Abstract
Rotavirus (RV) is the leading cause of infantile gastroenteritis worldwide. RV nonstructural protein 4 (NSP4), the first characterized viral enterotoxin, is a 28 kDa glycoprotein that has pleiotropic functions in RV infection and pathogenesis. NSP4 has multiple forms enabling it to perform its different functions. Dissecting such functions could be facilitated by use of epitope-specific antibodies. This work mapped the epitopes for the monoclonal antibody B4-2/55 and three polyclonal antisera generated against synthetic SA11 NSP4 peptides corresponding to residues 114–135, 120–147, and 150–175. The epitope for B4-2/55 mapped to residues 100–118, wherein residues E105, R108 and E111 are critical for antibody binding. Antiserum generated to two peptides (aa114–135 and aa120–147) with enterotoxin activity each recognize a single but distinct epitope. The epitope for the peptide antiserum to aa114–135 was mapped to residues 114–125 with highly conserved residues T117/T118, E120, and E122 being critical for antibody binding. The peptide antiserum to aa120–147 binds to NSP4 at residues 130–140 and residues Q137–T138 are critical for this epitope. Finally, the epitope for the antiserum to peptide aa150–175 mapped to residues 155–170, wherein residues E160 and E170 are critical for antibody binding. Knowledge of the binding sites of domain-specific antibodies can aid in further characterizing different functions of NSP4. To demonstrate this, we characterized the interaction between NSP4 and VP5* [Kd = 0.47µM] and show that binding of NSP4 to VP5* is blocked by antibody to NSP4 aa114–135 and aa120–147, but not aa150–175. The use of single epitope-specific antibodies to differentially block functions of NSP4 is a feasible approach to determine the functional domain structure of this important RV virulence factor.
Keywords: rotavirus, NSP4, viral enterotoxin, epitope-specific antibody, monoclonal antibody
Introduction
Rotaviruses (RV) are complex, glycosylated but nonenveloped viruses. The RV genome consists of 11 segments of double-stranded RNA that code for six structural and six nonstructural proteins (Estes and Kapikian, 2007). RV is the leading etiological agent of severe gastroenteritits in infants and young animals worldwide, which leads to the hospitalization of 1 in 60 children in the United States and approximately 600,000 fatalities worldwide (Fischer et al., 2007; Parashar et al., 2003). The product of one RV gene segment is the nonstructural protein 4 (NSP4), which is a pleiotropic protein involved in many aspects of RV infection including being the first characterized viral enterotoxin (Ball et al., 1996). NSP4 is initially produced in the endoplasmic reticulum (ER) as a transmembrane glycoprotein with high mannose carbohydrate moieties on amino acids (aa) 8 and 18 (Ericson et al., 1983b; Ericson et al., 1983a). Intracellularly expressed NSP4 causes an increase in the levels of intracellular calcium, which in-turn influences vesiculation of NSP4 and the association of NSP4-containing vesicles with viroplasms (Berkova et al., 2006). NSP4 also serves as an intracellular receptor for VP6 on newly formed double-layered particles (DLPs) (Au et al., 1993; Berkova et al., 2006; Meyer et al., 1989). In a morphological process unique to RV, nascent DLPs bud into the lumen of the ER, with particles acquiring a transient envelope as well as the outer coat protein VP7 and the spike protein VP4 (Petrie et al., 1983; Tian et al., 1996). Through an uncharacterized mechanism that involves NSP4 and VP7, the envelope is lost and VP4 and VP7 are retained to generate the mature virion (Cuadras et al., 2006; Lopez et al., 2005; Poruchynsky and Atkinson, 1991).
In addition to its role in RV morphogenesis, NSP4 is the only characterized viral enterotoxin (Ball et al., 1996; Jagannath et al., 2006; Mori Y et al., 2002; Sasaki et al., 2001; Zhang et al., 2000). From the ER, NSP4 traffics to the plasma membrane via a non-classical, Golgi-independent route. NSP4 is cleaved by a still uncharacterized protease and a C-terminal cleavage product is released from the infected cell (Zhang et al., 2000). This cleavage product consists of aa112–175 and contains the enterotoxin domain (aa114–135), which stimulates a phospholipase C-mediated Ca2+ mobilization and age-related Cl− secretion from neonatal but not adult intestinal cells. This is thought to be the mechanism behind the age-dependent diarrhea caused by NSP4 (Ball et al., 1996; Dong et al., 1997; Morris et al., 1999).
NSP4 is found in multiple forms. Several studies, including three crystal structures, indicate that NSP4 forms oligomers, including dimers, tetramers, and larger oligomers (Bowman et al., 2000; Deepa et al., 2007; Jagannath et al., 2006; Taylor et al., 1998). Computational and biochemical analyses indicate an extended coiled-coil domain from amino acids 85–135 directs the formation of NSP4 tetramers, but both hydrophobic (aa24–46) and amphipathic alpha helical (aa55–85) domains as well as the C-terminus (aa146–175) contribute to the formation of large aggregates of NSP4 (Jagannath et al., 2006; Lin and Tian, 2003). Further, NSP4 is found in multiple cellular compartments, as well as in viroplasms, and a cleavage product is released extracellularly (Berkova et al., 2006; Parr et al., 2006; Sapin et al., 2002; Zhang et al., 2000). Several cellular protein-binding sites have been mapped to the NSP4 cytoplasmic tail (Ball et al., 2005). The binding sites for the RV spike protein VP4 and intermediate capsid protein VP6 have been mapped to NSP4 aa112–148 and aa161–175, respectively (Au et al., 1993; O'Brien et al., 2000). The multitude of NSP4 forms and functions requires highly specific reagents to differentiate between various forms of NSP4 and determine the functional significance of a given form.
This manuscript reports the mapping of four epitope-specific antibodies to the NSP4 cytoplasmic tail: monoclonal antibody (MAb) B4-2/55 and antisera generated to synthetic NSP4 peptides corresponding to aa114–135 (αNSP4114–135), aa120–147 (αNSP4120–147), and aa150–175 (αNSP4150–175). For each, residues that are critical for the binding of the antibody to NSP4 were identified. The utility of these antibodies was demonstrated by characterizing the interaction between NSP4 and the VP4 spike protein, which is blocked by antibodies specific for the enterotoxin domain.
Materials and Methods
Cell Lines and Viruses
Simian SA11 clone 3 (cl. 3) (G3, P6[2]) and RRV (G3, P5B[3]); human S2 (G2, P1B[4]) and Ito (G3, P1A[8]); lapine Ala (G3, P11[14]) and C-11 (G3, P11[14]); porcine OSU (G5, P9[7]); murine ECTC (G3, P[19]); and avian Ty-1 (G7, P[17]) rotavirus strains were propagated in the African Green Monkey kidney cell line MA104 using DMEM supplemented with trypsin (Worthington Biochemical Corporation, Lakewood, NJ) as previously described (Ciarlet et al., 2002). The αNSP4 hybridoma cell line B4-2/55/17(1)/13 was generated and characterized as previously described (Petrie et al., 1984) and was a kind gift from Dr. Harry Greenberg (Stanford University Medical School).
Antibodies
Rabbit polyclonal antiserum to the SA11 NSP4 synthetic peptide aa113–149 (αNSP4113–149) was kindly provided by Dr. Judy Ball (Texas A&M)(Parr et al., 2006). For mouse αNSP4-FL, PCR-amplified fragments corresponding to SA11 NSP4 amino acids 1–175 were cloned into pFastBacHT (Invitrogen Corporation, Carlsbad, CA). Generation of the recombinant baculovirus and expression of HisNSP4-FL was done as previously described (Zhang et al., 1998). HisNSP4-FL was purified as described for bacterially expressed HisNSP4 except N-lauroylsarcosine was used in place of Trition-X100. The purity of HisNSP4-FL was determined by SDS-PAGE followed by Coomassie Blue staining and the concentration determined by the Bradford assay. Balb/C mice were primed with 30 µg HisNSP4-FL per mouse in Freund’s complete adjuvant followed by 3 boosts of 10 µg/mouse in Freund’s incomplete adjuvant. The anti-NSP4 titers were determined by ELISA. The antisera for the peptide specific antibodies αNSP4114–135 and αNSP4120–147 were generated as described previously (Ball et al., 1996), and antisera for the peptide specific αNSP4150–175 was generated similarly. The MAb B4-2/55/17(1)/13 hybridoma cell line was used to generate ascites in pristine-primed Balb/C mice as previously described (Burns et al., 1988).
Cloning of HisNSP4 Truncation Constructs
A 6x-histidine tag bacterial expression vector, pET46Ek/LIC (EMD Biosciences, Inc., San Diego), was used to express N- and C-terminal NSP4 deletion mutants. The primers used to generate these constructs are listed in Table 1. PCR-amplified fragments of NSP4 were generated using the appropriate primer pairs and cloned into pET46Ek/LIC by ligation independent cloning according to the manufacturer’s protocol. Recombinant vector was transformed into competent BL21(DE3) E. coli (EMD Biosciences, Inc., San Diego) and plated on LB agar supplemented with 100 µg/ml ampicillin. All constructs were confirmed by nucleotide sequencing (Lone Star Labs, Houston, TX).
Table 1.
Primers for N- and C-terminal Truncation Constructs
| Primer Name | Sequence |
|---|---|
| Fwda aa75 | 5′GACGACGACAAGATGATTTTTAATACGTTGTTAAAATTG |
| Fwd aa90 | 5′GACGACGACAAGATAACTACTAAAGATGAGATAG |
| Fwd aa95 | 5′GACGACGACAAGATAGAAAAGCAAATGGACAG |
| Fwd aa100 | 5′GACGACGACAAGATAGACAGAGTAGTCAAAGAAATG |
| Fwd aa105 | 5′GACGACGACAAGATAGAAATGAGACGCCAGCTAG |
| Fwd aa112 | 5′GACGACGACAAGATAATGATTGACAAATTGACTAC |
| Fwd aa130 | 5′GACGACGACAAGATGATTTACGATAAATTGACGGTGC |
| Revb aa175 | 5′GAGGAGAAGCCCGGTTTACATTGCTGCAGTCAC |
| Rev aa146 | 5′GAGGAGAAGCCCGGTTTATTTTGTCATATCTATTTCGCC |
| Rev aa140 | 5′GAGGAGAAGCCCGGTTTAGCCTGTCGTTTGCACCGT |
| Rev aa135 | 5′GAGGAGAAGCCCGGTTTACGTCAATTTATCGTAAATG |
| Rev aa130 | 5′GAGGAGAAGCCCGGTTTAAATGCGTTTAAGCAACTC |
| Rev aa125 | 5′GAGGAGAAGCCCGGTTTACTCTACTTGTTCAATTTC |
| Rev aa120 | 5′GAGGAGAAGCCCGGTTTATTCACGTGTAGTCAATTTG |
| Rev aa118 | 5′GAGGAGAAGCCCGGTTTATGTAGTCAATTTGTCAATC |
| Rev aa116 | 5′GAGGAGAAGCCCGGTTTACAATTTGTCAATCATTTC |
Forward (Fwda) and reverse (Revb) primers are listed and numbered according to the first or last NSP4 residue encoded, respectively. The underlined sequence is added according to the manufacturer’s protocol for the ligation-independent cloning procedure.
Site-Directed Mutagenesis of HisNSP490–175
The specified residues in HisNSP490–175 were altered using the Quickchange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA), using the primer pairs listed in Table 2, according to the manufacturer's instructions. Briefly, the primer pairs listed below containing the desired mutation were annealed to the complementary regions of the parental plasmid template and extended using Pfu polymerase to generate a mutated plasmid containing staggered nicks. The methylated parental DNA was digested by treatment with DpnI, and the newly synthesized nicked vector DNA was used to transform competent BL21(DE3) E. coli bacterial cells. Successful mutation of the desired residue was validated by nucleotide sequencing.
Table 2.
Mutagenesis Primers for Alanine Point Mutants
| Mutation | FORWARD SEQUENCE | REVERSE SEQUENCE |
|---|---|---|
| D100A | 5′GATGAGATAGAAAAGCAAATGGCCAGAGTAGTCAAAGAAATGAGAC | 5′GTCTCATTTCTTTGACTACTCTGGCCATTTGCTTTTCTATCTCATC |
| K104A | 5′GCAAATGGACAGAGTAGTCGCAGAAATGAGACGCCAGCTAG | 5′CTAGCTGGCGTCTCATTTCTGCGACTACTCTGTCCATTTGC |
| E105A | 5′CAAATGGACAGAGTAGTCAAAGCAATGAGACGCCAGCTAGAAATG | 5′CATTTCTAGCTGGCGTCTCATTGCTTTGACTACTCTGTCCATTTG |
| R107A | 5′GACAGAGTAGTCAAAGAAATGGCACGCCAGCTAGAAATGATTGAC | 5′GTCAATCATTTCTAGCTGGCGTGCCATTTCTTTGACTACTCTGTC |
| R108A | 5′CAGAGTAGTCAAAGAAATGAGAGCCCAGCTAGAAATGATTGACAAATTG | 5′CAATTTGTCAATCATTTCTAGCTGGGCTCTCATTTCTTTGACTACTCTG |
| E111A | 5′GTCAAAGAAATGAGACGCCAGCTAGCAATGATTGACAAATTGACTACACG | 5′CGTGTAGTCAATTTGTCAATCATTGCTAGCTGGCGTCTCATTTCTTTGAC |
| M112A | 5′GTCAAAGAAATGAGACGCCAGCTAGAAGCGATTGACAAATTGACTACACG | 5′CGTGTAGTCAATTTGTCAATCGCTTCTAGCTGGCGTCTCATTTCTTTGAC |
| E105A/R108A/E111A | 5′GGACAGAGTAGTCAAAGCAATGAGAGCCCAGCTAGCAATGATTGACAAATTG | 5′CAATTTGTCAATCATTGCTAGCTGGGCTCTCATTGCTTTGACTACTCTGTCC |
| T117A/T118A | 5′CTAGAAATGATTGACAAATTGGCCGCACGTGAAATTGAACAAGTAGAG | 5′CTCTACTTGTTCAATTTCACGTGCGGCCAATTTGTCAATCATTTCTAG |
| R119A | 5′GATTGACAAATTGACTACAGCTGAAATTGAACAAGTAGAG | 5′CTCTACTTGTTCAATTTCAGCTGTAGTCAATTTGTCAATC |
| E120A | 5′GACAAATTGACTACACGTGCAATTGAACAAGTAGAGTTG | 5′CAACTCTACTTGTTCAATTGCACGTGTAGTCAATTTGTC |
| E122A | 5′GACTACACGTGAAATTGCAGAACAAGTAGAGTTGC | 5′GCAACTCTACTTGTTCTGCAATTTCACGTGTAGTC |
| Q123A | 5′GACTACACGTGAAATTGAAGCTGTAGAGTTGCTTAAACGC | 5′ GCGTTTAAGCAACTCTACAGCTTCAATTTCACGTGTAGTC |
| L126A/L127A | 5′CGTGAAATTGAACAAGTAGAGGCGGCCAAACGCATTTACGATAAATTG | 5′CAATTTATCGTAAATGCGTTTGGCCGCCTCTACTTGTTCAATTTCACG |
| Y131A | 5′GTAGAGTTGCTTAAACGCATTGCAGATAAATTGACGGTGCAAACG | 5′CGTTTGCACCGTCAATTTATCTGCAATGCGTTTAAGCAACTCTAC |
| T135A | 5′CGCATTTACGATAAATTGGCGGTGCAAACGACAGGCGAAATAG | 5′CTATTTCGCCTGTCGTTTGCACCGCCAATTTATCGTAAATGCG |
| Q137A | 5′CGATAAATTGACGGTGGCAACGACAGGCGAAATAG | 5′CTATTTCGCCTGTCGTTGCCACCGTCAATTTATCG |
| T138A | 5′CGATAAATTGACGGTGCAAGCGACAGGCGAAATAGATATGAC | 5′GTCATATCTATTTCGCCTGTCGCTTGCACCGTCAATTTATCG |
| E160A | 5′GTGAGAACGCTAGAAGAATGGGCAAGTGGAAAAAATCCTTATG | 5′CATAAGGATTTTTTCCACTTGCCCATTCTTCTAGCGTTCTCAC |
| K163A | 5′GAAGAATGGGAAAGTGGAGCAAATCCTTATGAACCAAGAG | 5′CTCTTGGTTCATAAGGATTTGCTCCACTTTCCCATTCTTC |
| E170A | 5′GGAAAAAATCCTTATGAACCAAGAGCAGTGACTGCAGCAATG | 5′CATTGCTGCAGTCACTGCTCTTGGTTCATAAGGATTTTTTCC |
The amino acid position of the mutation(s) is listed on the left with the corresponding forward and reverse primers.
Expression and Purification of HisNSP4 Truncation and Mutant Constructs
Overnight cultures of BL21(DE3) cells transformed with each plasmid were diluted to a ratio of 1:10 in fresh LB broth supplemented with 100 µg/ml ampicillin. Cells were grown at 37°C for 3 hr or until a cell density of A600 =0.6 – 0.7 was reached. Recombinant protein expression was induced by addition of 1.0 mM isopropyl-β-D-thiogalactopyranoside (IPTG) (Invitrogen Corporation, Carlsbad, CA), and cultures were grown overnight at 25°C. Cells were harvested by centrifugation for 15 minutes at 13,800 × g at 4°C, and the cell pellet was suspended in lysis buffer (10mM sodium phosphate, 500mM sodium chloride, 10mM imidazole, and 1% Triton X-100, pH 8.0) and incubated for 15 minutes to lyse the cells. Insoluble material was pelleted by centrifugation for 30 minutes at 21,000 × g at 4°C. The supernatant was reserved and the pellet was re-extracted with lysis buffer two additional times. PBS-washed Ni2+-NTA beads (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) were added to the pooled supernatants and incubated with agitation for 1 hr at room temperature. The Ni2+-NTA beads were pelleted by centrifugation for 5 minutes at 3,200×g at 4°C, washed with wash buffer 1 (10 mM sodium phosphate, 500mM sodium chloride, 10 mM imidazole, pH 8.0), repelleted, and washed with wash buffer 2 (10 mM sodium phosphate, 500 mM sodium chloride, 50 mM imidazole, pH 8.0). The beads were again pelleted and the recombinant HisNSP4 proteins were eluted with elution buffer (10 mM sodium phosphate, 500 mM sodium chloride, 500 mM imidazole, pH 8.0). Each recombinant protein was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to a modified Laemmli protocol (Zhang et al., 2000) followed by Coomassie Blue (Sigma-Aldrich, St. Louis, MO) staining and protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Immunoblot Analysis of RV-infected Cell Lysates and HisNSP4 Constructs
Cell lysates or purified HisNSP4 protein was mixed with SDS-PAGE sample buffer, boiled for 5 minutes, and loaded onto 4–20% polyacryamide gradient gels (BioRad) to separate protein samples, which were then transferred onto a nitrocellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) as previously described (Zhang et al., 2000). Nitrocellulose membranes were stained with Ponceau S (Sigma-Aldrich, St. Louis, MO) to confirm equal loading of protein. Membranes were blocked using 5% Carnation Instant Milk in PBS (Blotto) for 10 minutes with agitation at room temperature. Proteins were detected with αNSP4113–149 serum at a dilution of 1:2000, αNSP4-FL serum at a dilution of 1:1000, MAb B4-2/55/17(1)/13 (B4-2/55) ascites at a dilution of 1:1000, αNSP4114–135 serum at a dilution of 1:10,000, αNSP4120–147 serum at a dilution of 1:10,000, and αNSP4150–175 serum at a dilution of 1:10,000 in 0.5% blotto. Alkaline phosphatase conjugated secondary antibodies (Sigma-Aldrich, St. Louis, MO) were used at a dilution of 1:3000 in 0.5% blotto. Membranes were incubated with the primary antibodies at room temperature overnight, washed 3 times for 5 minutes in 0.5% blotto, and incubated with the secondary antibodies for approximately 2 hours. The membranes were again washed 3 times for 5 minutes in 0.5% blotto and were developed using alkaline phosphatase (AP) detection solution (50 mM Tris, 3 mM MgCl2, 0.1 mg/mL p-nitro blue tetrazolium chloride, and 0.05 mg/mL 5-bromo-4-chloro-3-indolyl phosphate).
Dot Blot
Five micrograms of SA11 NSP4 peptides, aa113–149, aa114–135, aa120–147 in 5ul of 50 mM acetic acid, were separately spotted onto a nitrocellulose membrane that was then blocked with 3% Blotto. The peptides were detected with αNSP4114–135 or αNSP4120–147 sera at a dilution of 1:15,000 in 0.5% Blotto. Antibody-reactive dots were visualized with goat anti rabbit IgG(L+H)-alkaline phosphatase conjugate and the AP detection solution (as above).
Tris-Tricine Gel Electrophoresis and Immunoblots of Synthesized NSP4 Peptides
Synthesized NSP4 peptide aa128–142 of virus strains SA11 (3µg) or S2 (18ug), and their mutants Q137R (9µg), T135I/T138S (6µg), T138S (6µg), and T135I (6µg) were resolved by SDS-PAGE on a 16.5% Tris-Tricine gel (BioRad Laboratories Tris-tricine system) and transferred onto a nitrocellulose membrane that was then blocked with 3% blotto (see Figure 6 for peptide sequences). Approximately 15µg of synthesized SA11 NSP4 peptides aa150–165, aa155–170 and aa160–175 and their mutants were similarly resolved, transferred onto a nitrocellulose membrane and blocked with 3% blotto (see Figure 7 for peptide sequences). The blocked membrane was detected with αNSP4120–147 or αNSP4150–175 at a dilution of 1:5,000 in PBS containing 1% BSA and 1% glycerol for 72 hours at 4°C, then visualized with goat anti rabbit IgG(L+H)-alkaline phosphatase conjugate developed with AP substrate.
Figure 6. Identification of the Critical Residues for the αNSP4120–147 Epitope.
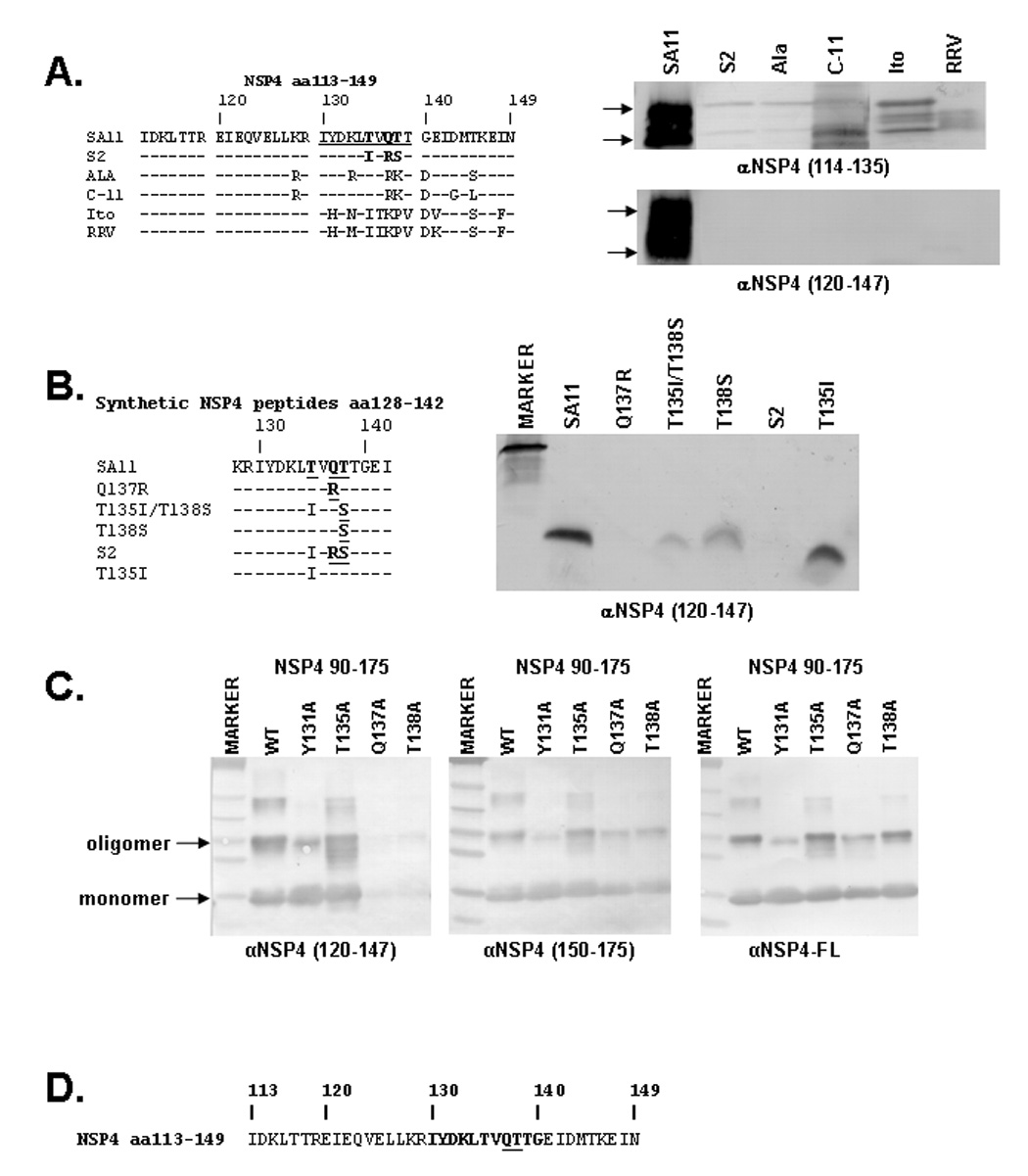
A: Multiple sequence alignment of the NSP4 sequence from aa113–149 for SA11, S2, Ala, C-11, Ito, and RRV (left) and immunoblot analysis of RV infected lysates of the above strains (right). The αNSP4120–147 epitope (aa130–140) is underlined in the SA11 sequence and residues hypothesized as critical for the αNSP4120–147 epitope are highlighted by bold font. Individual blots were detected by either αNSP4114–135 or αNSP4120–147. Arrows indicate the nonglycosylated (lower) and glycosylated (upper) NSP4 bands. B: Amino acid sequence alignment (left) and immunoblot (right) of synthetic NSP4 peptides from aa128–140. Residues identified as critical for αNSP4120–147 are highlighted by bold and underlined font. The immunoblot was detected by αNSP4120–147. C: Immunoblot analysis of alanine substitution mutants. Alanine mutants of NSP490–175 were expressed in BL21(DE3) E. coli, purified by Ni-NTA beads, separated by SDS-PAGE, and blotted onto nitrocellulose. Identical blots were detected with αNSP4120–147 (left), αNSP4150–175 (middle), or αNSP4-FL (right). D: The SA11 NSP4 sequence is shown, highlighting the αNSP4120–147 epitope (aa130–140) in bold with the critical residues (137 & 138) underlined.
Figure 7. Mapping and Identification of Critical Residues for the αNSP4 150–175 Epitope.

A: Multiple sequence alignment of the amino acid sequence from 150–175 for SA11, S2, Ala, C-11, Ito, and RRV (left) and immunoblot analysis of RV infected cell lysates from the above strains (right). Residues hypothesized as critical for the αNSP4150–175 epitope are highlighted by bold and underlined font. Individual blots were detected by either αNSP4114–135 or αNSP4150–175. Arrows indicate the nonglycosylated (lower) and glycosylated (upper) NSP4 bands. B: Sequence alignment (left) and immunoblot (right) of an array of synthetic NSP4 peptides from aa150–175. Residues identified as critical for the αNSP4150–175 are highlighted by bold and underlined font. The immunoblot was detected by αNSP4150–175. C: Immunoblot analysis of alanine substitution mutants. Alanine mutants of NSP490–175 were expressed in BL21(DE3) E. coli, purified by Ni-NTA beads, separated by SDS-PAGE, and blotted onto nitrocellulose. Identical blots were detected with αNSP4120–147 (left), αNSP4150–175 (middle), or αNSP4-FL (right). D: The SA11 NSP4 sequence is shown, highlighting the αNSP4150–175 epitope (aa155–170) in bold with the critical residues (160 & 170) underlined.
MAb B4-2/55 End-point Titer ELISA
Purified wild-type or mutant NSP490–175 was coated onto flat-bottomed polyvinylchloride 96-well microtiter plates (Dynatech Laboratories, Inc., Alexandria, VA) at 10 µg/mL in carbonate/bicarbonate solution, pH 9.6 (CBC) overnight at 4°C. The plates were blocked with 5% Blotto for 1 hour and then washed 3 times with PBS-0.05% Tween 20. MAb B4-2/55 or αNSP4-FL was diluted 1:50 in 0.5% Blotto, added to the appropriate wells of the microtiter plate, diluted 2-fold down the plate, and incubated for 1 hour. The plates were again washed with PBS-Tween. HRP-conjugated rabbit anti-mouse antibody was diluted 1:1000 in 0.5% blotto, added to the plates and incubated for 1 hour. After another wash, TMB substrate (K&PL, Gaithersburg, MD) was added, allowed to develop for 10 minutes before the reaction was stopped with 1 M H3PO4. Absorbance at 405 nm was measured using a Thermomax microplate reader (Molecular Devices Corp., Menlo Park, CA). End-point titers were determined to be the reciprocal of the last positive dilution. The cut-off optical density (OD) was 0.096, which was calculated as the mean of the OD of the blank plus 3 standard deviations. All steps were carried out at room temperature.
Surface Plasmon Resonance (SPR) Analysis of Interactions Between NSP4 Proteins and Peptides Binding to VP5* or VP8*
SPR analysis was performed at 25°C in a BIAcore 3000 system using an NTA sensor chip (BIAcore AB, Uppsala, Sweden). Briefly, baculovirus expressed and purified 5xHisVP5* or bacterially expressed and purified 6xHisVP8* was immobilized onto Ni2+ activated NTA sensor surface of flow cells up to 4500 resonance units (RU) on a BIAcore NTA sensor chip. For the blank control, one of the flow cells on the sensor chip lacked immobilized protein. Non-specific binding of the Ni2+-activated sensor surface of flow cells was blocked by BSA in HEPES-buffered saline (HBS: 10mM HEPES, 150mM NaCl, 50µM EDTA, 0.005% Surfactant P20, pH 7.4). Recombinant SA11 NSP4-FL in HBS containing 1mM MgCl2 was injected into the flow cells of the NTA sensor chip at a flow rate of 5 µl/min. The binding RU of NSP4 proteins to VP5* or VP8* were obtained by subtraction of the RU of the blank control from the RU of the reaction flow cells. Additionally, HisVP5* was covalently immobilized onto the sensor surface on a BIAcore CM5 sensor chip, according to the manufacturer’s protocol. Synthetic NSP4 peptides aa114–135 and aa150–175 in HBS were injected as above. Based on the correlation between the SPR response and change in soluble protein and peptide binding to the immobilized protein, values for the binding ratio, Vbound, and the concentration of free protein, [P]free, were calculated using equations described previously (Rich et al., 1999). The KD of NSP4-FL and NSP4 peptide aa114–135 for VP5* was calculated using a one-site-binding nonlinear regression model.
NSP4-VP5* Binding and Antibody Blocking ELISA
Microtiter wells (Dynex Technologies) were coated with 1 µg of purified recombinant 5xHisVP5* in CBC containing 1mM MgCl2 overnight. The wells were washed with Tris buffered saline + 1mM MgCl2 (TBS) and incubated with blocking solution (TBS containing 1%, w/v, BSA and 0.05%, v/v, Tween 20) for 1 h. Varying concentrations of the NSP4 peptide-specific antiserum, rabbit pre-immune serum, or no antibody as a positive control was combined with either 1µg of purified recombinant SA11 HisNSP4-FL in TBS or buffer alone as a negative control and added to the wells. After incubation overnight, the wells were washed with TBS + 0.05% Tween 20 (TBS-T). Mouse αNSP4-FL antiserum, diluted 1:3000, in TBS-T + 2.5% normal rabbit serum was added to the wells. After incubation for 1 h, the wells were washed and HRP-conjugated rabbit anti-mouse antibody diluted 1:3000 in TBS-T + 2.5% normal rabbit serum was added to the wells. After incubation for 1 h, the wells were again washed and developed as described above. Background was calculated as the mean OD plus 3 standard deviations of the negative control (no NSP4 added), and NSP4 binding to VP5* in the presence of the blocking antisera is presented as the percent of the positive control. All steps were carried out at room temperature.
Results
Mapping MAb B4-2/55
Previously published immunoelectron microscopy (IEM) data of thin sections of rotavirus-infected cells and immunoprecipitation of NSP4 transcribed in vitro in the presence of microsomes using MAb B4-2/55 suggested that the epitope recognized by this MAb is found on the long cytoplasmic C-terminus of NSP4, which corresponds to amino acids 47–175 (Au et al., 1993; Petrie et al., 1984). To further map MAb B4-2/55, a series of N- and C-terminal truncation constructs of NSP4 were constructed in a bacterial expression plasmid with an N-terminal 6x-histidine tag. A schematic of the His-tagged truncation proteins and summary of their reactivities with MAb B4-2/55 is illustrated in Figure 1. This summary is based on Western Blot analysis of the purified His-tagged NSP4 truncations using either MAb B4-2/55 (Figure 2A & C), polyclonal αNSP4-FL (Figure 2B & D), or by Ponceau S staining to demonstrate equal protein loading (Figure 2A–D, lower panels). B4-2/55 failed to recognize NSP4105–175 and NSP4112–175 N-terminal truncations (Figure 2A, lanes 5–6) and a NSP475–116 C-terminal truncation (Figure 2C, lane 9). The positive control αNSP4-FL serum recognized all of the truncation products as expected (Figure 2B & D). These data confirm that B4-2/55 detects a linear epitope within amino acids 100–118 of NSP4 (Figure 3D).
Figure 1. Schematic Diagram of Truncation Constructs of SA11 NSP4.

A linear diagram of full-length NSP4 is shown at the top, numbers indicating the corresponding amino acid position. The 3 hydrophobic domains (boxes), glycosylation sites (straight lines), and ER membrane domains (curved lines) are shown. The truncation constructs generated are illustrated below and aligned corresponding to the full-length protein. The name of each construct is listed to the left of the illustration and all have an N-terminal 6xHis-tag, (black box). A summary of the reactivity of MAb B4-2/55, αNSP4114–135, αNSP4120–147 (Figure 2 & 4C, respectively) is listed to the right of illustration where + indicates reactivity, − indicates no reactivity, ND indicates not done, and (+) indicates reactivity but the data is not shown.
Figure 2. Epitope Mapping of B4-2/55.

A – D: Immunoblot analysis of NSP4 truncation constructs. N- or C-terminal trunctions of NSP4 were expressed in BL21(DE3) E. coli and purified on Ni-NTA beads. Purified protein separated by SDS-PAGE and blotted onto nitrocellulose was stained with Ponceau S to confirm equal loading (lower panels). Duplicate blots were reacted with either MAb B4-2/55 (A & C) or mouse anti-NSP4-FL (B & D). E & F: Genogroup cross-reactivity of B4-2/55. MA104 cells were either mock- or rotavirus-infected with a MOI of 1 for SA11 cl. 3 (genogroup A), OSU (genogroup B), RRV (genogroup C), ECTC (gengroup D), or Ty-1 (gengroup E) and maintained for 48 hr. at 37°C. Cells were lysed in RIPA buffer and separated by SDS-PAGE without and with Endo H treatment for 2 hr at 37°C, as indicated by (−) and (+), respectively. Arrows (left of each blot) indicate the fully glycosylated ~28K (upper) and non-glycosylated ~20K (lower) NSP4, and the black dots indicate these two main forms for each strain. Cleavage products were present in all cell lysates. Identical blots were incubated with either MAb B4-2/55 (E) or rabbit anti-NSP4 peptide 113–149 (F).
Figure 3. Identification of Residues Critical for B4-2/55 Binding.

A: Multiple sequence alignment of NSP4 representing each genogroup. Primary NSP4 sequence corresponding to amino acids 99–119 for SA11 cl. 3 (genogroup A), Wa-attenuated (genogroup B and the immunogen for B4-2/55), OSU (genogroup B), RRV (genogroup C), ECTC (gengroup D) and Ty-1 (genogroup E) are shown. The B4-2/55 epitope (aa100–118) is highlighted in bold type on the SA11 cl. 3 sequence. Conservative changes relative to SA11 NSP4 are shown in bold type, and non-conservative changes are in underlined bold type. Residues that differ between genogroups A–D and genogroup E within the B4-2/55 epitope are indicated by arrows. B: Immunoblot analysis of alanine substitution mutants. Alanine mutants of NSP490–175 were expressed in BL21(DE3) E. coli, purified by Ni-NTA beads, separated by SDS-PAGE, blotted onto nitrocellulose, and stained by Ponceau S to determine equal loading (lower panels). Identical blots were detected with either MAb B4-2/55 or αNSP4-FL. Arrows indicate NSP4 monomers or oligomers in the immunoblot. C: ELISA end-point titer of B4-2/55 against NSP4 alanine mutants. * indicates p < 0.01 by students T-test. D: The SA11 NSP4 sequence is shown highlighting the B4-2/55 epitope (aa100–118) in bold with the critical residues (105, 108, & 111) underlined.
Group A rotavirus NSP4 sequences cluster into 5 distinct genogroups (Ciarlet M et al., 2000; Lin and Tian, 2003) and monoclonal antibody B4-2/55 was generated against the human rotavirus Wa, which has a genogroup B NSP4. We sought to determine the cross-reactivity of B4-2/55 against each NSP4 genogroup as one approach to identify residues potentially critical for B4-2/55 binding to NSP4. The reactivity of B4-2/55 was examined by Western blot with lysates from MA104 cells infected with SA11 Cl. 3 (genogroup A), OSU (genogroup B), RRV (genogroup C), ECTC (genogroup D), or Ty-1 (genogroup E) or mock-infected cells. Reactivity was also tested with an aliquot of each lysate treated with endoglycosidase H (Endo H) to remove the carbohydrates followed by Western blotting using either MAb B4-2/55 or a rabbit antiserum to a NSP4 peptide (aa113–149) as a positive control (Figure 2E & F). Western blot analysis using the rabbit anti-NSP4 peptide 113–149 showed that SA11 cl. 3 NSP4 in the Endo H-treated and untreated lysates migrates at the expected 20KDa and 28KDa, respectively (Figure 2F). The 113–149 peptide antiserum reacted with each NSP4 from genogroups A–D very well, although the apparent molecular sizes of the proteins differed slightly (identified by black dots). This antiserum also weakly detected Ty-1 NSP4. The weak reactivity of this antiserum against Ty-1 NSP4 is not surprising, given the greater genetic distance between SA11 and Ty-1 compared with the other RV strains used. Both the rabbit anti-NSP4 peptide 113–149 serum and MAb B4-2/55 reacted with SDS-PAGE resistant oligomers as well as bands that migrated faster than the unglycosylated 20KDa NSP4, which are likely cleavage products of NSP4. Western blot analysis using MAb B4-2/55 showed reactivity with both glycosylated and unglycosylated NSP4 from genogroups A–D; however, B4-2/55 did not react with Ty-1 (genogroup E) NSP4 (Figure 2E). Based on these results, we hypothesized that residues critical for B4-2/55 binding to NSP4 should be relatively well conserved among mammalian rotavirus NSP4s but not conserved in the avian NSP4 sequences.
Since B4-2/55 recognized NSP4 from genogroups A–D, but not genogroup E, (Figure 2E), we hypothesized that critical residues should be relatively well conserved between genogroups A–D, but differ when compared to genogroup E. A multiple sequence alignment (Figure 3A) illustrated amino acids 100, 104, 105, 107, 108, 111, 112, 115, and 118 show non-conservative changes (underlined bold font) that differ between genogroup A and genogroup E and are the most likely candidates to be the critical residues of the B4-2/55 epitope. The importance of these residues for B4-2/55 binding was tested by introducing single alanine substitution mutations at these positions in the NSP490–175 truncation construct background. Western blot analysis (Figure 3B, left) of these mutants using MAb B4-2/55 showed that B4-2/55 detected both monomer and oligomer of NSP490–175 D100A, K104A, R107A, M112A, and T117A/T118A. In contrast, B4-2/55 failed to detect the monomer of NSP490–175 E105A, R108A, and E111A. Interestingly, B4-2/55 detected the SDS-resistant oligomer of these mutants, though the detection of the E105A, R108A, and E111A oligomer was weaker than that of WT NSP490–175. B4-2/55 did not detect the monomer and oligomer of the triple mutant (E105A/R108A/E111A) suggesting that ablation of B4-2/55 binding to NSP4 requires mutation of at least two of these residues. All of the mutants were detected by αNSP4-FL (Figure 3B, right). There appeared to be less of the dimeric and trimeric oligomers for the E111A and M112A proteins despite similar protein loading (Figure 3B, Ponceau S). Yet, since the alanine substitutions are detected by the polyclonal antiserum, the mutations only changed the local structure of the B4-2/55 epitope, but did not perturb the overall structure of NSP4.
Together, these data suggested that residues E105, R108, and E111 are critical for recognition of the NSP4 monomer by B4-2/55; however, mutation of any one of these residues alone weakened, but did not ablate, detection of the NSP4 oligomer. The stronger detection of the NSP4 oligomer than the monomer by B4-2/55 suggested that there may be additional contacts between B4-2/55 and oligomeric NSP4 that are lost by denaturing NSP4 into a monomer. This led us to question if the B4-2/55 epitope was conformational, rather than linear, since for some proteins elements of secondary structure remain stable under SDS-PAGE conditions and conformational epitopes are detected by Western blot analysis (Parker et al., 2005; Zhou et al., 2007).
We tested if E105, R108, and/or E111 were also critical for the detection of native NSP4 by determining the end-point titer of B4-2/55 against each of the mutant constructs by ELISA (Figure 3C). For the ELISA, NSP490–175 and NSP4105–175 were used as positive and negative controls, respectively. The end-point titer for B4-2/55 for alanine substitution mutants of D100, K104, R107, or M112 was not statistically different than that of wild-type NSP490–175. In contrast, mutation of E105A, R108A, E111A, or all three amino acids in the triple mutant resulted in a statistically significant reduction in the end-point titer. This assay allowed us to assign a relative importance of each residue for binding by B4-2/55, such that E105≅R108>E111, since both E105A and R108A exhibited a similarly reduced end-point (50–100), whereas E111A had an intermediate end-point (6,400). Thus, B4-2/55 binds to the oligomerization domain of NSP4 within aa100–118, residues E105, R108, and E111 are critical for the interaction between B4-2/55 and either native or denatured NSP4, and mutation of these residues individually reduces the strength of the interaction but does not eliminate it.
αNSP4114–135 and αNSP4120–147 Detect Distinct NSP4 Epitopes
The two enterotoxic peptides (synthetic SA11 NSP4 peptides aa114–135 and aa120–147) used to generate two antisera differ only slightly in their sequence; however, previous studies using these two peptide-specific antisera suggested that αNSP4114–135 and αNSP4120–147 reacted to distinct epitopes on NSP4 (unpublished data). To determine the epitopes for the two antisera, we performed dot blot analysis to test their reactivities with three synthetic peptides, which correspond to NSP4 aa113–149, aa114–135, and aa120–147, respectively (Figure 4A). The synthetic peptides were spotted onto nitrocellulose membranes and detected with either αNSP4114–135 or αNSP4120–147. The peptide aa113–149, which encompasses both of the other peptide sequences, was used as a positive control and was detected by both antisera (Figure 4B). In contrast, the αNSP4114–135 only detected the aa114–135 peptide and not the aa120–147 peptide, and vice versa. Together, these data indicated that αNSP4114–135 and αNSP4120–147 are each specific for distinct epitopes within aa113–149.
Figure 4. Mapping the Epitopes for αNSP4114–135 and αNSP4120–147.
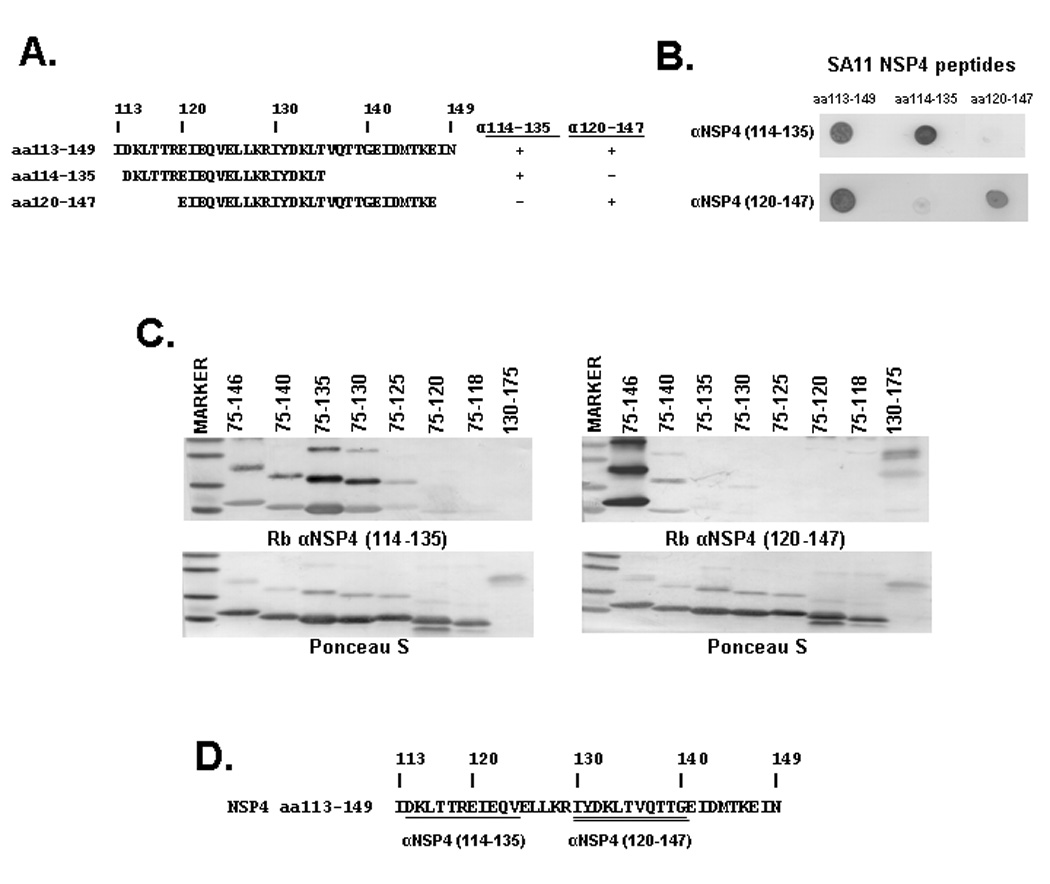
A: Sequence alignment and summary of the αNSP4114–135 and αNSP4120–147 reactivity to the SA11 NSP4 synthetic peptides used in the dot blot analysis (shown in B). + indicates reactivity and − indicates no reactivity. B: The synthetic peptides indicated on the top of the blots were spotted onto nitrocellulose and reacted with the antibody indicated to the right of the blot. C: Immunoblot analysis of NSP4 truncation constructs. N- or C-terminal trunctions of NSP4 were expressed in BL21(DE3) E. coli and purified on Ni-NTA beads. Purified protein separated by SDS-PAGE and blotted onto nitrocellulose was stained with Ponceau S to confirm equal loading (lower panels). Duplicate blots were reacted with either αNSP4114–135 (left) or mouse αNSP4120–147 (right). D: The SA11 NSP4 aa113–149 sequence highlighting the distinct epitopes detected by αNSP4114–135 (aa114–125—single underline) and αNSP4120–147 (aa130–140—double underline).
The minimal binding sequence for these two peptide antisera was further defined by Western blot analysis using αNSP4114–135 (Figure 4C, left), αNSP4120–147 (Figure 4C, right). Ponceau S staining of each blot demonstrated equal protein loading for each of the N- and C-terminal NSP4 truncations (Figure 4C, lower panels). The truncations used and summary of the antibody reactivities are shown in Figure 1. αNSP4114–135 detected C-terminal truncations NSP475–146 (lane 2) through NSP475–130 (lane 5), weakly detected NSP475–125 (lane 6), but was unable to or only faintly detected NSP475–120 or NSP475–118. This antiserum was also unable to detect the N-terminal truncation NSP4130–175 (lanes 7–9). αNSP4120–147 was only able to detect NSP475–146 and NSP475–140 (lanes 2 & 3), but not the other C-terminal truncations (lanes 4–8). NSP4130–175 was detected by αNSP4120–147 (lane 9). Based on the Western blot analysis, the minimal binding sequences for αNSP4114–135 and αNSP4120–147 were NSP4 aa114–125 and aa130–140, respectively. Thus, the differential detection of the N- and C-terminal truncations by these two antisera supports the dot blot data and indicates that αNSP4114–135 and αNSP4120–147 are each specific for distinct epitopes (Figure 4D, underlined).
Mapping the Critical Residues of αNSP4114–135
The epitope detected by αNSP4114–135 falls within a highly conserved domain of NSP4 that includes the putative calcium binding site (aa120–123) which is 100% conserved in all Group A RV NSP4 sequences (Figure 5A). Thus, as expected, αNSP4114–135 detected NSP4 from six different rotavirus strains: SA11 (simian), S2 (human), Ala (lapine), C-11 (lapine), Ito (human), and RRV (simian) using Western blot analysis of infected cell lysates (Figure 6A, right upper panel). To determine the amino acid residues within this epitope that are critical for the antibody antigen interaction, we again utilized alanine substitution mutations of the HisNSP490–175 construct. Based on the sequence conservation of the putative calcium binding site, we focused the mutagenesis on this motif and adjacent residues, and the purified His-tagged mutants were analyzed by Western blot analysis using αNSP4114–135 (Figure 5B, left), αNSP4120–147 (Figure 5B, middle), and αNSP4-FL (Figure 5B, right). Mutation of T117/T118, E120, or E122 to alanine eliminated the detection of the NSP490–175 monomer by αNSP4114–135; however, weak detection of the oligomer was seen for the T117A/T118A and E120A mutants. Mutation of R119, Q123, or LL126/127 had no effect on detection. Interestingly, αNSP4114–135 showed stronger detection of the NSP4 oligomers than the monomer for all of the constructs, especially when the relative amount of the monomer and oligomer detected by Ponceau S. This bias for detection of the oligomer is not apparent for either αNSP4120–147 or αNSP4-FL. These mutations do not disrupt the overall structure of NSP4, since both αNSP4120–147 and αNSP4-FL detected WT and mutant NSP4 similarly. Thus, while residue E122 was critical for detection of NSP4 by αNSP4114–135, mutation of T117/T118 or E120 reduced detection of NSP4 suggesting these residues are also important for this epitope (Figure 5C).
Figure 5. Identification of the Critical Residues of the αNSP4114–135 Epitope.

A: Multiple sequence alignment of the NSP4 sequence from aa113–149 for SA11, S2, Ala, C-11, Ito, and RRV. The αNSP4114–135 epitope (aa114–125) is underlined in the SA11 sequence and residues 100% conserved in all Group A RV NSP4 sequences are highlighted by bold font. B: Immunoblot analysis of alanine substitution mutants. Alanine mutants of NSP490–175 were expressed in BL21(DE3) E. coli, purified by Ni-NTA beads, separated by SDS-PAGE, blotted onto nitrocellulose, and stained by Ponceau S to determine equal loading (lower panel). Identical blots were detected with αNSP4114–135 (left), αNSP4120–147 (middle), or αNSP4-FL (right). Arrows indicate NSP4 monomers or oligomers in the immunoblot. C: The SA11 NSP4 sequence is shown, highlighting the αNSP4114–135 epitope (aa114–125) in bold with the important residues (117, 118, 120, & 122) underlined and the critical E122 indicated by the asterisk (*).
Mapping the Critical Residues of αNSP4120–147
We next sought to further map the epitope detected by αNSP4120–147. The Western blot analysis of truncation constructs (Figure 4C, right), indicated the epitope fell between aa130–140, which is a region of NSP4 that shows sequence diversity between rotavirus strains. To identify candidate residues critical for the detection of NSP4 by αNSP4120–147, we tested the reactivity of this antiserum against NSP4 from lysates of the six different rotavirus strains previously discussed. An alignment of the amino acid sequence for the six NSP4s and immunoblot analysis of the lysates is shown in Figure 6A. Immunoblot analysis showed that while αNSP4114–135 detected NSP4 from all six rotavirus strains, αNSP4120–147 only detected SA11 NSP4. By comparing the sequences in Figure 6A we predicted that the critical residues for this epitope are T135, Q137 and/or T138 because αNSP4120–147 is unable to detect S2 NSP4 and those residues are the only changes between the S2 and SA11 NSP4 sequences.
We investigated the importance of these three residues by testing the reactivity of αNSP4120–147 against various synthetic NSP4 peptides, listed in Figure 6B. These peptides represent either the SA11 or S2 amino acid sequence as well as substitution of one or two residues (Figure 6B, left). The peptides were resolved on a Tris-Tricine SDS-polyacrylamide gel and blotted onto a nitrocellulose membrane and then detected with αNSP4120–147 (Figure 6B, right). The αNSP4120–147 serum detected the SA11 peptide (lane 2), but not the S2 peptide (lane 6). The Q137R mutation completely abolished detection of this peptide by αNSP4120–147 (lane 3), indicating that Q137 is critical for antibody binding. Mutation of T138S (lanes 4 & 5) reduced the level of detection by αNSP4120–147, suggesting that T138 is also important for antibody binding, yet the conservative change from threonine to serine weakened, but did not abolish, antibody binding. In contrast, the T135I mutation did not affect the level of detection by αNSP4120–147, so this residue is not essential for antibody binding. To confirm the peptide blot results, alanine mutation of residues Y131, T135, Q137, and T138 were generated in HisNSP490–175 and analyzed by immunoblot as before. Purified His-tagged NSP4 mutants were detected with αNSP4120–147 (Figure 6C, left), αNSP4150–175 (Figure 6C, middle), and αNSP4-FL (Figure 6C, right). αNSP4120–147 detected Y131A and T135A mutants similar to WT NSP490–175, but mutation of Q137 or T138 to alanine abolished detection of NSP4. These mutations did not disrupt the overall structure of NSP490–175 because both αNSP4150–175 and αNSP4-FL detected all mutant constructs to similar levels. Thus, the epitope for αNSP4120–147 falls between amino acids 130–140 and Q137 and T138 are critical residues for antibody binding (Figure 6D).
Mapping αNSP4150–175
Lastly, we used similar methods to map a peptide-specific antibody to NSP4 aa150–175. As before, we utilized the naturally occurring sequence divergence within this region of NSP4 to identify the epitope and potentially critical residues for the αNSP4150–175 antiserum. Figure 7A shows a multiple sequence alignment for NSP4 aa150–175 and corresponding immunoblot analysis of infected cell lysates of the six RV strains used previously and αNSP4114–135 was used as the positive control. αNSP4150–175 detected NSP4 from SA11, S2, Ala, Ito, had weak detection of C-11 NSP4, and no detection of RRV NSP4. By comparing this profile with the multiple sequence alignment (Figure 7A), a number of changes between the detected and undetected NSP4 sequences were evident. First, the weaker detection of C-11 NSP4 compared to that of Ala NSP4 suggested that the single amino acid change between these two sequences, E170K, might be involved in antibody binding. Additionally, there are numerous changes between the detected NSP4s and RRV NSP4. These changes are N152Y, V153F, E157N, E160A, S161E and K163E (using the SA11 and RRV sequences), and are highlighted by bold and underlined fonts in the sequence alignment.
To further map the minimal binding sequence for αNSP4150–175 and determine which of these residues was critical for αNSP4150–175 binding to NSP4, three nested sets of synthetic peptides incorporating these changes were generated (Figure 7B, left). NSP4112–175 and NSP4112–150 were used as positive and negative controls, respectively. Analysis of the synthetic peptides indicated that αNSP4150–175 was unable to detect neither the SA11 peptide aa150–165 nor the N152Y or V153F mutant peptides (Figure 7B, lanes 4–6). Peptides corresponding to SA11 NSP4 aa155–170 and aa160–175 were both detected (lanes 7 & 13) by the antiserum, suggesting that the minimal binding sequence fall within residues 155–170. Amino acid changes of S161E or K163E resulted in a slightly reduced detection of the aa155–170 peptide by αNSP4150–175 (lanes 9–10). In contrast, the changes of E160A (in peptide aa155–170) and E170K (in peptide aa160–175) both dramatically reduced the detection of the corresponding peptide by αNSP4150–175, suggesting that these two residues are critical for antibody binding. As above, the peptide blot results were confirmed by immunoblot analysis of single alanine mutants of residues E160, K163, or E170 in the HisNSP490–175 construct using αNSP4120–147 (Figure 7C, left), αNSP4150–175 (Figure 7C, middle), and αNSP4-FL (Figure 7C, right). As with the peptide bolt, mutation of E160 or E170 caused a loss of NSP4 detection by αNSP4150–175, but the K163A mutant was detected similar to wild-type NSP490–175. None of the mutants affected the ability of αNSP4120–147 or αNSP4-FL to detect NSP4, demonstrating that these mutants disrupt this epitope and not the overall structure of NSP490–175. Thus, αNSP4150–175 binds to NSP4 within aa155–170 and E160 and E170 are critical for antibody binding.
The Binding of NSP4 to VP5* is Blocked by αNSP4114–135 & αNSP4120–147
We hypothesize that the peptide-specific antisera are each specific for a single epitope on NSP4, since the mutation of a single critical residue within the epitope is sufficient to ablate detection of NSP4. Thus, the specificity of these antisera makes them useful for the mapping and characterization of different functional and protein-protein binding domains of NSP4, most of which reside in the cytoplasmic tail. The RV spike protein VP4 is one suggested NSP4 binding partner; however, this interaction has not been investigated in detail (Au et al., 1993). We used the antisera mapped above to further characterize the interaction between NSP4 and VP4.
In order to show a direct interaction between NSP4 and VP4, as well as to determine which proteolytic fragment of VP4 (either VP5* or VP8*) binds to NSP4, we analyzed the binding of untagged SA11 NSP4-FL to His-tagged VP5* or VP8* by Surface Plasmon Resonance (SPR) using a BIAcore 3000 system. Various concentrations of SA11 NSP4-FL protein were passed over flow cells of NTA sensor chips upon which HisVP5* (Figure 8A) or HisVP8* (Figure 8B) had been immobilized. NSP4-FL was capable of binding to immobilized HisVP5* (Figure 8A) but not HisVP8* (Figure 8B). The NSP4 site that binds VP5* was determined by testing the ability of synthetic NSP4 peptides corresponding to aa114–135 or aa150–175 to bind to VP5*. Various concentrations of NSP4 aa114–135 and 1.6µM NSP4 aa150–175 were passed over flow cells of a CM5 sensor chip that had been chemically conjugated to HisVP5* (Figure 8C). NSP4 aa114–135, but not NSP4 aa150–175 bound to VP5*, confirming the previously published data that the VP4 binding site on NSP4 mapped to the enterotoxin domain. The dissociation constant (KD) for the interaction between NSP4-FL or NSP4 aa114–135 and HisVP5* was determined by flowing increasing concentrations of NSP4-FL (0.38—6.0µM) and NSP4 aa114–135 (0.4—1.6µM) passed over immobilized HisVP5* and the KD calculated using a one-site-binding nonlinear regression model. Both full-length and aa114–135 of NSP4 bound VP5* with affinity (NSP4-FL—VP5*: KD ~0.47±0.1µM; aa114–135—VP5*: 0.43±0.28µM).
Figure 8. The Binding of NSP4 to VP5* is Blocked by αNSP4114–135 or αNSP4120–147, but by αNSP4150–175.

A & B: Representative profiles of the relative SPR response of the binding of increasing concentrations of NSP4-FL to HisVP5* (A) or HisVP8* (B). Background binding detected in a control cell was subtracted. y axis values are resonance units (RU) normalized to the maximum resonance units of NSP4-FL binding to HisVP5* or HisVP8*, respectively. C: Representative profile of the relative SPR response of the binding of increasing concentrations of SA11 NSP4 peptide aa114–135 or peptide aa150–175 to HisVP5*. Background binding detected in a control cell was subtracted. y axis values are resonance units (RU) normalized to the maximum resonance units of NSP4 peptide aa114–135 binding to HisVP5*. D: An ELISA-based NSP4-VP5* binding assay was used to test the ability of αNSP4114–135, αNSP4120–147, and αNSP4150–175 to block the NSP4-VP5* interaction. The results are plotted as the percent NSP4 bound relative to the positive control (without antibody) versus the serum dilution. Statistical difference (p< 0.01, students T-test) between the pre-immune serum and the NSP4-specific antiserum was performed.
Finally, an ELISA-based NSP4-VP5* binding assay was developed to test the ability of the three peptide-specific antisera to block this interaction (Figure 8D). Various concentrations of a rabbit pre-immune serum (negative control), αNSP4114–135, αNSP4120–147, or αNSP4150–175 were mixed with SA11 NSP4 and then added to VP5*-coated wells of a microtiter plate. Bound NSP4 was detected by mouse αNSP4-FL antisera and a HRP-conjugated secondary antibody. The positive control, NSP4 -VP5* binding without antibody was set to 100%. The data are expressed as percent of the positive control. Neither the pre-immune sera, nor αNSP4150–175 significantly reduced the binding of NSP4 to VP5*. In contrast, both αNSP4114–135 and αNSP4120–147 significantly blocked the binding of NSP4 to VP5* (p < 0.01) in a dose-dependent manner. Together, these data show that the interaction between the enterotoxin domain of NSP4 (aa114–140) and VP5* is direct, strong, and specific.
Discussion
Identification of multiple and novel functions of NSP4 in RV replication and pathogenesis has made this molecule a focus of study. However, since NSP4 has multiple forms, occupies several intracellular compartments and is released extracellularly, it is challenging to specifically correlate a given form of NSP4 to a distinct function (Ball et al., 2005). The ability of the NSP4 cleavage product to elicit diarrhea as well as to induce an immune response that is protective has made pursuit of the immunological properties of NSP4 important (Choi NW et al., 2005).
The work presented here describes the detailed mapping of four epitope specific antibodies to the SA11 NSP4 cytoplasmic tail. A summary (Figure 9A) of the binding on a linear schematic shows the epitopes and critical residues for the four antibodies studied. Within each epitope we identified key residues important for antibody binding. It is possible that some of the key residues identified are critical for the folding of the epitope, but may not be directly involved in the antibody-antigen interaction. However, this is unlikely to be the case for the following reasons: [1] all of the antibodies had linear epitopes and detected denatured wild-type NSP4, thus protein folding should not be a consideration. [2] For each epitope, alanine mutations near the critical residues did not affect detection of NSP4 by the corresponding antibody. [3] Mutation of residues critical for one epitope, i.e. E122, did not affect detection of NSP4 by either αNSP4-FL or the other epitope-specific antibodies, and [4] analysis of wild-type NSP4 and the E120A and Q123A mutants by circular dichroism showed no difference in secondary structural elements (data not shown). Thus, any alanine mutation in our studies would only affect the structure of the epitope if it is directly involved in the antibody-antigen interaction.
Figure 9. Summary of the Antibody Mapping in the NSP4 Cytoplasmic Tail.

A: Linear schematic diagram of the four epitope specific antibodies. The epitopes recognized by the four antibodies are shown in the blue (B4-2/55), orange (αNSP4114–135), magenta (αNSP4120–147), and green (αNSP4150–175) boxes. The critical residues identified for each are highlighted in red. B: Organization of the B4-2/55, αNSP4114–135 and αNSP4120–147 epitopes on the NSP4 crystal structure (PDB accession number 1G1I (Bowman et al., 2000)). Each epitope is color coded as above and the critical residues are highlighted in red. E105 is on the reverse face of the structure. C: Localization of the critical residues on the NSP4 tetramer (PDB accession numbers 1G1I and 1G1J (Bowman et al., 2000)). The critical residues are highlighted according to their respective epitope as in A. D: Localization of the VP5* binding domain on the tetramer structure of NSP495–137. The minimal VP5 binding site (NSP4 aa114–135) is highlighted in green, while the DLP binding site is indicated by the blue box.
Each of these four antibodies has interesting properties that will facilitate further characterization of the multifaceted NSP4 domain structure, illustrated in part by the crystal structure shown in Figure 9B (Bowman et al., 2000). The three epitopes present within the crystal structure are tightly packed together, with few, if any, residues between them. Given this tightly packed yet distinct epitope structure, these antibodies can be used to differentiate different forms of NSP4 and determine which domains are present on a given form or intracellular pool of NSP4. Further, it is possible that the overall epitope structure is similar for NSP4 from other RV strains.
One previous study mapped epitopes of the pigeon rotavirus PO-13 NSP4 by the production of MAbs to that protein (Borgan et al., 2003). In those studies, the authors describe MAbs that map to four antigenic sites, though the minimal binding sequence was not determined. When aligned to the SA11 NSP4 sequence, the epitopes detected by 3 of the PO-13 MAbs correspond to aa112–133, aa136–151, and aa155–175 and are similar to the epitopes mapped in the current study for αNSP4114–135, αNSP4120–147, and αNSP4150–175, respectively. An epitope analogous to the B4-2/55 MAb epitope was not isolated in the PO-13 MAb study; however, given the seeming importance of the NSP4 oligomer for B4-2/55 binding, it is tempting to speculate that this domain on avian NSP4 is less immunogenic due to its lack of a coiled-coil domain (Lin and Tian, 2003). Thus, while our peptide antisera were made to synthetic peptides rather than full-length NSP4, it is compelling that the overall epitope structure of the cytoplasmic tail is similar for both SA11 NSP4 and PO-13 NSP4, suggesting that similar domains of divergent NSP4s are immunogenic irrespective of the primary sequence. This idea lends credence to the theory that NSP4 from divergent RV stains has evolved to maintain a structure/function relationship, as suggested by the biochemical and functional similarities between Group A and Group C RV NSP4 proteins, in the absence of any significant primary sequence conservation (Horie et al., 1997; Sasaki et al., 2001). It would be interesting to extend these studies by determining if Group C RV NSP4s has a similar epitope structure.
There are a number of important differences between the epitopes found in the PO-13 MAb study and the present work. The PO-13 NSP4 MAb mapping to aa136–151 showed cross-reactivity against NSP4 from all genogroups, despite this region having high amino acid diversity. The corresponding antibody described here, αNSP4120–147, showed the opposite phenotype, as it was specific to SA11, whereas αNSP4114–135 had the highest genogroup cross-reactivity. The broad cross-reactivity of αNSP4114–135 is likely due to the high degree of sequence identity for this epitope across NSP4 genogroups, and two of the critical residues (E120 and E122) are 100% conserved in all reported sequences. In contrast, since few reported NSP4 sequences contain the Q137 residue, as does SA11 NSP4 (aa137 is K/R on most NSP4s), it is likely that αNSP4120–147 was specific to SA11 due to the glutamine 137 and did not react with other NSP4s due to the difference in charge of this position.
Since NSP4 forms oligomers, we also visualized the critical residues for each epitope in the context of a tetramer (Figure 9C). In the tetramer, the residues critical for antibody binding to their respective epitopes are clustered together and surface exposed. For the B4-2/55 epitope, residue K115 was identified as a possible candidate, but the alanine substitution of this residue was not tested in this study. It seemed unlikely that K115 was involved in the epitope, since it falls on the edge of the minimal binding sequence of the B4-2/55 epitope and mutation of neither M112A nor T117A/T118A affected the detection of NSP4 by B4-2/55. However, in the crystal structure K115 does cluster with E105, R108, and E111 so it remains possible that this residue also contributes to the interaction. In the αNSP4114–135 epitope, residue E120, which contributes to the αNSP4114–135 epitope, is only partially exposed and antibody binding may induce localized conformational changes to expose E120. Both B4-2/55 and αNSP4114–135 bind within the tetramerization coiled-coil domain, and showed a biased detection of the SDS-resistant NSP4 oligomers (Figure 3B & Figure 5B) over the monomer.
This bias was often more apparent for the point mutants that disrupted antibody binding, since the oligomer but not the monomer was detected. This led us to question whether or not the categorization of these epitopes as linear was appropriate or if conformational elements of the epitope were assembled upon oligomerization of NSP4. Both antibodies detected the denatured monomeric form of wild-type NSP4 and the epitopes were 18 and 11 residues for B4-2/55 and αNSP4114–135, respectively, suggesting that these are true linear epitopes. While oligomerization is not essential for antibody binding, it appears to enhance the affinity of the antibody-antigen interaction. This suggests that the presentation of this epitope is slightly different between the denatured monomer and oligomeric form or additional bonds are present between the antibody and the NSP4 oligomer that strengthen the overall affinity of the interaction. The enhanced antibody binding afforded by NSP4 oligomerization partially compensated for the mutation of any single, but not multiple, critical residues within the B4-2/55 and αNSP4114–135 epitopes. Together, these data suggest that these antibodies bind to the NSP4 oligomer with a higher affinity than to the NSP4 monomer. Similar MAbs to the HIV envelope protein have been characterized, indicating that B4-2/55 and αNSP4114–135 recognize a novel type of linear epitope that is sensitive to the quaternary structure of NSP4 (Broder et al., 1994; Gorny et al., 2000).
This underscores the importance of oligomerization to the structure and antigenicity of NSP4. Recent work to characterize the biochemical mechanism of oligomerization of NSP4 suggests that the amphipathic alpha helical domain (aa55–85), coiled-coil domain (aa95–137), and the C-terminus (aa147–175) all contribute to the formation of NSP4 oligomers and high molecular weight aggregates (Jagannath et al., 2006). Despite much in vitro biochemical analysis of NSP4 oligomerization, mutations that disrupt oligomer formation have not been reported. Moreover, little is known about NSP4 oligomerization during a RV infection. Given that B4-2/55 and αNSP4114–135 have higher affinity for the NSP4 oligomer than the monomer, it may be possible to use these antibodies to differentially stain the NSP4 oligomer pool from the monomer pool in RV-infected cells and determine if NSP4 oligomerization is linked to the formation of the calcium-triggered NSP4 vesicules associated with viroplasms (Berkova et al., 2006).
In the recent crystal structures of bacterially expressed NSP4 95–146, residues 138–146 are disordered, and larger fragments of NSP4 containing either the amphipathic alpha helix or the C-terminal 29 residues did not crystallize (Deepa et al., 2007). B4-2/55 and αNSP4114–135 may be useful for screening conditions for the assembly of monodispersed NSP4 oligomers of larger fragments or full-length NSP4 that would be more amenable to crystalization. Alternatively, co-crystalization of NSP4 bound to the B4-2/55 Fab fragment may prevent the formation of high molecular weight soluble aggregates of NSP4 (Jagannath et al., 2006). The use of Fab fragments of αNSP4120–147 or αNSP4150–175—if they can be sufficiently purified—may force the disordered residues of the NSP4 C-terminus into a more ordered state that could be visualized in the crystal structure.
The detailed mapping of these antibodies to NSP4 will also enable further dissection of NSP4 functional domains. Using the NSP4 peptide specific antibodies, we characterized the interaction between NSP4 and the RV spike protein VP4. Previous studies have shown that NSP4 aa112–148 binds VP4. We have refined this to show that the VP5* fragment of the spike protein binds NSP4 and aa114–135 are sufficient for binding. The interaction is specific because αNSP4114–135 and αNSP4120–147 block binding, but αNSP4150–175 did not, confirming previous data that the C-terminus is dispensable for the VP5*-NSP4 interaction (Au et al., 1993).
Multiple pools of VP4 and NSP4 have been described in RV-infected cells, including a fraction of each that trafficks to the plasma membrane, and both proteins localize to detergent-resistant rafts (Berkova et al., 2006; Cuadras et al., 2006; Sapin et al., 2002; Storey et al., 2007). These two proteins might work in concert to establish the non-classical, apically targeted release of RV in polarized cells (Sapin et al., 2002). siRNA knock-down or tunicamycin treatment of RV-infected cells, which affects the production and maturation of NSP4, respectively, also blocked the associated of VP4 with mature virions and detergent-resistant rafts. Thus, the interaction between NSP4 and VP5* appears to be critical for assembly of VP4 spikes during particle formation in the ER (Cuadras et al., 2006). If VP4 has to be assembled onto the DLP before VP7, as recently suggested, the high affinity interactions between the entertoxin domain of NSP4 (aa114–135) and VP5* and the DLP binding domain of NSP4 (aa161–175) and DLPs during particle maturation could correctly orient the spike onto maturing particles before VP7 assembly, illustrated in Figure 9D (Cuadras et al., 2006).
Lastly, since αNSP4150–175 binds to the extreme C-terminus of NSP4, previously shown to bind nacent DLPs and facilitate their budding into the ER, then use of αNSP4150–175 Fab fragments to block the binding of NSP4 to DLPs may further elucidate the mechanism for the NSP4-DLP interaction (Au et al., 1993; Meyer et al., 1989; O'Brien et al., 2000). Additionally, αNSP4120–147 and αNSP4150–175 may block the interaction between NSP4 and tubulin, reported to bind NSP4 aa120–175, further mapping and confirming the specificity of NSP4-tubulin interaction (Xu et al., 2000).
In summary, our detailed epitope map of the SA11 NSP4 cytoplasmic tail using both MAb and epitope-specific peptide antibodies supports a model of NSP4 in which distinct domains are closely juxtaposed within the primary sequence yet they can be distinguished by examining the global epitope structure of the molecule. Mapping binding of the VP5* fragment of the RV spike to NSP4 aa114–135 by use of the antibodies characterized here demonstrates one application for functional analysis of the multifunctional NSP4 virulence factor.
Acknowledgements
The authors would like to thank Xi-Lei Zeng for the purification of His-tagged VP5* and VP8* proteins and Sue Crawford for her help with experimental design and analysis.
This work was supported in part by NIH grant DK30144, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center, and NIH Training Grant in Molecular Virology, AI007471.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Au KS, Mattion NM, Estes MK. A subviral particle binding domain on the rotavirus nonstructural glycoprotein NS28. Virology. 1993;194:665–673. doi: 10.1006/viro.1993.1306. [DOI] [PubMed] [Google Scholar]
- Ball JM, Mitchell DM, Gibbons TF, Parr RD. Rotavirus NSP4: A multifunctional viral enterotoxin. Viral Immunol. 2005;18:27–40. doi: 10.1089/vim.2005.18.27. [DOI] [PubMed] [Google Scholar]
- Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- Berkova Z, Crawford SE, Trugnan G, Yoshimori T, Morris AP, Estes MK. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. J. Virol. 2006;80:6061–6071. doi: 10.1128/JVI.02167-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgan MA, Mori Y, Ito N, Sugiyama M, Minamoto N. Antigenic analysis of nonstructural protein (NSP) 4 of group A avian rotavirus strain PO-13. Microbiol Immunol. 2003;47:661–668. doi: 10.1111/j.1348-0421.2003.tb03429.x. [DOI] [PubMed] [Google Scholar]
- Bowman G, Nodelman I, Levy O, Lin S, Tian P, Zamb T, Udem S, Venkataraghavan B, Schutt C. Crystal structure of the oligomerization domain of NSP4 from rotavirus reveals a core metal-binding site. J Mol Biol. 2000;304:861–871. doi: 10.1006/jmbi.2000.4250. [DOI] [PubMed] [Google Scholar]
- Broder CC, Earl PL, Long D, Abedon ST, Moss B, Doms RW. Antigenic Implications of Human-Immunodeficiency-Virus Type-1 Envelope Quaternary Structure - Oligomer-Specific and Oligomer-Sensitive Monoclonal-Antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JW, Greenberg HB, Shaw RD, Estes MK. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J. Virol. 1988;62:2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NW, Estes MK, Langridge WHR. Oral immunization with a shiga toxin B subunit: rotavirus NSP4(90) fusion protein protects mice against gastroenteritis. Vaccine. 2005;23:5168–5176. doi: 10.1016/j.vaccine.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Liprandi F, Conner ME, Estes MK. Species specificity and interspecies relatedness of NSP4 genetic groups by comparative NSP4 sequence analyses of animal rotaviruses. Arch Virol. 2000;145:371–383. doi: 10.1007/s007050050029. [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Conner ME, Finegold MJ, Estes MK. Group A rotavirus infection and age-dependent diarrheal disease in rats: A new animal model to study the pathophysiology of rotavirus infection. J Virol. 2002;76:41–57. doi: 10.1128/JVI.76.1.41-57.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadras MA, Bordier BB, Zambrano J, Ludert JE, Greenberg HB. Dissecting rotavirus particle-raft interaction with small interfering RNAs: Insights into rotavirus transit through the secretory pathway. J Virol. 2006;80:3935–3946. doi: 10.1128/JVI.80.8.3935-3946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa R, Rao CD, Suguna K. Structure of the extended diarrhea-inducing domain of rotavirus enterotoxigenic protein NSP4. Archives of Virology. 2007;152:847–859. doi: 10.1007/s00705-006-0921-x. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zeng CQ-Y, Ball JM, Estes MK, Morris AP. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. PNAS. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson BL, Graham DY, Mason BB, Hanssen HH, Estes MK. Two types of glycoprotein precursors are produced by the Simian rotavirus SA11. Virology. 1983a;127:320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Ericson BL, Petrie BL, Graham DY, Mason BB, Estes MK. Rotaviruses code for two types of glycoprotein precursors. J Cell Biochem. 1983b;22:151–160. doi: 10.1002/jcb.240220304. [DOI] [PubMed] [Google Scholar]
- Estes MK, Kapikian AZ. Rotaviruses. 5th. 2007. pp. 1917–1974. [Google Scholar]
- Fischer TK, Viboud C, Parashar U, Malek M, Steiner C, Glass R, Simonsen L. Hospitalizations and Deaths from Diarrhea and Rotavirus among Children <5 Years of Age in the United States, 1993–2003. J Infect Dis. 2007;195:1117–1125. doi: 10.1086/512863. [DOI] [PubMed] [Google Scholar]
- Gorny MK, VanCott TC, Williams C, Revesz K, Zolla-Pazner S. Effects of oligomerization on the epitopes of the human immunodeficiency virus type 1 envelope glycoproteins. Virology. 2000;267:220–228. doi: 10.1006/viro.1999.0095. [DOI] [PubMed] [Google Scholar]
- Horie Y, Nakagomi T, Oseto M, Masamune O, Nakagomi O. Conserved structural features of nonstructural glycoprotein NSP4 between group A and group C rotaviruses. Archives of Virology. 1997;142:1865–1872. doi: 10.1007/s007050050204. [DOI] [PubMed] [Google Scholar]
- Jagannath MR, Kesavulu MM, Deepa R, Sastri PN, Kumar SS, Suguna K, Rao CD. N- and C-terminal cooperation in rotavirus enterotoxin: Novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain. J Virol. 2006;80:412–425. doi: 10.1128/JVI.80.1.412-425.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Tian P. Detailed computational analysis of a comprehensive set of group a rotavirus NSP4 proteins. Virus Genes. 2003;26:271–282. doi: 10.1023/a:1024451314534. [DOI] [PubMed] [Google Scholar]
- Lopez T, Camacho M, Zayas M, Najera R, Sanchez R, Arias CF, Lopez S. Silencing the morphogenesis of rotavirus. J. Virol. 2005;79:184–192. doi: 10.1128/JVI.79.1.184-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JC, Bergmann CC, Bellamy AR. Interaction of rotavirus cores with the nonstructural glycoprotein NS28. Virology. 1989;171:98–107. doi: 10.1016/0042-6822(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Diarrhea-inducing activity of avian rotavirus NSP4 glycoproteins, which differ greatly from mamalian Rotavirus NSP4 glycoproteins in deduced amino acid sequence, in suckling mice. J Virol. 2002;76:5829–5834. doi: 10.1128/JVI.76.11.5829-5834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Scott JK, Ball JM, Zeng CQ-Y, O'Neal WK, Estes MK. NSP4 elicits age-dependent diarrhea and Ca2+-mediated I- influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Taylor JA, Bellamy AR. Probing the structure of rotavirus NSP4: A short sequence at the extreme C terminus mediates binding to the inner capsid particle. J Virol. 2000;74:5388–5394. doi: 10.1128/jvi.74.11.5388-5394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TD, Kitamoto N, Tanaka T, Hutson AM, Estes MK. Identification of genogroup I and genogroup II broadly reactive epitopes on the norovirus capsid. Journal of Virology. 2005;79:7402–7409. doi: 10.1128/JVI.79.12.7402-7409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr RD, Storey SM, Mitchell DM, McIntosh AL, Zhou ML, Mir KD, Ball JM. The rotavirus enterotoxin NSP4 directly interacts with the caveolar structural protein caveolin-1. J Virol. 2006;80:2842–2854. doi: 10.1128/JVI.80.6.2842-2854.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie BL, Estes MK, Graham DY. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J. Virol. 1983;46:270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie BL, Greenberg HB, Graham DY, Estes MK. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus. Res. 1984;1:133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Poruchynsky MS, Atkinson PH. Rotavirus protein rearrangements in purified membrane-enveloped intermediate particles. J Virol. 1991;65:4720–4727. doi: 10.1128/jvi.65.9.4720-4727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RL, Deivanayagam CCS, Owens RT, Carson M, Hook A, Moore D, Yang VWC, Narayana SVL, Hook M. Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors, alpha(1)beta(1) integrin and Staphylococcus aureus Cna MSCRAMM. Journal of Biological Chemistry. 1999;274:24906–24913. doi: 10.1074/jbc.274.35.24906. [DOI] [PubMed] [Google Scholar]
- Sapin C, Colard O, Delmas O, Tessier C, Breton M, Enouf V, Chwetzoff S, Ouanich J, Cohen J, Wolf C, Trugnan G. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J Virol. 2002;76:4591–4602. doi: 10.1128/JVI.76.9.4591-4602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Horie Y, Nakagomi TOM, Nakagomi O. Group C Rotavirus NSP4 Induces Diarrhea in Neonatal Mice. Arch Virol. 2001;146:801–806. doi: 10.1007/s007050170148. [DOI] [PubMed] [Google Scholar]
- Storey SM, Gibbons TF, Williams CV, Parr RD, Schroeder F, Ball JM. Full-length, glycosylated NSP4 is localized to plasma membrane caveolae by a novel raft isolation technique. Journal of Virology. 2007;81:5472–5483. doi: 10.1128/JVI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, O'Brien JA, Yeager M. The cytoplasmic tail of NSP4, the endoplasmic reticulum-localized non-structural glycoprotein of rotavirus, contains distinct virus binding and coiled coil domains. EMBO. J. 1998;15:4469–4476. [PMC free article] [PubMed] [Google Scholar]
- Tian P, Ball JM, Zeng CQ-Y, Estes MK. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J Virol. 1996;70:6973–6981. doi: 10.1128/jvi.70.10.6973-6981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Bellamy AR, Taylor JA. Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules. EMBO J. 2000;19:6465–6474. doi: 10.1093/emboj/19.23.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zeng CQ-Y, Dong Y, Ball JM, Saif LJ, Morris AP, Estes MK. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zeng CQ-Y, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J. Virol. 2000;74:11663–11670. doi: 10.1128/jvi.74.24.11663-11670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Chen ZC, Purcell RH, Emerson SU. Positive reactions on Western blots do not necessarily indicate the epitopes on antigens are continuous. Immunology and Cell Biology. 2007;85:73–78. doi: 10.1038/sj.icb.7100004. [DOI] [PubMed] [Google Scholar]


