Abstract
Vulval intraepithelial neoplasia (VIN) is defined histopathologically by distinctive abnormalities of cellular maturation and differentiation. To investigate the functional properties of VIN, the expression of several proteins involved in the regulation of the cell cycle as well as in situ DNA replication competence was analysed by immunohistochemistry. Snap-frozen vulval biopsies were graded as normal squamous epithelium (n=6), undifferentiated HPV positive VIN 1 (n=3), VIN 2 (n=8) and VIN 3 (n=20). Immunohistochemistry was performed using the following markers: cyclin D1 (expressed in middle/late G1), cyclin B1 (expressed in G2/early M), phosphorylated histone H3 (expressed during mitosis) and minichromosome maintenance (Mcm) proteins 2 and 5 (expressed during the cell cycle, but not in differentiated or quiescent cells). In situ DNA replication competence was used to identify S-phase cells. The percentage of positively stained nuclei in three representative microscopic fields was calculated per biopsy. In normal vulva, the expression of all markers was restricted to the proliferative compartment of the basal layer of the epithelium. In contrast in high-grade VIN, the majority of epithelial cells expressed the Mcm proteins from basal to superficial layer. The detection of cyclins B1 and D1, phospho-histone H3 and in situ DNA replication was also found through the full thickness of these lesions but by a lower proportion of the cells. This is consistent with these markers providing a series of ‘snapshots’ of the cell cycle status of individual cells. The low-grade VIN showed reduced expression of the cell cycle markers in relation to the level of dysplasia. The combination of these analyses establishes that the majority of VIN cells remain in a functional replicative or prereplicative state of the cell cycle. Clinical application of these analyses may provide a basis for improved diagnosis of VIN.
Keywords: vulval intraepithelial neoplasia (VIN), minichromosome maintenance (Mcm) proteins, cyclin D1, cyclin B1, in situ DNA synthesis, phosphohistone H3, immunostaining
Vulval intraepithelial neoplasia (VIN) is a dysplastic condition of the squamous epithelium of the vulva (Buckley et al, 1984). It is characterised histopathologically by abnormalities of cellular maturation and differentiation. Mitotic figures, sometimes of abnormal or bizarre form, cellular and nuclear pleomorphism and cells with abnormally high nuclear to cytoplasmic ratios are observed throughout the various layers of the epithelium (Scully et al, 1994). Lesions are classified as VIN 1, VIN 2 and VIN 3 according to the proportion of cells from the basement membrane to the surface that are affected by these changes (Wilkinson et al, 1986). While the histopathological features associated with VIN are well described, however, little is currently understood about the functional properties of dysplastic vulval cells. One important factor is the association of undifferentiated VIN with high-risk human papillomavirus (HPV) infection (van Beurden et al, 1995). In HPV-infected epithelium, the viral oncogenes E6 and E7 drive the dysplasia by dysregulating the cell cycle checkpoints through interaction with the p53 and Rb regulatory proteins (Zur Hausen, 2002).
The cell cycle is the orchestrated series of molecular events, which coordinate the production of two daughter cells. It is divided into four distinct phases: G1, S, G2 and M. Progression through the successive stages of the cell cycle is accompanied by the alternate expression and lack of expression of specific regulatory proteins (Viallard et al, 2001). Dysregulation of the cell cycle is known to play an important role in the oncogenic transformation of cells (Johnson et al, 1995) and abnormal expression patterns of cell-cycle-associated cyclin D1 and retinoblastoma protein (Rb) have previously been documented in high-grade VIN lesions (Rolfe et al, 2001; Zamparelli et al, 2001). Other studies have demonstrated increased cellular proliferation in high-grade VIN using proliferation markers Ki-67 and proliferating cell nuclear antigen (PCNA) (van Hoeven and Kovatich, 1996; Modesitt et al, 2000). However, the scope of these analyses is restricted to high-grade VIN lesions and to the analysis of cell cycle proteins normally expressed during a single phase of the cell cycle (G1).
In the present study, we have investigated the expression of a number of cell cycle regulatory proteins and proliferation markers across the full spectrum of vulval dysplasia, from normal vulval epithelium to VIN 3. The approach used has been verified in a larger study of cell cycle kinetics in colorectal cancer (Scott et al, in submission). The markers used were cyclin D1, cyclin B1 and phosphorylated histone H3, all of which are normally expressed during distinct phases of the cell cycle (Viallard et al, 2001). Cyclin D1 is expressed in middle/late G1 and with CDK4/6 phosphorylates Rb, the inactivation of which is completed by cyclin E/CDK2 (Adams, 2001). Cyclin B1 is expressed in G2/early M and with CDK1 facilitates the processes involved in mitosis (Pines and Hunter, 1989; Ito, 2000; Taylor and Stark, 2001). Histone H3 is phosphorylated in mitosis and is associated with mitotic chromosome condensation (Mahadevan et al, 1991; Goto et al, 1999). To identify S-phase cells, in situ DNA replication competence was used. This technique has previously been validated in a variety of normal and neoplastic tissues, including normal squamous epithelium of the cervix and cervical intraepithelial neoplasia (CIN) 3 (Mills et al, 2000).
We have also compared the use of proliferation markers Mcm 2 and Mcm 5 in VIN. The Mcm proteins form an essential prereplicative complex by binding to DNA sites at which origin recognition complex and cdc6 proteins have sequentially bound. Mcm proteins are only present during the cell cycle and are lost in quiescent and differentiating cells (Ritzi et al, 1998). The use of an antibody directed against Mcm 5 has been shown to improve the specificity and sensitivity of cytological screening for CIN compared with the use of conventional cervical cytology alone (Freeman et al, 1999). Furthermore, antibodies against Mcm 2 and Mcm 5 have consistently been shown to identify a greater number of cycling cells than antibodies against Ki-67 or PCNA (Williams et al, 1998; Freeman et al, 1999). Here we report the expression pattern of Mcm proteins 2 and 5, cyclins D1 and B1, phosphohistone H3 and in situ DNA replication competence in normal squamous epithelium of the vulva, VIN 1, VIN 2 and VIN 3. The Mcm proteins identify all cells actively involved in the cell cycle, while cyclins D1 and B1, phosphohistone H3 and in situ DNA replication detect only a fraction of cells within a particular phase of the cell cycle.
Materials and methods
Patient material
In all, 37 vulval biopsies were obtained from women attending the Vulva Clinic at St Mary's Hospital in Manchester, UK. The Central Manchester Local Research Ethics Committee approved the study and all patients gave written informed consent. The tissue was coated in OCT and snap-frozen in liquid nitrogen. Seven and 10 μm sections were cut onto aminopropyltriethoxysilane (APES)-coated slides and stained with haematoxylin and eosin (H/E) for histopathological analysis or stored at −70°C until required. Histological grading of all vulval specimens was performed by two independent histopathologists at St Mary's Hospital. Biopsies were graded as normal vulval epithelium (n=6), VIN 1 (n=3), VIN 2 (n=8) and VIN 3 (n=20). Only undifferentiated VIN associated with high-risk HPV infection as determined by PCR was included in this study. For HPV typing, DNA was extracted from the vulval biopsies by incubating the samples overnight at 37°C on a rotary mixer in 500 μl of guanidinium isothyocyanate (GuScn) Lysis Buffer (12 g GuScn, 635 μl 1 M sodium citrate, 425 μl 30% sarcosyl, 50 μl glycogen, 250 μl 0.1 M DTT, up to 25.5 ml with double-distilled H2O). Samples were spun for 1 min and DNA was precipitated from the supernatant using isopropanol. The mixture was centrifuged at 13 000 rpm for 15 min, the pellet washed with 70% ethanol for 5 min and then dried at 56°C for 15 min before being dissolved in 400 μl of double-distilled H2O. All specimens were positive for β-globin. HPV DNA was detected using GP5+/GP6+ consensus primers and genotyped using type specific primers for HPV 6/11, 16, 18, 31 and 33 (Van den Brule et al, 1990). Amplification products were resolved on 2% agarose gels and visualised by ethidium bromide staining.
Immunohistochemical staining of specimens
Sections, 5-μm, were quick-thawed and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min (Freeman et al, 1999). Endogenous peroxidase activity was quenched by incubation in 0.6% hydrogen peroxide in Tris-buffered saline (TBS) for 30 min. Sections were washed in TBS and blocked with 10% normal goat serum (DAKO, Ely, UK) and 10% bovine serum albumin (BSA) in TBS for 90 min at room temperature. The antibody directed against cyclin D1 was preabsorbed overnight at 4°C with 10% BSA in TBS. The antibodies against Mcm proteins 2 and 5, cyclin B1 and phospho-histone H3 did not require preabsorption. Final dilutions of primary antibody were prepared in 1% BSA and 0.1% Triton X-100 in TBS. In total, 50 μl of antibody was added to each section and the slides were incubated at 4°C overnight in a humidified chamber. The following concentrations were found to produce optimal staining: Mcm 2 1 : 25, Mcm 5 1 : 25, cyclin D1 1 : 50, cyclin B1 1 : 600, phosphohistone H3 1 : 500. The primary antibody layer was omitted from a single section of each biopsy to serve as a negative control.
The slides were washed in 0.1% Tween 20 in TBS and incubated with biotinylated goat antimouse secondary antibody (Mcm 2 & 5 and cyclins B1 & D1) or biotinylated goat antirabbit secondary antibody (phosphohistone H3) (both from DAKO) at 1 : 500 in 1% BSA in TBS for 1 h at room temperature. Streptavidin horseradish peroxidase (HRP) (DAKO) was added for 30 min, followed by the substrate diaminobenzidine (DAB) for 10 min at room temperature and the reaction stopped with running water. The slides were counterstained with Harris' haematoxylin, dehydrated through graded ethanols and cleared in xylene. Coverslips were applied with DEPEX mounting medium (Gurr, BDH, Poole, Dorset, UK).
In situ DNA replication competence
Frozen sections, 10 μm, were quick-thawed, covered with 40 μl incubation buffer (30 μl SuNaSp/BSA with protease-inhibitor cocktail and 10 μl 50 μM Dig-dUTP nucleotide mix (Boehringer Mannheim)) and incubated at 37°C for 45 min in a humidified chamber. Slides were washed in PBS and fixed with 4% paraformaldehyde in PBS for 2 min (Mills et al, 2000). Endogenous peroxidase activity was quenched by incubation in 0.6% hydrogen peroxide in TBS for 3 min. Sections were coated with 50 μl of rabbit anti-DIG/HRP antibody at 1 : 50 in 1% BSA in TBS and incubated at room temperature for 45 min. The slides were washed in TBS and DAB was used to develop the stain. The slides were counterstained with Harris' haematoxylin, dehydrated with ethanol and cleared in xylene. Coverslips were applied with DEPEX mounting medium.
Scoring of stained sections
Scoring was carried out by two independent observers (EJD and PLS). The percentage of positively stained nuclei in three representative high-powered microscopic fields (approximately 1000 cells) was calculated per biopsy. In addition, the distribution of staining from basal layer to the surface layer of the epithelium was scored. Negative control sections from each biopsy showed negative immunostaining.
Statistical analysis
The Spearman rank correlation coefficient (rs) was calculated for each marker across the spectrum of vulval dysplasia (normal, VIN 1, VIN 2 and VIN 3) using the raw data values from each patient. A value of P<0.05 was considered statistically significant.
Results
Expression of cell cycle markers and in situ DNA replication competence in normal squamous epithelium of the vulva
In normal vulva, the expression of all cell cycle proteins and in situ DNA replication competence was restricted to the nuclei of the basal epithelial cells (Figure 1). Differentiating cells in the more superficial layers of the epithelium were negative for all cell cycle markers. Mcm proteins 2 and 5 were found in the majority of cells in the basal epithelial layer (Table 1 ). In contrast, cells positively stained for cyclins B1, D1 and phosphohistone H3 and those exhibiting in situ DNA replication competence were found in a subset of the basal epithelial cell layer. Less than 5% of cells in the basal layer of the epithelium scored positive for cyclin D1 (data not shown).
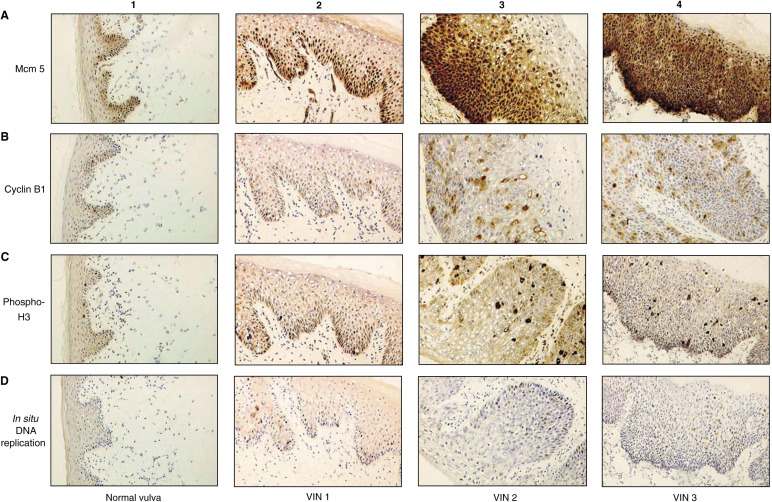
Figure 1.
Immunostaining of normal vulval epithelium and VIN 1, VIN 2 and VIN 3 with cell cycle markers (x40): (A1) normal vulval epithelium shows that Mcm 5 expression is restricted to the basal epithelial layer; (A2) in VIN 1, >40% of cells express Mcm 5 in the basal and suprabasal epithelial layers; (A3) in VIN 2, >85% of cells express Mcm 5 and positive nuclei are detected in basal and superficial epithelial layers; (A4) VIN 3 shows >90% of cells express Mcm 5, extending throughout the full thickness of the epithelium; (B1) in normal vulval epithelium, expression of cyclin; (B1) is restricted to the basal epithelial layer. In VIN 1 (B2), VIN 2 (B3) and VIN 3 (B4), a greater proportion of cells express cyclin B1 compared with normal skin and positively stained nuclei are found in progressively more superficial epithelial layers, correlating with the level of dysplasia. C1–4 and D1–4 show a similar pattern of expression of phosphohistone H3 in normal vulva (C1), VIN 1 (C2), VIN 2 (C3) and VIN 3 (C4), and by in situ DNA replication in normal vulva (D1), VIN 1 (D2), VIN 2 (D3) and VIN 3 (D4).
Table 1. Percentage of cells expressing each cell cycle marker in normal vulval epithelium and VIN 1, VIN 2 and VIN 3.
| Normal (n=6) | VIN 1 (n=3) | VIN 2 (n=8) | VIN 3 (n=20) | Correlation coefficient | |
|---|---|---|---|---|---|
| Mcm 2 | 14.7 (9.9–19.4) | 32.3 (19.2–52.5) | 85.0 (80.5–94.1) | 93.4 (82.9–97.8) | rs=+0.91 |
| P<0.001 | |||||
| Mcm 5 | 16.3 (9.7–21.7) | 44.2 (33.6–61.0) | 85.1 (79.6–91.7) | 94.1 (84.5–98.9) | rs=+0.90 |
| P<0.001 | |||||
| Cyclin B1 | 1.9 (0.6–3.9) | 2.5 (1.1–4.8) | 9.5 (6.0–13.6) | 9.7 (3.4–16.2) | rs=+0.68 |
| P<0.001 | |||||
| Phospho-H3 | 2.7 (0.5–4.7) | 3.4 (1.0–7.3) | 7.2 (1.8–14.1) | 10.0 (3.0–26.2) | rs=+0.65 |
| P<0.001 | |||||
| In situ DNA replication | 1.6 (1.8–3.4) | 3.4 (2.0–5.3) | 8.1 (4.7–12.3) | 13.4 (5.5–30.2) | rs=+0.81 |
| P<0.001 |
This table shows the mean labelling indices (percentage of stained cells) for normal vulval epithelium and VIN 1, VIN 2 and VIN 3 with each of the cell cycle markers. Similar patterns of staining were observed for cyclins B1 and D1, but precise quantification of the latter was not possible. Ranges are given in parentheses. The proportion of positively stained nuclei increases with the level of dysplasia with each of the markers studied. The Spearman rank correlation coefficients and level of statistical significance are shown.
Expression of cell cycle markers and in situ DNA replication competence in VIN 1, VIN 2 and VIN 3
Positively stained nuclei were present in the superficial epithelial layers of all VIN lesions with antibodies against Mcm proteins 2 and 5, cyclin B1 and D1, phosphohistone H3 and by in situ DNA replication (Figure 1). The proportion of positively stained nuclei in the superficial epithelial layers correlated with the degree of dysplasia. The lowest proportion was found in the VIN 1 lesions, where positively stained nuclei were seen only in the suprabasal epithelial layers ( Table 1). A similar pattern of staining was seen for cyclins B1 and D1, but precise quantification of the latter was not possible because of cytoplasmic background staining.
In VIN 2 lesions, staining with all markers was seen in superficial layers of the epithelium and by a higher proportion of the cells overall compared with VIN 1. In VIN 3, full thickness staining of epithelial cells from basal to superficial layers was seen with all cell cycle markers. The majority of cells from the basement membrane to the surface of the epithelium expressed Mcm proteins 2 (93.4%) and 5 (94.1%). The detection of cyclins B1 and D1, phosphohistone H3 and in situ DNA replication was also found through the full thickness of these lesions, but by a lower proportion of cells.
There was a statistically significant correlation between the level of dysplasia and the proportion of positively stained nuclei with every marker investigated (Table 1). The highest Spearman rank correlation coefficients were observed for Mcm proteins 2 (rs=+0.91) and 5 (rs=+0.90), P<0.001. The correlation coefficients for cyclin B1 (rs=+0.68), phosphohistone H3 (rs=+0.65) and in situ DNA replication (rs=+0.81) were also statistically significant at the P<0.001 level.
Discussion
In this study we have analysed the expression patterns of several cell cycle regulatory proteins and proliferation markers across the spectrum of vulval dysplasia, from normal squamous epithelium to VIN 3. The expression of all cell cycle markers and in situ DNA replication competence was restricted to the proliferative compartment of the basal layer of normal vulval squamous epithelium. In contrast, the majority of cells from basal to superficial layer expressed Mcm proteins 2 and 5 in VIN 3. Cyclin D1, B1, phosphohistone H3 and in situ DNA replication were also seen through the full thickness of VIN 3 lesions, but in a lower proportion of the cells. This is consistent with each marker providing a series of ‘snapshots’ of the cell cycle status of individual cells within the lesion. In low-grade VIN, reduced expression of all markers was seen in relation to the level of dysplasia, with positively stained nuclei found in the suprabasal layers of the epithelium in VIN 1 and extending into more superficial layers of the epithelium in VIN 2. There was a statistically significant correlation between an increasing level of dysplasia and a higher proportion of positively stained cells with each of the markers tested. The best markers of increasing dysplasia were the Mcm proteins 2 and 5, which gave correlation coefficients of rs=+0.91 and +0.90, respectively. Cyclin D1 expression was similar to that observed for cyclin B1, but was more difficult to quantify precisely because of cytoplasmic background staining.
The proportion of cells positive for Mcm proteins 2 and 5 was similar to that previously observed in normal squamous epithelium of the cervix and in low- and high-grade CIN (Williams et al, 1998). We also found that the proportion of cells demonstrating in situ DNA replication competence was consistent with that previously observed in the cervix (Mills et al, 2000). It should be noted, however, that those women who had ‘normal vulval epithelium’ by histology had presented with symptoms which necessitated a vulval biopsy. Whether this tissue is functionally normal is not known, but at least in histopathological terms, there was no evidence of VIN in these sections.
This study establishes that the majority of VIN cells remain in a functional replicative or prereplicative state of the cell cycle. All cells actively involved in the cell cycle are detected with antibodies against the Mcm proteins and a subset of these with each additional cell cycle marker. As a consequence of this, immunostaining of VIN lesions, particularly with antibodies against the Mcm proteins, may have a number of clinical applications. VIN 3 is a chronic condition with a significant risk of progression to invasive vulval carcinoma (Jones, 1995). Conventional therapy, including laser and surgical excision, is typically unsatisfactory since lesions are multifocal and show high rates of recurrence (Modesitt et al, 1998). Histological grading of VIN based on H/E sections does not always accurately distinguish between VIN 2 and VIN 3 (van Beurden et al, 1999). This may have significant clinical implications since the decision to treat rather than continue with conservative management may critically depend on this distinction (van Beurden et al, 1998). The application of these approaches may therefore provide a means of improved diagnosis of high-grade VIN.
In cervical dysplasia, persistent infection with high-risk HPV types potentiates the outgrowth of cervical intraepithelial neoplasia (CIN) by the inactivation of pRb and p53 by high-risk HPV E7 and E6, respectively. Since pRb inhibits transcription of the cyclin-dependent kinase inhibitor p16INK4a, p16 is markedly overexpressed in HPV-associated CIN and cervical cancers (Klaes et al, 2001). It is therefore possible that p16INK4a may be a useful marker for dysplasia in VIN. Contrary to this expectation, Chan et al, (1998) claimed that the proportion of lesions with either undetectable p16 and/or pRb increased from benign lesions, through increasing grade of VIN to squamous carcinoma of the vulva. The latter study did not investigate the HPV status of the lesions and given the pathobiological differences between HPV positive and negative cancers (Crum, 1992), the precise relation of VIN and p16 status is still unclear. Inactivation of p16 has previously been shown to confer a loss of tumour-suppressor function in high-grade gliomas (Nishikawa et al, 1995). Another investigation of the inactivation of p16INK4a and 14-3-3σ suggested the latter as early events in vulval squamous neoplasia (Gacso et al, 2002). Downregulation of 14-3-3σ in keratinocytes allows escape from replicative senescence and this is also accompanied by loss of p16INK4a. It has been shown that loss of 14-3-3σ, via methylation-dependent transcriptional silencing, occurs commonly in vulval neoplasia, and that it frequently coexists with silencing of p16 early in the natural history of disease. A closer examination of these data shows that most HPV-16-positive VIN 2/3 (11/12) do not have methylated p16INK4a, whereas in HPV-negative lesions, 8/11 VIN 3, 2/5 VIN 2 and 0/4 VIN 1 show p16 methylation. It is obvious that there is heterogeneity in the mechanisms underlying VIN in respect of viral and other influences on p16. The expression of Mcm proteins is a measure of licence for the cell cycle, which is independent of the consequences, or presence of HPV. Thus, it seems likely that combinations of these approaches will provide a useful synergism in the study of VIN. Mcm staining may also be useful in directing laser capture analysis of expressed transcripts encoding relevant viral and immunological factors in the ongoing biological analysis of VIN (Wong et al, 2000). Further work in long-term follow-up studies is required to establish the clinical relevance of these analyses.
Acknowledgments
We thank Garry Ashton for cutting the frozen sections. Cancer Research UK supported EJD and PLS; the Wigan Cancer Research Fund Joseph Starkey Clinical Research Fellowship supported EJD. Other authors were supported by the Medical Research Council and Cancer Research UK.
References
- Adams PD (2001) Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim Biophys Acta 1471(3): M123–M133 [DOI] [PubMed] [Google Scholar]
- Buckley CH, Butler EB, Fox H (1984) Vulvar intraepithelial neoplasia and microinvasive carcinoma of the vulva. J Clin Pathol 37: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MK, Cheung TH, Chung TK, Bao SY, Zhao CL, Nobori T, Wong YF (1998) Expression of p16INK4 and retinoblastoma protein Rb in vulvar lesions of Chinese women. Gynecol Oncol 68(2): 156–161 [DOI] [PubMed] [Google Scholar]
- Crum CP (1992) Carcinoma of the vulva: epidemiology and pathogenesis. Obstet Gynecol 79: 448–454 [DOI] [PubMed] [Google Scholar]
- Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, Coleman N (1999) Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res 5: 2121–2132 [PubMed] [Google Scholar]
- Gacso M, Sullivan A, Repellin C, Brooks L, Farrell PJ, Tidy JA, Dunne B, Gusterson B, Evans DJ, Crook T (2002) Coincident inactivation of 14.3.3σ and p16INK4A is an early event in vulval squamous neoplasia. Oncogene 21: 1876–1881 [DOI] [PubMed] [Google Scholar]
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M (1999) Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 274(36): 25543–25549 [DOI] [PubMed] [Google Scholar]
- Ito M (2000) Factors controlling cyclin B expression. Plant Mol Biol 43(5–6): 677–690 [DOI] [PubMed] [Google Scholar]
- Johnson DG, Richie E, Conti C (1995) The cell cycle and cancer. Cancer Bull 47: 480–485 [Google Scholar]
- Jones RW (1995) The natural history of vulvar intraepithelial neoplasia. Br J Obstet Gynaecol 102: 764–766 [DOI] [PubMed] [Google Scholar]
- Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schimdt D, von Knebel Doeberitz M (2001) Overexpression pf p16(INK4A) as a specific marker for dyplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 92: 276–284 [DOI] [PubMed] [Google Scholar]
- Mahadevan LC, Willis AC, Barratt MJ (1991) Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid and protein synthesis inhibitors. Cell 65: 775–783 [DOI] [PubMed] [Google Scholar]
- Mills AD, Coleman N, Morris LS, Laskey RA (2000) Detection of S-phase cells in tissue sections by in situ DNA replication. Nat Cell Biol 2: 244–245 [DOI] [PubMed] [Google Scholar]
- Modesitt SC, Groben PA, Walton LA, Fowler WCJ, Van Le L (2000) Expression of Ki-67 in vulvar carcinoma and vulvar intraepithelial neoplasia III: correlation with clinical prognostic factors. Gynecol Oncol 76(1): 51–55 [DOI] [PubMed] [Google Scholar]
- Modesitt SC, Waters AB, Walton L, Fowler WC, Van Le L (1998) Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstet Gynecol 92: 962–966 [DOI] [PubMed] [Google Scholar]
- Nishikawa R, Furnari FB, Lin H, Arap W, Berger MS, Cavennee WK (1995) Loss of p16INK4A expression is frequent in high-grade gliomas. Cancer Res 55: 1941–1945 [PubMed] [Google Scholar]
- Pines J, Hunter T (1989) Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and interaction with p34cdc2. Cell 58(5): 833–846 [DOI] [PubMed] [Google Scholar]
- Ritzi M, Baack M, Musahl C, Romanowski P, Laskey RA, Knippers R (1998) Human minichromosome maintenance proteins and human origin recognition complex 2 protein on chromatin. J Biol Chem 273(38): 24543–24549 [DOI] [PubMed] [Google Scholar]
- Rolfe KJ, Crow JC, Benjamin E, Reid WMN, Maclean AB, Perrett CW (2001) Cyclin D1 and retinoblastoma protein in vulvar cancer and adjacent lesions. Int J Gynecol Cancer 11: 381–386 [DOI] [PubMed] [Google Scholar]
- Scully RE, Boniglio TA, Kurman RT, Silverberg SG, Wilkinson EJ (Eds) (1994) Vulva. Epithelial tumors and related lesions. In Histological Typing of Female Genital Tract Tumors, pp. 64. Berlin/Heidelberg: Springer-Verlag [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20(15): 1803–1815 [DOI] [PubMed] [Google Scholar]
- van Beurden M, de Craen AJM, de Vet HCW, Blaauwgeers JLG, Drillenburg P, Gallee MPW, de Kraker NW, Lammes FB, ten Kate FJW (1999) The contribution of MIB 1 in the accurate grading of vulvar intraepithelial neoplasia. J Clin Pathol 52: 820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beurden M, ten Kate F J, Smits HL, Berkhout RJ, de Craen AJ, van der Vange N, Lammes FB, ter Schegget J (1995) Multifocal vulvar intraepithelial neoplasia grade III and multicentric lower genital tract neoplasia is associated with transcriptionally active human papillomavirus. Cancer 75: 2879–2884 [DOI] [PubMed] [Google Scholar]
- van Beurden M, van der Vange N, ten Kate FJW, de Craen AJ, Schilthuis MS, Lammes FB (1998) Restricted surgical management of vulvar intraepithelial neoplasia 3: focus on exclusion of invasion and on relief of symptoms. Int J Gynecol Cancer 8: 73–77 [DOI] [PubMed] [Google Scholar]
- Van den Brule AJC, Meijer CJLM, Bakels V, Kenemans P, Walboomers JMM (1990) Rapid human papillomavirus detection in cervical scrapes by combined general primers mediated and type specific polymerase chain reaction. J Clin Microbiol 28: 2739–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoeven KH, Kovatich AJ (1996) Immunohistochemical staining for proliferating cell nuclear antigen, BCL2 and Ki-67 in vulvar tissues. Int J Gynecol Pathol 15(1): 10–16 [DOI] [PubMed] [Google Scholar]
- Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J (2001) Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology. Cancer Radiother 5(2): 109–129 [DOI] [PubMed] [Google Scholar]
- Wilkinson EJ, Kneale B, Lynch PJ (1986) Report of the ISSVD Terminology Committee. Proceedings of the Eighth World Congress of the International Society for the Study of Vulvar Diseases. Mariehamn, Finland, September 9–15, 1986. J Reprod Med 31: 973. [PubMed] [Google Scholar]
- Williams GH, Romanowski P, Morris LS, Madine M, Mills AD, Stoeber K, Marr J, Laskey RA, Coleman N (1998) Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA 95: 14932–14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH, Saam JR, Stappenbeck TS, Rexer CH, Gordon JI (2000) Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc Natl Acad Sci USA 97(23): 12601–12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamparelli A, Masciullo MV, Bovicelli A, Santini D, Ferrandina G, Minimo C, Terzano P, Costa S, Cinti C, Ceccarelli C, Mancuso S, Scambia G, Bovicelli L, Giordano A (2001) Expression of cell-cycle-associated proteins pRB2/p130 and p27kip in vulvar squamous cell carcinomas. Hum Pathol 32(1): 4–9 [DOI] [PubMed] [Google Scholar]
- Zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342–350 [DOI] [PubMed] [Google Scholar]