Abstract
Lauryl gallate (antioxidant food additive E-312) prevents the formation of dimethylbenzanthracene-induced skin tumours in mice, and kills, selectively, tumoral cells on established tumours. This results in total remission, after topical application of the compound on the tumoral mass, without affecting the surrounding tissue.
Keywords: lauryl gallate, antioxidants, protein tyrosine kinase inhibitors, antitumoral agents, food additives
We have reported that triphenols such as gallic acid (3,4,5-trihydroxybenzoic acid) and its esters (methyl, propyl (E-310), octyl (E-311) and lauryl (E-312)) inhibit protein tyrosine kinases (PTKs) (Lázaro et al, 1995; Palacios et al, 2002). Similarly to other PTK inhibitors such as genistein (Uckun et al, 1995), herbymicin (Otani et al, 1993), staurosporin (Bossy-Wetzel et al, 1998), tyrphostins (Burger et al, 1995), etc., they show an antiproliferative effect on tumoral cell lines (Serrano et al, 1998). The most hydrophobic of the alkyl gallates (octyl and lauryl) exhibit a proapoptotic potency between 50 and 250 times higher than gallic acid for the lines tested (Serrano et al, 1998). The study of lauryl gallate (LG) on the pre-B cell lymphoma Wehi 231 line shows that it inhibits PTKs both in crude extracts and whole cells, disrupts the mitochondrial membrane potential, promotes the efflux of cytochrome c to the cytosol, activates the cascade of caspases and induces oligonucleosomal breakdown of DNA (Roy et al, 2000). Also these types of compounds seem to show a selectivity for rapidly growing cells, opening the possibility of their study as potential antitumoral agents (Serrano et al, 1998). Here, we report that LG not only prevents the formation of chemically induced skin tumours in mice but is also able to kill, selectively, tumoral cells in established tumours.
MATERIALS AND METHODS
Chemicals
Lauryl gallate (Fluka), 7,12-dimethylbenzanthracene, phorbol myristate acetate, dimethylsulphoxide, acetone and glycerol were supplied by Sigma-Aldrich Química (Spain).
Mice
IRC mice (6–8 weeks old) were from the Charles River Laboratories (Saint Aubin les Elbeuf, Rouen, France). All experiments involving ‘in vivo’ testing were carried out with ethical committee approval and met the standards required by the guidelines for the welfare of animals in experimental neoplasia (Workman et al, 1998).
Prevention of tumour formation
Thirty-two IRC mice were individualised and subdivided into four groups (four females and four males per group), as shown in Table 1 . The induction of the tumours was performed following the protocol described (Segal et al, 1978) with a slight modification. The solvent used was a mixture of dimethylsulphoxide and glycerol (1 : 1), in which all the compounds were soluble. After a single application of 25 μg of 7,12-dimethylbenz[a]anthracene (DMBA) in a volume of 20 μl, and 15 days without treatment, a solution of 5 μg of phorbol-12-myristate-13-acetate (PMA) with different doses of LG (Fluka) was applied topically three times per week. The doses of LG were 0 (controls), 2.5, 10 and 50 μg in a volume of 20 μl per application. After 6–7 weeks of treatment, tumours appeared on 90% of the control mice, and the behaviour of LG was evaluated as shown in Table 1.
Table 1. Effect of different doses of LG on the prevention of tumour formation and regression of established tumours.
|
Prevention of tumour formation |
Regression of established tumours |
|||||
|---|---|---|---|---|---|---|
| Group | LG 2.5 | LG 10 | LG 50 | LG 100 | LG 250 | LG 500 |
| Sex | ||||||
| Male | 1/4 | 2/4 | 4/4 | 0/2 | 2/4 | 3/3 |
| Female | 0/4 | 2/4 | 3/4 | 0/3 | 2/4 | 2/4 |
Regression of established tumours
Twenty-four individualised mice were treated with a single application of 25 μg of DMBA, and after 15 days with no application of compounds, 5 μg of PMA was applied three times a week (6–7 weeks) until the tumour appeared. The mice were subdivided into three different groups (LG 100, LG 250 and LG 500, four males and four females per group), depending on the treatment dose after tumour formation. The volume per application was 20 μl, which was sufficient to cover the whole tumour surface and was applied three times a week for a period of 8 weeks.
RESULTS AND DISCUSSION
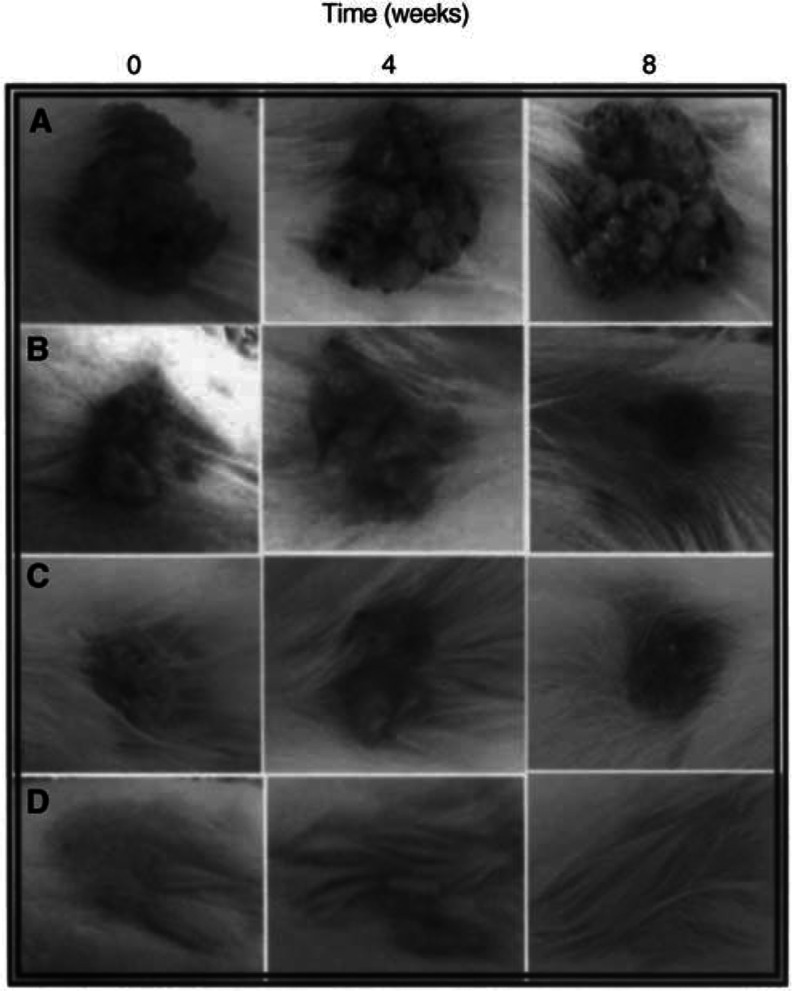
To test for the effect of LG on chemically induced skin tumours, we have used the two-stage carcinogenesis model (Segal et al, 1978) consisting in the single application of the carcinogen DMBA followed by repetitive applications of the noncarcinogen promoter PMA. We have slightly modified the published procedure by substituting the solvent currently used (acetone) for a mixture of dimethylsulphoxide/glycerol (1 : 1). This results in an easier application and better spreading of the compounds on the mouse skin and avoids the drawback of itching, uneasiness, and sore formation in mice because of the delipidisation produced by the continuous use of acetone. Consequently, mice are easier to handle and are less aggressive. Also, this change does not affect the growth of tumours since more than 90% of mice developed papillomas. Table 1 summarises the results obtained in two types of experiments. In one of them we investigated the effect that increasing amounts of LG added together with the PMA solution have on the prevention of tumour formation. In the other, we studied the effect that topical application of an LG solution has on already established tumours. In both cases, partial inhibition (i.e. tumour size, number of papillomas, etc.) was not considered. Only those mice in which there is complete prevention of tumour formation or total remission of established tumours are recorded in the table. The term ‘full remission’ (arbitrarily defined) refers to those cases in which no papillomas were visually present after 8 weeks of treatment with LG and that if left untreated for a period of up to 3 months they did not exhibit the growth of new papillomas. In both series there is a clear dose–response relation, although the amounts of LG needed for the prevention of tumour formation are roughly 10 times lower than those needed for attaining full regression of established tumours. Figure 1 shows the effect that topical application of the solution of LG has on the regression of tumours. Time 0 is the time at which treatment was installed (all tumours had a developing time, under standard conditions, of around 8 weeks). The treatment was evaluated after 4 and 8 weeks of the initiation (see Materials and Methods). At a dose of 100 μg per application, the effect observed is only partial after 8 weeks (Figure 1A). However, when the dose was increased to 250 μg (Figure 1B) or 500 μg (Figure 1C), full remission was reached, in most cases, after the same period of time. In all cases, it was noticeable that during the treatment, tumours changed in size and shape, passing from a smoother surface at the beginning to a cauliflower-like surface with multiple indentations and pedunculations which later fall without bleeding. However, the surrounding nontumoral tissue is not affected by the treatment. Indeed, the application of the highest dose (500 μg) on healthy mice does not produce any effect during the same period of time (Figure 1D).
Figure 1.
Effect of different doses of LG on the regression of DMBA+PMA induced tumours. (A) 100 μg, (B) 250 μg, (C) 500 μg and (D) 500 μg on healthy skin.
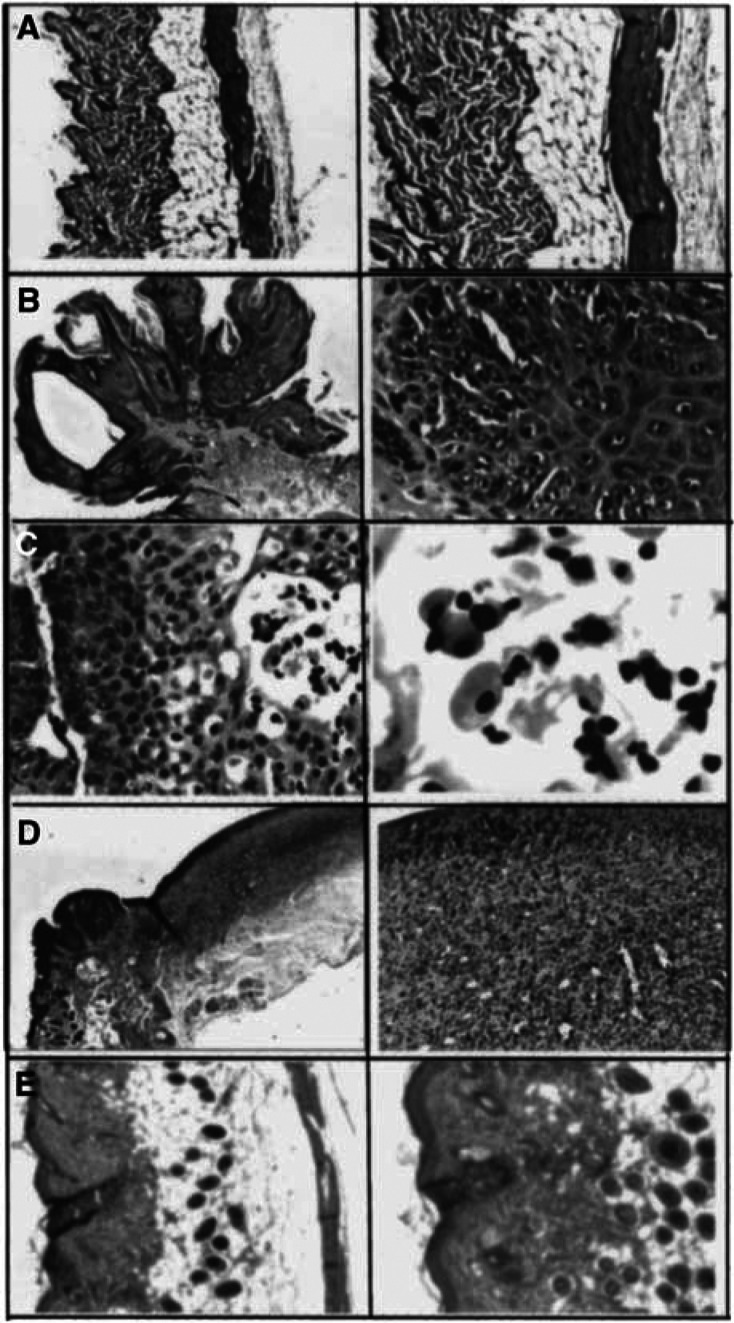
To analyse the anatomopathological characteristics of the tumours undergoing LG treatment, mice were killed under anaesthesia after different periods of time. Figure 2A shows the normal skin, without significant lesions, of a healthy mouse. The untreated tumoral mass induced by DMBA and PMA shows an exophytic squamous lesion with a central core. The epidermal surface presents orthokeratotic hyperkeratosis with clumps of red cells and neutrophils. The tumour infiltrates the superficial dermis, and marked nuclear atypia and frequent mitosis are also found (Figure 2B). The characteristics of the tumoral mass after 4 weeks of treatment with LG show features similar to the untreated tumour. However, an increase in the number of apoptotic cells was found (Figure 2C). The residual lesion after 8 weeks of LG treatment (the tumoral mass has already disappeared) shows a deep ulcer penetrating the muscular layer and granulation tissue at the base. Also there is reactive hyperplasia of the epidermal borders of the ulcer (Figure 2D). Finally, the treatment with LG on healthy mice (analogue to Figure 1D) does not produce significant alterations on the skin (Figure 2E).
Figure 2.
Anatomopathological studies. (A) Normal skin, (B) untreated tumour, (C) treated tumour for 4 weeks with 500 μg of LG, (D) skin after tumour regression (treatment with 500 μg of LG for 8 weeks) and (E) normal skin treated with 500 μg of LG for 8 weeks.
As a complement or substitution of the traditional armamentarium against tumoral growth and chemoprevention, based mainly in cytotoxic chemotherapy, because of its toxicity, new approaches dealing with the manipulation of signal transduction pathways have been the centre of research during the last years. Protein tyrosine phosphorylation is an early and key step activated by receptor and nonreceptor PTKs which have been implicated in carcinogenesis, tumour progression and metastasis when their activities are not properly regulated (Kolibaba and Druker, 1997; Porter and Vaillancourt, 1998; Blume-Jensen and Hunter, 2001). An intensive search for inhibitors of these enzymes has proven to be fruitful and some of these compounds are already at the preclinical and clinical stages (Levitt and Koty, 1999). One would expect that competitive inhibitors towards the tyrosine (acceptor substrate) could be more efficient than inhibitors towards ATP (donor substrate) because of the high intracellular ATP concentration (in the mM range). However, most of the current strategies are designed to inhibit the ATP binding by specific PTKs which are overexpressed in certain tumours (i.e. imatinib mesylate (formerly STI571) for Bcr-Abl in chronic myeloid leukaemia (Thiesing et al, 2000); ZD1839 (Iressa) for epidermal growth factor receptor (Albanell et al, 2002) or WHI-PI 131 for Janus 3 kinase in acute lymphobastic leukaemia (Uckun et al, 1999), among others). However, sometimes the aberrant PTK is not identified or the growth of the tumour may depend on the concerted action or partial contribution of several PTKs. In such cases, the use of a general nonspecific PTK inhibitor would be worthwhile considering. It may be argued that these kinds of inhibitors might have a significant effect on normal cells as well. Nevertheless, the activity of PTKs in normal cells is usually in the ‘off’ state, which is switched to the ‘on’ state by means of specific ligand, activation of transduction cascades, aberrant overexpression of the oncogenic protein or expression of a mutant protein that escapes the tight regulation of these enzymes. In our opinion, the use of LG (or other alkyl gallates) as one of such inhibitors could be worth investigating considering the following.
First, LG inhibits PTKs in whole cells, crude extracts and purified form (PTK c-Src) without inhibiting serine/threonine kinases (Palacios et al, 2002) and are not affected by high ATP concentrations. This compound and other gallic acid derivatives are able to induce apoptosis in tumoral cell lines showing selectivity for rapidly growing cells (resting lymphocytes can stand much higher concentrations than mitogen-stimulated lymphocytes (Serrano et al, 1998).
Second, as shown in this report, LG is able to prevent the tumour formation of skin tumours induced by DMBA and PMA in mice and is able to induce full regression after topical application, on already established tumours.
Third, because of their low toxicity, alkyl gallates have been widely used as food additive antioxidants for over 50 years. Thus, much of the work on their metabolism, toxicological effect and pharmacokinetic studies has already been performed. Long-term toxicity studies in rats and mice have shown that no effects were observed at doses as high as 1000 mg kg−1 feed. In 1976, the FAO/WHO Joint Expert Committee on Food Additives (JECFA) established an acceptable daily intake (ADI) for man of 0.2 mg kg−1 body weight as a sum of propyl, octyl and LG (Van der Heijden et al, 1986).
Also, their simple chemical structures and availability in unlimited amounts at a very low commercial cost are important aspects to consider.
Acknowledgments
This work was supported by Grants FIS 00/410 from the Fondo de Investigaciones Sanitarias de la Seguridad Social and CAM/08.8/0005/1997 from the Comunidad Autónoma de Madrid.
References
- Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, Nicholson RI, Baselga J (2002) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20: 110–124 [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T (2001). Oncogenic kinase signaling. Nature 411: 355–365 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AM, Kaur G, Alley MC, Supko JG, Malspeis L, Grever MR, Sausville EA (1995) Tyrphostin AG17, [(3,5- di- tert- butyl-4-hydroxybenzylidene)-malonitrile], inhibits cell growth by disrupting mitochondria. Cancer Res 55: 2794–2799 [PubMed] [Google Scholar]
- Kolibaba KS, Druker BJ (1997) Protein tyrosine kinases and cancer. Biochim Biophys Acta 1333: F217–F248 [DOI] [PubMed] [Google Scholar]
- Lázaro I, Palacios C, González M, González-Porqué P (1995) Inhibition of human spleen protein tyrosine kinases by phenolic compounds. Anal Biochem 225: 180–183 [DOI] [PubMed] [Google Scholar]
- Levitt ML, Koty PP (1999) Tyrosine kinase inhibitors in preclinical development. Invest New Drugs 17: 213–226 [DOI] [PubMed] [Google Scholar]
- Otani H, Erdos M, Leonard WJ (1993) Tyrosine kinase(s) regulate apoptosis and bcl-2 expression in a growth factor dependent cell line. J Biol Chem 268: 22 733–22 736 [PubMed] [Google Scholar]
- Palacios C, Cespon C, Martin de la Vega C, Roy G, Serrano A, Salinas M, Gonzalez-Porque P (2002) Lauryl gallate inhibits the activity of protein tyrosine kinase c-Src purified from human platelets. J Enzyme Inhibition 16: 527–533 [DOI] [PubMed] [Google Scholar]
- Porter AC, Vaillancourt RR (1998) Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene 17: 1343–1352 [DOI] [PubMed] [Google Scholar]
- Roy G, Lombardia M, Palacios C, Serrano A, Cespon C, Ortega E, Eiras P, Lujan S, Revilla Y, Gonzalez-Porque P (2000) Mechanistic aspects of the induction of apoptosis by lauryl gallate in the murine B-cell lymphoma line Wehi 231. Arch Biochem Biophys 383: 206–214 [DOI] [PubMed] [Google Scholar]
- Segal A, Van Duuren BL, Mate U, Solomon JJ, Seidman I, Smith A, Melchionne S (1978) Tumor-promoting activity of 2,3-dihydrophorbol myristate acetate and phorbol myristate acetate in mouse skin. Cancer Res 38: 921–925 [PubMed] [Google Scholar]
- Serrano A, Palacios C, Roy G, Cespon C, Villar LM, Nocito M, Gonzalez-Porque P (1998) Derivatives of gallic acid induce apoptosis in tumoral cell lines and inhibit lymphocyte proliferation. Arch Biochem Biophys 350: 49–54 [DOI] [PubMed] [Google Scholar]
- Thiesing JT, Ohno-Jones S, Kolibaba KS, Druker BJ (2000) Efficacy of STI571, an Abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against Bcr-Abl-positive cells. Blood 96: 3195–3199 [PubMed] [Google Scholar]
- Uckun FM, Evans WW, Forsyth CJ, Waddick KG, Ahlgren LT, Chelstrom LM, Buskhardt A, Bolen J, Myers DE (1995) Biotherapy of B cell precursor by targeting genistein to CD19 associated tyrosine kinases. Science 267: 886–891 [DOI] [PubMed] [Google Scholar]
- Uckun FM, Ek O, Liu XP, Chen CL (1999) In vivo toxicity and pharmacokinetic features of the Janus kinase 3 inhibitor WHI-P131 [4-(4′ Hydroxyphenyl)-amino-6,7-dimethoxyquinazoline]. Clin Cancer Res 5: 2954–2962 [PubMed] [Google Scholar]
- Van der Heijden CA, Janssen PJCM, Strik JJTWA (1986) Toxicology of gallates: a review and evaluation. Food Chem Toxic 24: 1067–1070 [DOI] [PubMed] [Google Scholar]
- Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia, 2nd ed. Br J Cancer 77: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]