Abstract
Purpose
The purpose of the present study was to elucidate the role of the polyol pathway enzyme, aldose reductase (AR) in the mediation of ocular inflammation in rat model of endotoxin-induced uveitis (EIU).
Methods
EIU was induced by a subcutaneous injection of lipopolysaccharide (LPS) (200 μg) in male Lewis rats treated with AR inhibitor, zopolrestat (25 mg/kg body weight, i.p.) or carrier. The rats were sacrificed 24 h after LPS injection, eyes were enucleated immediately and aqueous humor (AqH) was collected. The number of infiltrating cells, protein concentration, and the levels of nitric oxide (NO), tumor necrosis factor (TNF)-α, and prostaglandin E2 (PGE2) in the AqH were determined. Immunohistochemical analysis was performed in para-formaldehyde-fixed eye sections by staining with antibodies against iNOS, Cox-2, TNF-α, NF-κB, and AR. The levels of ROS in rat eye sections were determined by dihydroethidium (hydroethidine) fluorescence staining.
Results
In the EIU rat eye AqH, both the infiltrating cell number and protein concentration as well as inflammatory markers, TNF-α, NO and PGE2 were significantly higher than in the control rats and inhibition of AR by zopolrestat suppressed the LPS-induced increases. The LPS-induced increased expression of AR, TNF-α, iNOS and Cox-2 proteins in ciliary body, corneal epithelium and retinal wall were also significantly inhibited by zopolrestat. Furthermore, AR inhibition also prevented the LPS-induced increased levels of ROS and activation of NF-κB in the ciliary body, corneal epithelium as well as in the retinal wall of rat eyes. The AR inhibition also prevented the LPS-induced activation of NF-κB and expression of Cox-2 and iNOS in human monocyte cells U-937.
Conclusion
The results indicate that AR inhibition suppresses the inflammation in EIU by blocking the inflammatory markers expression and release in ocular tissues along with attenuation of NF-κB activation. This suggests that AR inhibition could be a novel therapeutic target for the treatment of uveitis and associated ocular inflammation.
Keywords: Aldose reductase, inflammation, LPS, uveitis, NF-κB
Introduction
Uveitis is the major cause of severe visual impairment and has been estimated to account for 5–15% of all cases of total blindness in the US1,2. It’s even more prevalent in developing nations with limited access to health care3. The uvea has good vasculature that nourishes the eye and inflammation in uvea can affect ocular functions. Although the cause of uveitis can include autoimmune disorders, infection, or exposure to toxins, in a number of cases the etiology remains unknown4. However the ocular inflammation due to autoimmune diseases and infections is considered the major source5. Steroids and other drugs that suppress the immune response are currently used to control the inflammation have many serious side effects including severely diminishing patient’s quality of life 6, 7. In uveitis cytokine levels significantly increase in ocular tissues and initiate distinct intracellular signaling cascades that lead to both acute physiological effects and long-term changes in inflammatory gene expression1,2,8. Therefore, elucidation of cytokine signaling is critical for understanding uveitis. Endotoxin-induced uveitis (EIU) is an acute anterior segment intraocular inflammation that can be induced by lipopolysaccharide (LPS) in rodents9. Although EIU was originally used as a model of anterior uveitis, increasing evidences suggest that it also involves inflammation in the posterior segment of the eye with recruitment of leukocytes that adhere to the retinal vasculature and infiltrate the vitreous cavity1,9,10. This phenomenon could serve as a model for certain types of human uveitis such as those associated with seronegative arthritis, where gram-negative bacteria may play a role major in the pathogenesis9. LPS enhances the expression of various inflammatory cytokines and chemokines such as TNF-α, IL-6, MIF, IFN-γ, MCP-1 as well as the production of PGE2 and nitric oxide resulting in the breakdown of the blood–ocular barrier and in the infiltration of leukocytes and monocytes in ocular tissues which contribute to the development of EIU9.
Various reports show that reactive oxygen species (ROS) are obligatory mediators of inflammation induced by cytokines and chemokines11,12 which in turn induce intracellular ROS generation by a) mitochondrial respiratory chain reaction, b) the arachidonic metabolic reactions, and c) the membrane-bound superoxide-generating enzyme NADPH oxidase. Further, ROS activate redox-sensitive transcription factors such as NF-κB and AP-1 which play a central and crucial role in inflammation13–15. This is probably due to the over-expression of inflammatory cytokines and iNOS and Cox-2 enzymes resulting in increased NO and PGE216,17. These local messenger molecules act further in autocrine and paracrine fashion and amplify ROS effects. The ROS in turn activate various genes involved in the cytotoxicity. For example the pro-inflammatory cytokines TNF-α, IL-1, IL-6 play important role at initial stages of cell growth or apoptosis. Among the proinflammatory cytokines, TNF-α is known to be recognized as a central mediator in the pathophysiology of chronic inflammatory bowel diseases such as Crohn’s and ulcerative colitis which cause increased risk of uveitis18,19. Recent studies have shown the use of anti-TNF-α therapy to treat uveitis20–23. However, it is not clear how inflammation-associated increase in free radicals could cause activation of NF-κB.
Our recent studies suggest that, the polyol pathway enzyme- aldose reductase (AR; AKR1B1) besides reducing aldo-sugars reduces various lipid aldehydes and their glutathione conjugates and is an obligatory mediator of ROS signals24. Further, we have shown that inhibition or siRNA ablation of AR prevents the cytokines-, growth factors- and hyperglycemia-induced cytotoxic signals in vascular smooth muscle cells (VSMC), vascular endothelial cells (VEC) and macrophages25–28. We have also demonstrated that TNF-α- and high glucose -induced activation of NF-κB and apoptosis of human lens epithelial cells (HLEC) are significantly prevented by AR inhibition29. Further, AR inhibition prevents LPS-induced expression of TNF-α, MMP2, MMP9 and Cox-2 in HLEC which indicate the role of AR in mediating inflammatory signals in lens epithelial cells30. However, the role of AR in mediating ocular inflammation leading to uveitis is not known. In the present study, we therefore investigated the effect of AR inhibition on the ocular inflammation caused by LPS during EIU in Lewis rats. Our results show that inhibition of AR prevents EIU-induced activation of NF-κB and production of inflammatory markers such as NO, PGE2, Cox-2, and TNF-α, and accumulation of infiltrating cells in various ocular tissues suggesting possible therapeutic applications of AR inhibitors in ocular inflammation.
Materials and Methods
Materials
RPMI-1640 medium, phosphate-buffered saline (PBS), gentamicin sulfate solution, trypsin/EDTA solution and fetal bovine serum (FBS) were purchased from GIBCO BRL Life Technologies (Grand Island, NY). Zopolrestat was obtained as gift from Pfizer (New York, NY). Dimethyl sulfoxide (DMSO) was obtained from Fischer scientific (Pittsburg, PA). Nitrite/Nitrate and PGE2 assay kits were obtained from Cayman Chemical Inc (Ann Arbor, MI). Rat TNF-α ELISA kit was obtained from BD Biosciences (San Diego, CA). LPS from Escherichia coli was obtained from Sigma (Sigma-Aldrich, Saint Louise, MO). Antibodies against TNF-α, and phospho-p65 (serine 536) were purchased from cell signaling (Danvers, MA), iNOS was from Cayman Chemicals (Ann Arbor, MI), Cox-2 and GAPDH were from Santacruz biotech inc. (Santa Cruz, CA), and polyclonal antibodies against human recombinant AR were made for us by Alpha diagnostic intl. San Antonio, TX. All other reagents used were of analytical grade.
Animal groups and EIU
Six to eight-weeks-old male Lewis rats weighing approximately 150–160 g were used in this study (n=6). All animals were kept in the UTMB’s Animal Care Center. All the animal studies were conducted in compliance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research. EIU was induced by a subcutaneous injection of Escherichia coli LPS (200 μg) dissolved in phosphate-buffered saline (100 μl PBS, pH 7.4) at two different locations. Rats in ARI and EIU + ARI groups were injected intraperitoneally with AR inhibitor zopolrestat (25 mg/kg body weight) dissolved in dimethyl- sulfoxide (DMSO) 24 h before and immediately after LPS injection. Rats of control group received carrier (PBS + 20% DMSO) injection.
Infiltrating cells and proteins in aqueous humor
The rats were euthanized after 3, 6, and 24 h after LPS injection and the aqueous humor (AqH) was collected immediately from eye by an anterior chamber puncture using a 30-gauge needle under the surgical microscope. For cell counting, the AqH samples were suspended in an equal amount of Trypan-blue solution, and the cells were counted using a Hemocytometer under a light microscope (Olympus Optical Ltd). The total protein concentration in the AqH samples was measured using a Biorad protein assay kit (Biorad, CA, USA). The AqH samples were stored in ice water until testing, cell counts and total protein concentrations were measured on the day of sample collection. Rest of the AqH was stored at −80°C until used.
TNF-α, NO and PGE2 in aqueous humor
The levels of TNF-α in the AqH (stored at −80°C) were assessed with commercially available ELISA kit, according to the manufacturer’s instructions. The total level of nitrate plus nitrite in the AqH was measured by using a total nitrite colorimetric assay (LDH) kit according to the manufacturer’s instructions. PGE2 production was measured by enzyme immunoassay kit following the manufacturer’s instructions.
Histopathological evaluation
Rats were euthanized 24 h after LPS injection and the eyes were enucleated immediately and stored in 4% para-formaldehyde solution for 48 h at 4° C. The eyes were washed in ice-cold PBS twice and kept in 70% alcohol at 4° C until they were embedded in paraffin. Sagittal sections (5 μm) were cut and stained with hematoxylin and eosin (H&E). The iris-ciliary body complex, anterior chamber, vitreous and retina were observed under light microscope.
Immunohistochemical studies
The paraffin sections were warmed at 60° C for 1 h and deparafinized in xylene, followed by rehydration by passing through 100%, 95%, 80% and 70% ethanol and finally washed in deionozed water. After peroxidase blocking with 3% H2O2 the sections were rinsed in PBS twice and incubated with blocking buffer (2% BSA, 0.1% Triton-X100, 2% normal rabbit IgG and 2% normal goat serum) for overnight at 4° C. Sections were incubated with antibodies against TNF-α, iNOS, Cox-2, phospho-p65 antibodies (Ser536), and AR for 1 h at room temperature. The sections were stained using universal LSAB+System-HRP (DakoCytomation, CF, USA). The sections were examined under bright field light microscopy (EPI-800 microscope) and photographed with Nikon camera fitted to EPI-800 microscope.
Measurement of ROS
The levels of ROS in rat eye were quantified by dihydroethidium (DHE) (Molecular Probes, Eugene, OR, U.S.A.) which gives red fluorescence when oxidized to ethidium in the presence of ROS. Serial sections (5 μM) of para-formaldehyde fixed rat eyes were deparafenized, rehydrated and incubated with ROS- sensitive dye (5 μM) for 30 min at 37° C followed by acquisition of images using a fluorescence microscope.
Cell Culture and LPS treatment
U-937, a human monocytic cell line, was obtained from ATCC (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 25 mM HEPES, antibiotics (100U/mL penicillin and 100 μg/mL streptomycin) and 10% heat-inactivated fetal bovine serum and maintained at 37° C in a humidified incubator containing 95% O2 and 5% CO2. The cells were pretreated with 10 μM AR inhibitor, zopolrestat for overnight in serum-free medium and subsequently stimulated with 1 μg/mL LPS from E. coli for 24 h, unless otherwise stated.
Western Blot Analysis
U-937 cells were washed twice with ice-cold PBS and lysed in ice-cold lysis buffer containing 50 mM HEPES [pH 7.6], 10 mM KCl, 0.5% NP-40, 1 mM DTT, 1 mM phenylmethylsulfonylfluoride (PMSF), and 1:100 dilution of protease inhibitor cocktail (Sigma, Saint Louise, MO) for 15 min with occasional vortexing at maximum speed at 4° C. The crude lysates were cleared by centrifugation at 12,000 g for 10 min at 4° C. Aliquots of the lysates were diluted with 2X SDS sample buffer and boiled for 5 minutes. Lysates were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were then incubated in blocking solution containing 5% wt/vol dried fat-free milk and 0.1% vol/vol Tween-20 in Tris-buffered Saline. Subsequently, the membranes were incubated with anti-Cox-2, -iNOS, and -GAPDH antibodies. The membranes were then probed with horseradish peroxidase- conjugated secondary antibody (GE Healthcare, Piscataway, NJ) and visualized by chemiluminescence (Pierce biotechnology, Rockford, IL).
Transient transfection and NF-κB-Dependent Secretory Alkaline Phosphatase (SEAP) Expression Assay
To examine NF-κB promoter activity in U-937 cells in response to LPS treatment, U-937 cells (2.5×106 cells/well in 6-well plate) in RPMI-1640 (with 10% FBS) were transfected with pNF-κB-SEAP2-construct and pTAL-SEAP control plasmid (Clontech, USA) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following suppliers instructions. The, cells were harvested and plated in 24-well plate in serum-free medium, treated with AR inhibitor for 6 h and then stimulated with LPS (1 μg/ml) for 48 h. The cell culture media were centrifuged at 5000 rpm and supernatants were stored at −80° C. The media was thawed and used for chemiluminescent secretory alkaline phosphatase (SEAP) assay using Great EscAPe™ SEAP reporter assay system according to protocol essentially as described by the manufacturer, (BD Biosciences, Palo Alto, CA) using a 96-well chemiluminescence plate reader. All the suggested controls by manufacturers were used in the assay.
Statistical analysis
Data are expressed as the mean ± SD. All the Data were analyzed by student’s t-test using Microsoft Excel 2003 software. P<0.05 was considered as statistically significant.
Results
Effect of AR inhibition on Leukocyte infiltration and protein concentration induced by EIU
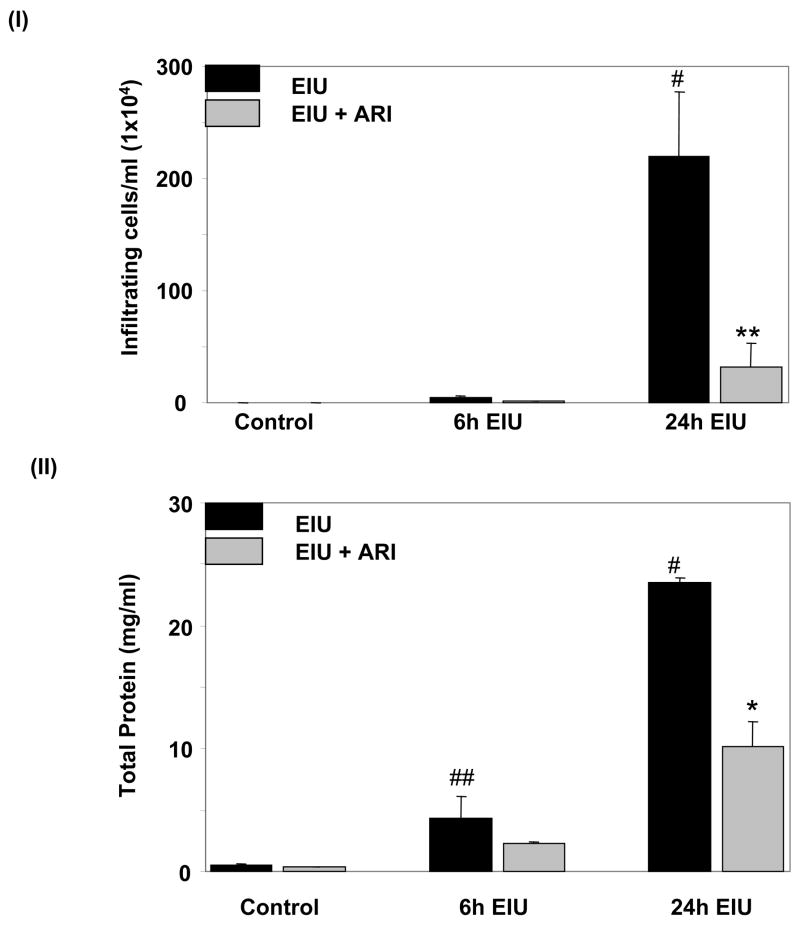
To investigate the effect of AR inhibitor on EIU–induced infiltration of inflammatory cells such as leukocytes and monocytes in the anterior chamber and aqueous humor (AqH) of the eye, saggital sections of rat eyes were stained with H&E (Fig. 1) and examined under bright-field microscope. EIU caused infiltration of a large number of cells which was significantly prevented by AR inhibitor. No significant infiltration of cells was observed in either carrier or zopolrestat alone- treated groups (data not shown). In EIU-rat eyes a few infiltrating cells were also present in the vitreous chamber (VC) but none were observed in AR inhibitor + EIU or control rats. The accumulation of infiltrating cells in AqH was also confirmed by manually counting the cells in AqH by using a hemocytometer (Fig. 1. I). As observed in histological examination, the manual cell counting also demonstrated a significant (>200-folds) increase in the infiltration of inflammatory cells in the aqueous humor of EIU-rat eyes which was significantly (>80%) prevented by AR inhibitor treatment of the EIU-rats (Fig. 1 II). In addition, the total protein concentration in the AqH of EIU-rat eyes was increased up to 23–fold as compared to control rat eyes and inhibition of AR prevented it by >60% (Fig. 1. II). These results suggest that AR inhibition prevents EIU-induced infiltration of inflammatory cells as well as release of inflammatory proteins in the AqH of rat eyes.
Fig. 1. Inhibition of AR prevents LPS-induced inflammatory cell infiltration and protein concentration in aqueous humour.
(I) The inflammatory cells and (II) total protein concentration in the AqH were measured using trypan-blue exclusion cell counting and Bradford methods, respectively as described in the Methods. Results are given as mean ± SD (n=6); #p<0.001 and ##p<0.05 Vs Control group; *p<0.01 and **p<0.001 Vs 24 h EIU- group. AqH, Aqueous humor; CB, ciliary body; I, Iris; C, cornea; R, Retina; V, Vitrious.
Effect of AR inhibition on EIU-induced inflammatory markers in AqH
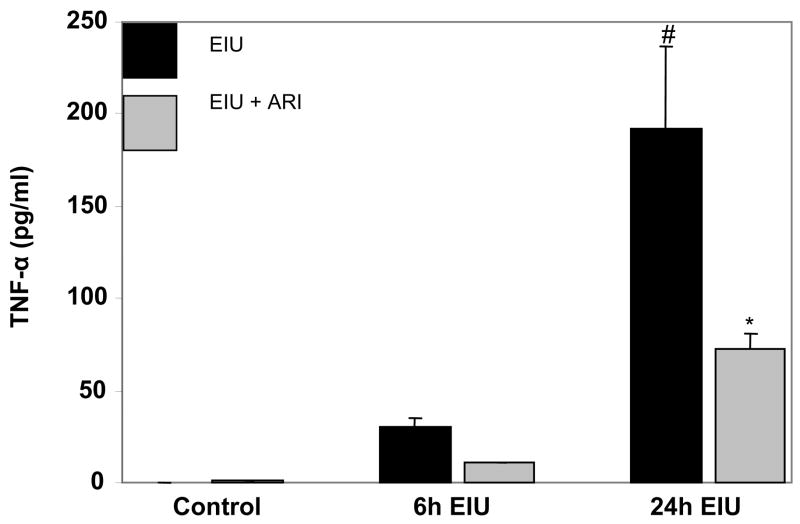
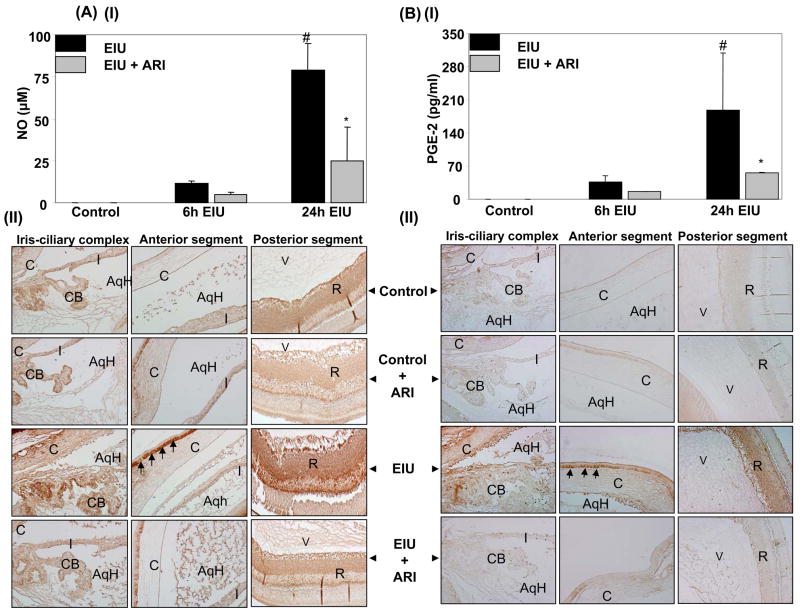
We next examined the effect of AR inhibition on the levels of inflammatory markers (TNF-α, NO and PGE2) in the AqH of EIU-rat eyes. TNF-α was non-detectable in the AqH of control animals while in EIU – rats the TNF-α levels were approximately 30 and 190 ng/ml after 6 and 24 h of EIU- induction (Fig. 2). Treatment of rats with zopolrestat followed by EIU significantly (>60%) reduced the TNF-α concentration in AqH during both time points. These results were further confirmed by immunohistochemistry using antibodies against TNF-α. The EIU – rats showed a significant intensity of antibody staining in iris-ciliary complex, and AqH region whereas, AR inhibitor-treated animals showed diminished antibody staining, indicating that AR inhibition prevents accumulation of TNF-α (data not shown). Since EIU-induced acute inflammation is not restricted to anterior chamber only9,10, we immunohistochemically examined the levels of TNF-α in vitreous region as well. The results demonstrate increased levels of TNF-α in vitreous, and retina of LPS-treated rats as compared to control groups which were significantly prevented by AR inhibitor (data no shown) suggesting anti-inflammatory role of AR inhibition in EIU. Similarly, the levels of NO and PGE2 (Fig. 3AI & 3BI) significantly increased in the aqueous humor of EIU rat eyes as compared to control and treatment with AR inhibitor significantly (>70%) reduced their levels. Since NO and PGE2 are produced by inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (Cox-2) enzymes respectively, we immunohistochemically examined the expression of iNOS and Cox-2 proteins in the various regions of eye. The EIU rat eyes showed increased expression of iNOS and Cox-2 proteins in the iris-ciliary body complex, corneal epithelium in anterior segment and retinal wall in the posterior segment (Fig. 3AII and Fig. 3BII). AR inhibitor significantly prevented the expression of iNOS as well as Cox-2 in the ocular tissues.
Fig. 2. Inhibition of AR prevents TNF-α secretion in EIU.
TNF-α levels in the AqH collected 6 and 24 h after LPS injection were measured by using ELISA kit as described in the Methods. Each value represents mean ± SD (n=4); #p<0.001 vs control group and * p<0.001 vs 24 h EIU- group.
Fig 3. Inhibition of AR prevents NO and PGE2 secretion in EIU.
(AI and BI) NO and PGE2 levels in the AqH collected 6 and 24 h after LPS injection were measured by using ELISA kits as described in Methods. Each value represents the mean ± SD (n=4), #p <0.001 vs control group and *p<0.01 vs 24 h EIU- group. (AII and BII) Serial sections of para-formaldehyde -fixed rat eyes were immuno-stained with antibodies against iNOS (AII) and Cox-2 (BII) and observed under EPI-800 microscope (A representative picture is shown (n=4); Magnification 200X). AqH, aqueous humor; C, Cornea; CB, ciliary body; I, Iris; V, Vitreous; R, Retina.
Effect of AR inhibitor on the expression of AR in EIU rat eyes
Since AR is oxidative stress response protein and increased AR protein levels have been observed in many of the pathogenesis31–33, we next examined the AR expression in EIU - rat ocular tissues. Immunohistochemical staining of EIU -rat eye sections using antibodies against AR showed a strong staining for AR in cells at iris-ciliary body, corneal epithelium layer, and retina (Fig. 4A) as compared to control eyes. However, the AR inhibitor in endotoxin-injected rat ocular tissues significantly inhibited the expression of AR, suggesting that AR inhibition prevents signaling events responsible for its own gene expression.
Fig 4. Inhibition of AR prevents expression of AR and activation of NF-κB in EIU.
(A) Serial sections of para-formaldehyde -fixed rat eyes, enucleated 24 h after EIU- induction, were immuno-stained with antibodies against AR and (B) serial sections of para-formaldehyde- fixed rat eyes, enucleated 3 h after EIU-induction, were immuno-stained with antibodies against active NF-κB (phospho-p65) as described in the Methods. The antibody staining intensity was observed under EPI-800 microscope (A representative picture is shown (n=4); Magnification 200X (A) and 400X (B)). AqH, Aqueous humor; CB, ciliary body; C, Cornea; R, Retina.
Effect of inhibition of AR on NF-κB activity in EIU rat eyes
Since redox-sensitive transcription factor NF-κB transcribes various inflammatory markers genes including that of TNF-α, iNOS and Cox-2, and AR 33,34, we next examined the effect of AR inhibition on endotoxin-induced activation of NF-κB in rat eyes. The eye sections were immuno-stained with antibodies against active subunit of NF-κB (phospho-p65) which is released subsequent to degradation of the inhibitory protein IκB and does not cross react with the inactive NF-κB complex. After 3 h of EIU, NF-κB positive cells were observed in the iris-ciliary body complex, corneal epithelium in anterior segment, and retina in posterior segment of the eye (Fig. 4B). In contrast, the number of NF-κB positive cells in the anterior as well as posterior chambers of AR inhibitor-treated EIU eyes was significantly decreased.
Effect of AR inhibition on endotoxin-induced oxidative stress
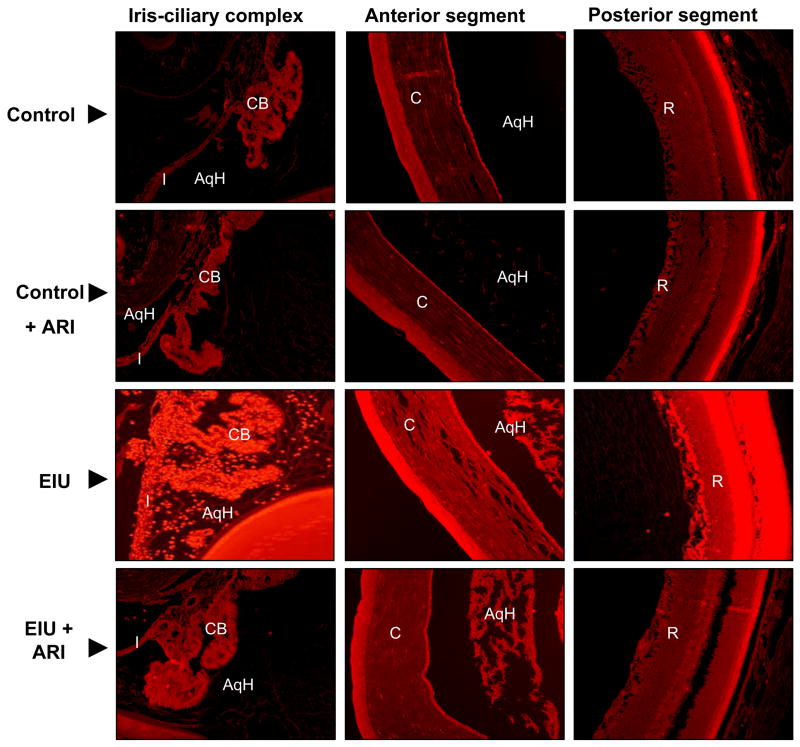
Since NF-κB is a ROS sensitive transcription factor and AR inhibition prevents EIU-induced NF-κB activation, we next examined the effect of AR inhibition on ROS generation in EIU rat eyes. As shown in Fig 5, the increased fluorescence corresponding to the increased level of ROS was observed in the iris-ciliary body complex, corneal epithelium in the anterior segment and inhibition of AR significantly prevented LPS–induced increased in ROS. Further, LPS-also increased the ROS levels in the retinal region of the posterior segment of the rat eyes and the increase was prevented by AR inhibitor.
Fig 5. Inhibition of AR prevents ROS generation in EIU.
Serial sections of para-formaldehyde -fixed rat eyes were stained with ROS-sensitive dye dihydroethidium (DHE) for 30 min at 37°C followed by acquisition of images using a fluorescence microscope (A representative picture is shown (n=4); Magnification 200X). AqH, Aqueous humor; I, Iris; CB, Ciliary body; C, Cornea; R, Retina.
Effect of AR inhibition on LPS-induced NF-κB –dependent inflammatory protein expression in human monocytic cell line
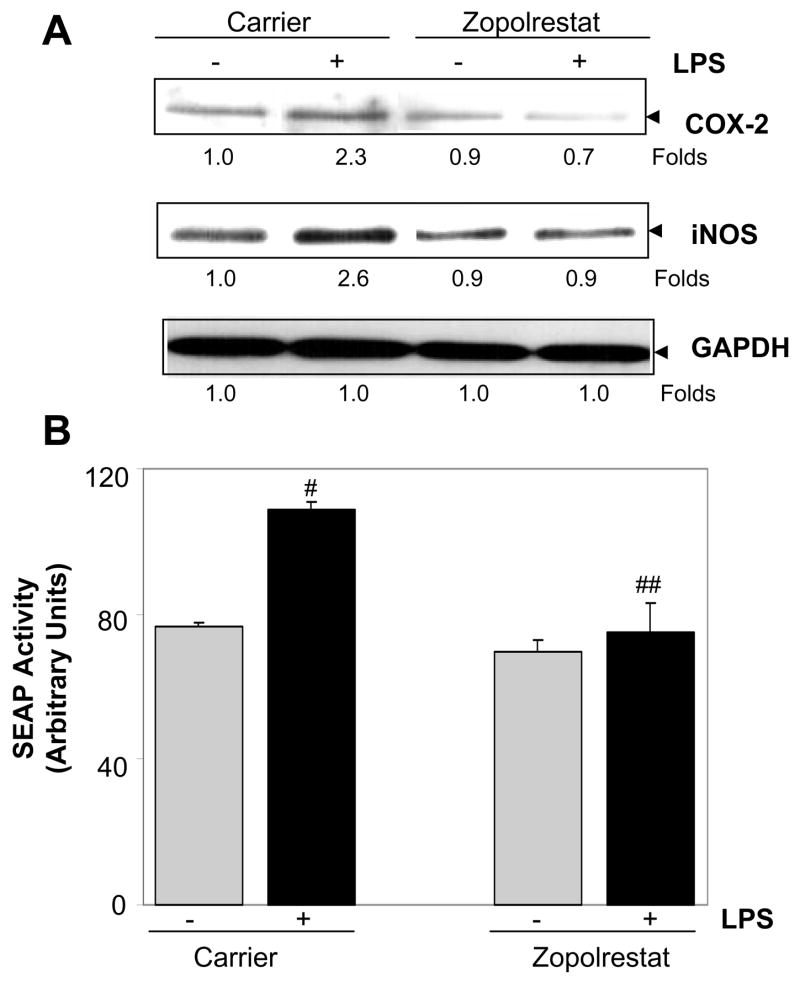
The in-vivo observations made in EIU were confirmed by in-vitro studies using U-937 (human monocytic cells) cell line as these are one of the major infiltrating cells in AqH during EIU. Incubation of U-937 cells with LPS caused 2-, and 3-fold increase in the expression of Cox-2 and iNOS proteins, respectively compared to control cells. However, in the presence of AR inhibitor, LPS-induced increase in Cox-2 and iNOS proteins in monocytic cells was significantly prevented (Fig. 6A). Furthermore, we tested the activation of NF-κB in LPS-treated cells by secretary alkaline phosphatase (SEAP) -reporter assay and the results showed ~40% increase in the SEAP activity (corresponding to NF-κB activation) as compared to controls, which was significantly prevented by AR inhibition (Fig. 6B).
Fig. 6. Effect of inhibition of AR on the LPS-induced inflammatory response in human monocytic cells.
(A). Growth- arrested U-937 cells without or with zopolrestat (10 μM each) were incubated with 1 μg/ml of LPS for 24 h. The expression of Cox-2 and iNOS proteins was determined by Western blot analysis using specific antibodies as described in the Methods. (B). U-937 cells were transiently transfected with pNF-κB-SEAP reporter vector. The cells treated without or with sorbinil and zopolrestat (10 μM each) were incubated with 1 μg/ml of LPS. After 24 h the culture supernatants were assayed for SEAP activity using chemiluminescence kit according to supplier’s instructions. Data represents mean ± SD (n = 6). #p<0.01 vs control group; ##p<0.01 vs LPS group.
Discussion
Despite significant research efforts and advances in diagnosis and therapy, ocular inflammatory diseases, which cover a variety of ocular diseases with varying clinical symptoms and pathogenicity, remain significant cause of visual impairment in humans 1–3. The disease may be of infectious or putative autoimmune etiology. Since uveitis frequently leads to severe vision loss and blindness with retinal vasculitis, retinal detachment, and glaucoma, it is important to elucidate the mechanisms that cause ocular inflammation 1–3. The present study is the first to demonstrate that the inhibition of polyol pathway enzyme leads to the suppression of ocular inflammation (uveitis) including leukocyte infiltration and protein leakage in the AqH and expression of various inflammatory markers in eye tissues.
Oxidative stress –induced ROS generation is the major factor in triggering inflammation and tissue damage during the inflammatory process induced by LPS35,36. The rationale for this study stems from our previous work showing that AR inhibitors could prevent cytokines and chemokines signals downstream to ROS that activate various transcription factors25–30. This has been attributed to the involvement of AR in a number of inflammatory mechanisms responsible for carcinogenesis and sepsis. Our studies have shown that inhibition of AR prevents production of inflammatory markers such as PGE2, Cox-2, TNF-α, IL-6 and NO in murine macrophages stimulated with LPS37 and in human colon cancer cells stimulated with growth factors38. We have also demonstrated that treatment of mouse macrophages or VSMC with 4-hydroxynonenal (HNE), GS-HNE and GS-DHN caused cytotoxicity via NF-κB- dependent signaling38,39. AR inhibitors prevented HNE and glutathione-HNE (GS-HNE)–induced cytotoxic effects but not glutathione-dihydroxynonane (GS-DHN)38,39. These observations assigned an important role to AR- catalyzed reduced product, GS-DHN in the inflammatory signaling and indicate that AR inhibition could be anti-inflammatory. Our most recent study shows that LPS- induced cytotoxicty in HLEC is mediated by AR and inhibition of AR prevents LPS-induced activation of redox-sensitive transcription factor NF-κB and production of inflammatory markers such as TNF-α, MMP2 and MMP9 in HLEC30. Since the microenvironment in the uveitis eye is characterized by high expression of inflammatory factors including cytokines, iNOS, Cox-2 and their products, PGE2 and NO1–3, inhibition of AR could represents a useful approach for prevention and/or treatment of ocular inflammatory response such as uveitis. In past several years, many AR inhibitors have been tested for potential therapy for diabetic complications such as diabetic neuropathy and retinopathy 40, 41. AR inhibitor, epalrestat, is currently marketed in Japan for treatment to diabetic neuropathy 42. AR inhibitors such as fidarestat, zenarestat and minalrestat are currently in phase-3 clinical trials 43. Common limitations to these drugs include critical hepatic and renal toxicity 43. AR inhibitor used in this study, zopolrestat, is a new AR inhibitor synthesized by Pfizer Inc 44. Zopolrestat has already gone through Phase-3 clinical trails for long term (several years) use in the therapy of diabetic neuropathy and phase-2 clinical trails for diabetic cardiomyopathy and nephropathy 40. No major side effects have been observed during clinical trails. Johnson et al (2004) 45, have shown that in patients of diabetic neuropathy receiving 500 mg to 1000 mg zopolrestat daily for 1 year did not show any major toxic effects and it reversed cardiac abnormalities observed in these patients. Our recent studies suggest the use of AR inhibitors as anti-inflammatory drugs since they can prevent generation of NF-kB dependent inflammatory cytokines and chemokines and their signals 37–39. Ocular inflammation is the major cause of uveitis and related complications 7–9.
The ocular inflammation during EIU is characterized by a breakdown of the blood-aqueous barrier with an increase of total protein content in the aqueous humor and cellular infiltration of leukocytes into the anterior chamber of the eye46. Our results indicate that AR inhibition suppressed the endotoxin -induced ocular inflammation, which is evident from significantly reduced number of leucocyte infiltration and protein concentration in the AqH of rat eyes (Fig 1). Similarly, other investigators have also shown that treatment with anti-oxidants such as vitamin E47,48, pyrrolidine dithiocarbamate49, astaxanthin50 prevents endotoxin-induced ocular inflammation. Therefore the anti-oxidative property of AR inhibitors could be used for preventing ocular inflammation. Increased protein concentration in the AqH during EIU is contributed by various inflammatory markers which include cytokines, chemokines and PGE2 and NO 51–54. High levels of TNF-α have been associated with a recurrent pattern of uveitis51,54. TNF-α is a pleiotropic cytokine produced by activated macrophages and monocytes during immune response to various infectious agents or other oxidant stimuli 54 and has also been detected in human eyes of patients with Behçet’s disease 55. Recent studies suggest that administering anti-TNF-α chimeric monoclonal antibodies (infliximab) in patients with acute uveitis and Behçet’s disease ameliorated ocular inflammation22,23,55. The role of TNF-α has been further substantiated by decreased inflammation in TNF-receptor–deficient mice in immune-complex–induced uveitis56. A number of studies have documented that LPS increases TNF-α levels in the aqueous humor during uveitis51,54. In the present study also we demonstrate that LPS significantly increased TNF-α levels in the AqH of rats with uveitis, and zopolrestat decreased the TNF-α levels. The level of the inhibition of TNF-α in the AqH corresponded to the inhibition of TNF-α expression in various tissues of rat eyes such as ciliary body, corneal epithelial cells, vitreous humor, and retina. Further, AR inhibition also prevented iNOS expression localized in epithelial cells in the iris-ciliary body, corneal epithelial cells, retina of EIU eye and NO levels in AqH (Fig 3A). Increased NO levels have been detected in the aqueous humor in Behçet’s disease patients with uveitis57. Mandai et al have shown that inhibition of iNOS activity by an iNOS inhibitor, NG-nitro-L-arginine (L-NAME), inhibits development of EIU58. Earlier studies suggest that NO could activates Cox enzymes and thereby lead to a marked increase in PGE2 production59. Our results are in agreement with the previous studies57–59, as we found that zopolrestat inhibited LPS-induced PGE2 as well as NO levels in AqH. In addition, our results suggest that the AR inhibition prevents the expression of the Cox-2 enzyme which is responsible for the production of PGE2.
Redox-sensitive transcription factor NF-κB is involved in the induction of Cox-2, iNOS and other inflammatory markers such as TNF-α 60. Also it is well known that in several cell types, LPS is the major inducer of redox sensitive transcription factor NF-κB, which regulates the expression of a variety of genes essential for cellular immune response such as inflammation, growth, development, and apoptotic processes 61–63. Alexander et al have shown that LPS activates NF-κB in the mice lens64 and Dudek et al have shown that the TNF-α activates NF-κB in HLEC 65. Moreover, there are several reports that show elevation of NF-κB in various eye tissues of animals with uveitis 62,63. Therefore, a possible mechanism for the inhibition of Cox-2, iNOS and TNF-α expression by AR inhibition could be due to inhibition of LPS-induced NF-κB activation 60. Indeed our results suggest that AR inhibition prevents NF-κB activation in rat eye tissues (Fig 4 B). We and others have earlier shown that inhibition of AR prevents PKC and NF-κB activation by a variety of stimuli such as TNF-α, FGF, PDGF, angiotensin-II and high glucose and hyperglycemia-induced MAPK and JAK2 25–29, 66–68. This would suggest that AR could be an obligatory mediator of stress -response including the activation of NF-κB and other ROS-sensitive transcription factors. Although the mechanisms by which AR activates NF-κB remain unclear, we propose that inhibition of AR prevents the events that could lead to the activation of PLC and PKC isozymes which are activated by LPS as observed in our in-vitro cell culture studies25–28. In summary, our results provide evidence for an unanticipated role of AR in mediating NF-κB-dependent inflammatory response during acute inflammatory conditions and provide a novel concept that inhibition of AR could be therapeutically useful in preventing ocular inflammation such as uveitis.
Acknowledgments
This work was supported in part by NIH grants GM71036 (KVR) and DK36118 (SKS).
References
- 1.Read RW. Uveitis: advances in understanding of pathogenesis. Curr Rheumatol Rep. 2006;8:260–266. doi: 10.1007/s11926-006-0006-6. [DOI] [PubMed] [Google Scholar]
- 2.Curi A, Matos K, Pavesio C. Acute anterior uveitis. Clin Evid. 2005;14:739–743. [PubMed] [Google Scholar]
- 3.Rathinam SR, Cunningham ET., Jr Infectious causes of uveitis in the developing world. Int Ophthalmol Clin. 2000;40:137–152. doi: 10.1097/00004397-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Murray PI. Chronic non-infectious uveitis in the elderly: epidemiology, pathophysiology and management. Drugs Aging. 2006;23:535–558. doi: 10.2165/00002512-200623070-00001. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 6.Dukes MNG. Corticotrophins and corticosteroids. In: Dukes MNG, editor. Meyler’s Side Effects of Drugs. Amsterdam: Elsevier; 1996. pp. 1189–1209. [Google Scholar]
- 7.Samudre SS, Lattanzio FA, Jr, Williams PB, Sheppard JD., Jr Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004;20(6):533–47. doi: 10.1089/jop.2004.20.533. [DOI] [PubMed] [Google Scholar]
- 8.Curnow SJ, Murray PI. Inflammatory mediators of uveitis: cytokines and chemokines. Curr Opin Ophthalmol. 2006;17:532–537. doi: 10.1097/ICU.0b013e32801094b5. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 10.Altan-Yaycioglu R, Akova YA, Akca S, Yilmaz G. Inflammation of the posterior uvea: findings on fundus fluorescein and indocyanine green angiography. Ocul Immunol Inflamm. 2006;14:171–179. doi: 10.1080/09273940600660524. [DOI] [PubMed] [Google Scholar]
- 11.Nagata M. Inflammatory cells and oxygen radicals. Curr Drug Targets Inflamm Allergy. 2005;4:503–504. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Fisher AB. ROS to the rescue. Am J Physiol Lung Cell Mol Physiol. 2004;287:L704–L705. doi: 10.1152/ajplung.00182.2004. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol. 2002;2:1509–1520. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 14.Kitamei H, Iwabuchi K, Namba K, Yoshida K, Yanagawa Y, Kitaichi N, Kitamura M, Ohno S, Onoe K. Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-kappaB (NF-kappaB), pyrrolidine dithiocarbamate. J Leukoc Biol. 2006;79:1193–1201. doi: 10.1189/jlb.0805453. [DOI] [PubMed] [Google Scholar]
- 15.Fang IM, Yang CH, Lin CP, Yang CM, Chen MS. Effects of pyrrolidine dithiocarbamate, an NF-kappaB inhibitor, on cytokine expression and ocular inflammation in experimental autoimmune anterior uveitis. J Ocul Pharmacol Ther. 2005;21:95–106. doi: 10.1089/jop.2005.21.95. [DOI] [PubMed] [Google Scholar]
- 16.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 17.Fraser CC. Exploring the positive and negative consequences of NF-kappaB inhibition for the treatment of human disease. Cell Cycle. 2006;5:1160–1163. doi: 10.4161/cc.5.11.2773. [DOI] [PubMed] [Google Scholar]
- 18.Lin P, Tessler HH, Goldstein DA. Family history of inflammatory bowel disease in patients with idiopathic ocular inflammation. Am J Ophthalmol. 2006;141:1097–104. doi: 10.1016/j.ajo.2006.01.075. [DOI] [PubMed] [Google Scholar]
- 19.Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714–718. doi: 10.1053/gast.2002.35396. [DOI] [PubMed] [Google Scholar]
- 20.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Prog Reti Eye Res. 2004;23:617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Hale S, Lightman S. Anti-TNF therapies in the management of acute and chronic uveitis. Cytokine. 2006;33:231–237. doi: 10.1016/j.cyto.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 22.El-Shabrawi Y, Hermann J. Anti-tumor necrosis factor-alpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology. 2002;109:2342–2346. doi: 10.1016/s0161-6420(02)01292-7. [DOI] [PubMed] [Google Scholar]
- 23.Joseph A, Raj D, Dua HS, Powell PT, Lanyon PC, Powell RJ. Infliximab in the treatment of refractory posterior uveitis. Ophthalmology. 2003;110:1449–1453. doi: 10.1016/S0161-6420(03)00406-8. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 25.Ramana KV, Chandra D, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase Mediates Mitogenic Signaling in Vascular Smooth Muscle Cells. J Biol Chem. 2002;275:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 26.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 27.Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-alpha-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;28:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 29.Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 30.Pladzyk A, Reddy ABM, Yadav UCS, Tammali R, Ramana KV, Srivastava SK. Inhibition of Aldose Reductase Prevents Lipopolysaccharide- Induced inflammatory response in Human Lens Epithelial Cells. Invest Opthamol Vis Sci. 2006;47:5395–403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- 31.Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF, Lavandero S. Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem. 2003;278:38484–38494. doi: 10.1074/jbc.M211824200. [DOI] [PubMed] [Google Scholar]
- 32.El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- 33.Iwata T, Sato S, Jimenez J, McGowan M, Moroni M, Dey A, Ibaraki N, Reddy VN, Carper D. Osmotic response element is required for the induction of aldose reductase by tumor necrosis factor-alpha. J Biol Chem. 1999;274:7993–8001. doi: 10.1074/jbc.274.12.7993. [DOI] [PubMed] [Google Scholar]
- 34.Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;1:425–435. [PubMed] [Google Scholar]
- 35.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579:22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 38.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin e2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 39.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 40.Hamada Y, Nakamura J. Clinical potential of aldose reductase inhibitors in diabetic neuropathy. Treat Endocrinol. 2004;3:245–255. doi: 10.2165/00024677-200403040-00006. [DOI] [PubMed] [Google Scholar]
- 41.Porta M, Allione A. Current approaches and perspectives in the medical treatment of diabetic retinopathy. Pharmacol Ther. 2004;103:167–177. doi: 10.1016/j.pharmthera.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Hotta N, Sakamoto N, Shigeta Y, Kikkawa R, Goto Y the Diabetic Neuropathy Study Group in Japan. Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. J Diabetes Complications. 1996;10:168–172. doi: 10.1016/1056-8727(96)00113-4. [DOI] [PubMed] [Google Scholar]
- 43.El-Kabbani O, Ruiz F, Darmanin C, Chung RP. Aldose reductase structures: implications for mechanism and inhibition. Cell Mol Life Sci. 2004;61:750–762. doi: 10.1007/s00018-003-3403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inskeep PB, Reed AE, Ronfeld RA. Pharmacokinetics of zopolrestat, a carboxylic acid aldose reductase inhibitor, in normal and diabetic rats. Pharm Res. 1991;8:1511–1515. doi: 10.1023/a:1015894300247. [DOI] [PubMed] [Google Scholar]
- 45.Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, Law G, Wackers FJ, Young LH. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27:448–454. doi: 10.2337/diacare.27.2.448. [DOI] [PubMed] [Google Scholar]
- 46.Streilein JW, Ohta K, Mo JS, Taylor AW. Ocular immune privilege and the impact of intraocular inflammation. DNA Cell Biol. 2002;21:453–459. doi: 10.1089/10445490260099746. [DOI] [PubMed] [Google Scholar]
- 47.Kukner A, Colakoglu N, Serin D, Alagoz G, Celebi S, Kukner AS. Effects of intraperitoneal vitamin E, melatonin and aprotinin on leptin expression in the guinea pig eye during experimental uveitis. Acta Ophthalmol Scand. 2006;84:54–61. doi: 10.1111/j.1600-0420.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 48.Nussenblatt RB, Kim J, Thompson DJ, Davis MD, Chew E, Ferris FL, Buggage R. Vitamin E in the treatment of uveitis-associated macular edema. Am J Ophthalmol. 2006;141:193–194. doi: 10.1016/j.ajo.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Ohta K, Nakayama K, Kurokawa T, Kikuchi T, Yoshimura N. Inhibitory effects of pyrrolidine dithiocarbamate on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2002;43:744–750. [PubMed] [Google Scholar]
- 50.Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, Yazawa K, Yoshida K, Kase S, Ohno S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–281. doi: 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Santos Lacomba M, Marcos Martin C, Gallardo Galera JM, Gomez Vidal MA, Collantes Estevez E, Ramirez Chamond R, Omar M. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–255. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 52.Koga T, Koshiyama Y, Gotoh T, Yonemura N, Hirata A, Tanihara H, Negi A, Mori M. Coinduction of nitric oxide synthase and arginine metabolic enzymes in endotoxin-induced uveitis rats. Exp Eye Res. 2002;75:659–667. doi: 10.1006/exer.2002.2062. [DOI] [PubMed] [Google Scholar]
- 53.Williams RN, Paterson CA. PMN accumulation in aqueous humor and iris-ciliary body during intraocular inflammation. Invest Ophthalmol Vis Sci. 1984;25:105–108. [PubMed] [Google Scholar]
- 54.Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chignell AH, Dumonde DC. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11:187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- 55.Tugal-Tutkun I, Mudun A, Urgancioglu M, Kamali S, Kasapoglu E, Inanc M, Gul A. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behcet’s disease: an open-label trial. Arthritis Rheum. 2005;52:2478–2484. doi: 10.1002/art.21231. [DOI] [PubMed] [Google Scholar]
- 56.Brito BE, O’Rourke LM, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40:2583–2589. [PubMed] [Google Scholar]
- 57.Duygulu F, Evereklioglu C, Calis M, Borlu M, Cekmen M, Ascioglu O. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active Behcet’s disease: a pilot study. Clin Rheumatol. 2005;24:324–330. doi: 10.1007/s10067-004-1015-3. [DOI] [PubMed] [Google Scholar]
- 58.Mandai M, Yoshimura N, Yoshida M, Iwaki M, Honda Y. The role of nitric oxide synthase in endotoxin-induced uveitis: effects of NG-nitro L-arginine. Invest Ophthalmol Vis Sci. 1994;35:3673–3680. [PubMed] [Google Scholar]
- 59.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–68. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 61.Shiratori K, Ohgami K, Ilieva IB, Koyama Y, Yoshida K, Ohno S. Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by alpha-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci. 2004;45:159–164. doi: 10.1167/iovs.03-0492. [DOI] [PubMed] [Google Scholar]
- 62.Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 63.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander G, Carlsen H, Blomhoff R. Strong in vivo activation of NF-kappaB in mouse lenses by classic stressors. Invest Ophthalmol Vis Sci. 2003;44:2683–2688. doi: 10.1167/iovs.02-0829. [DOI] [PubMed] [Google Scholar]
- 65.Dudek EJ, Shang F, Taylor A. H2O2-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med. 2001;31:651–658. doi: 10.1016/s0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 66.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 67.Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem. 2003;278:30634–30641. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- 68.Hwang YC, Shaw S, Kaneko M, Redd H, Marrero MB, Ramasamy R. Aldose reductase pathway mediates JAK-STAT signaling: a novel axis in myocardial ischemic injury. FASEB J. 2005;19:795–797. doi: 10.1096/fj.04-2780fje. [DOI] [PubMed] [Google Scholar]