responsiveness to melatonin. We hypothesize that the circadian rhythm of melatonin synthesis reflects a beneficial role during the dark period, but is a potential hazard during exposure to light. In light of the evidence presented in this study, we suggest that it would be prudent for individuals to avoid chronic self-administration of melatonin in the presence of high levels of environmental illumination. The unanticipated observation that melatonin treatment potentially induces photoreceptor cell death during the night, when endogenous retinal melatonin levels are highest, raises new questions regarding the regulation and function of melatonin receptors in the retina.
Acknowledgements
The authors thank Rajendra Dhote and Radhika Dighe for their excellent technical assistance with the ocular morphometric measurements and analysis. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. This research was also supported by National Institutes of Health grants EY13686 (AFW), EY12191 (NEI Core), RR017713 (COBRE), and the Oklahoma Center for Science and Technology (OCAST: HR02−135R) (AFW). The housing and irradiation of the rats were provided under a National Institute of Environmental Health Sciences (NIEHS) contract NO1-ES-65406 at Battelle Memorial Institute, Ohio. The design and implementation of these studies were conducted in the Laboratory of Pharmacology and Chemistry at the NIEHS. All retinal analysis was conducted at the University of Oklahoma.
References
- Aldeghi R, Lissoni P, Barni S, Ardrizzoia A, Tancini G, Pozzi M, Ricci G, Conto A, Maestroni GJ. Low-dose interleukin-2 subcutaneous immunotherapy in association with the pineal hormone melatonin as a first-line therapy in locally advanced or metastatic hepatocellular carcinoma. Eur J Cancer. 1994;30A:167–170. doi: 10.1016/0959-8049(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Armstrong SM. Melatonin. The Internal Zeitgeber of Mammals? Pineal Res. Rev. 1098;7:157–202. [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1342. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: The role of GABA. Vis. Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Purtill RA. The role of melatonin and dopamine in retinal physiology. Can. J. Physiol. Pharmacol. 1980;58:1457–1462. doi: 10.1139/y80-220. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis. Neurosci. 1992;8:487–490. doi: 10.1017/s0952523800005009. [DOI] [PubMed] [Google Scholar]
- Claustrat B, Brun J, David M, Sassolas G, Chazot G. Melatonin and jet lag: Confirmatory result using a simplified protocol. Biol. Psychiatry. 1992;32:705–711. doi: 10.1016/0006-3223(92)90300-o. [DOI] [PubMed] [Google Scholar]
- Cosci B, Longoni B, Marchiafava PL. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sci. 1997;60:1885–1889. doi: 10.1016/s0024-3205(97)00150-1. [DOI] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Takahashi JS. Use of 2-[125]iodomelatonin to characterize melatonin binding sites in chicken retina. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3916–3920. doi: 10.1073/pnas.84.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Tryptophan hydroxylase is expressed by photoreceptors in Xenopus laevis retina. Vis. Neurosci. 1995;12:663–670. doi: 10.1017/s0952523800008956. [DOI] [PubMed] [Google Scholar]
- Guerlotte J, Greve P, Bernard M, Grechez-Cassiau A, Collin JP. Hydroxyindole-O-methyltransferase in the chicken retina: Immunocytochemical localization and daily rhythm of mRNA. Eur. J. Neurosci. 1996;8:710–715. doi: 10.1111/j.1460-9568.1996.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Krizaj D, Witkovsky P. Effects of submicromolar concentrations of dopamine on photoreceptor to horizontal cell communication. Brain Res. 1993;627:122–128. doi: 10.1016/0006-8993(93)90755-c. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Liang F-Q, Aleman TS, Yang Z, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001;12:1011–1014. doi: 10.1097/00001756-200104170-00029. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Barni S, Tancini G, Ardizzoia A, Ricci G, Aldeghi R, Brivio F, Tisi E, Rovelli F, Rescaldani R. A randomized study with subcutaneous low dose interleukin 2 alone vs interleukin 2 plus the pineal neurohormone melatonin in advanced solid neoplasms other than renal cancer and melanoma. Br. J. Cancer. 1994;69:196–199. doi: 10.1038/bjc.1994.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon JJ, Li Z-L, Shiouro N, Anderson RJ, Tso MOM. A comparative study of methods of photoreceptor morphometry. Invest. Ophthalmol. Vis. Sci. 1991;32:280–284. [PubMed] [Google Scholar]
- Niki T, Hamanda T, Ohtomi M. The localization of the site of arylalklamine N-acetyltransferase circadian expression in the photoreceptor cells of mammalian retina. Biochem. Biophys. Res. Commun. 1998;248:115–120. doi: 10.1006/bbrc.1998.8916. [DOI] [PubMed] [Google Scholar]
- Pang SF, Yu HS, Suen HC, Brown GM. Melatonin in the retina of rats: a diurnal rhythm. J. Endocrinol. 1980;87:89–93. doi: 10.1677/joe.0.0870089. [DOI] [PubMed] [Google Scholar]
- Petrie K, Conaglen JV, Thompson L, Chamberlain K. Effect of melatonin on jet lag after long haul flights. Br. Med. J. 1989;298:705–707. doi: 10.1136/bmj.298.6675.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp LM, Williams TP. The role of ocular pigmentation in protecting against retinal light damage. Vision Res. 1980;20:1127–1131. doi: 10.1016/0042-6989(80)90050-4. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The pineal gland and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Reuss S, Kiefer W. Melatonin administered systematically alters the properties of visual cortex cells in cat: further evidence for a role in visual information processing. Vision Res. 1989;29:1089–1093. doi: 10.1016/0042-6989(89)90057-6. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. The MIXED procedure. SAS/STAT User's Guide, Version 8. Vol. 2. Cary; North Carolina: 1999a. [Google Scholar]
- SAS Institute, Inc. The MULTTEST procedure. SAS/STAT User's Guide, Version 8. Vol. 2. Cary; North Carolina: 1999b. [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT(1) melatonin receptor in the human retina: expression and localization. Invest. Ophthalmol. Vis. Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- Semm P, Vollrath L. Alterations in the spontaneous activity of cells in the guinea pig pineal gland and visual system produced by pineal indoles. J. Neural Trans. 1982;53:265–275. doi: 10.1007/BF01252038. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Sieving PA, Iuvone PM, Bush RA. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Invest. Ophthalmol. Vis. Sci. 1998;39:2458–2465. [PubMed] [Google Scholar]
- White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989;29:167–179. doi: 10.1016/0042-6989(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF. Hydroxyindole-O-methyltransferase is expressed in a subpopulation of photoreceptors in the chicken retina. J. Pineal Res. 1996;20:217–225. doi: 10.1111/j.1600-079x.1996.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Craft CM. Localization of mRNA encoding the indolamine synthesizing enzyme, hydroxyindole-O-methyltransferase, in chicken pineal gland and retina by in situ hybridization. Neurosci. Lett. 1993;150:207–211. doi: 10.1016/0304-3940(93)90537-u. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O'Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest. Opthalmol. Vis. Sci. 1992;33:1894–1902. [PubMed] [Google Scholar]
- Wiechmann AF, Wirsig-Wiechmann CR. Multiple cell targets for melatonin action in Xenopus laevis retina: Distribution of melatonin receptor immunoreactivity. Vis. Neuro. 2001;18:1–8. doi: 10.1017/s0952523801185032. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Bok D, Horwitz J. Melatonin binding in the frog retina: autoradiographic and biochemical analysis. Invest. Ophthalmol. Vis. Sci. 1986;27:153–163. [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe RK, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel1c melatonin receptor in transgenic Xenopus laevis retina. Invest. Opthalmol. Vis. Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Yang X-L, Wu SM, Hollyfield JG. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988;453:377–380. doi: 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, Morabito C, Matheson JK, Schomer DL. Sleep-inducing effects of low-doses of melatonin ingested in the evening. Clin. Pharmacol. Ther. 1995;57:552–558. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]