Abstract
A recombinant fusion protein of colon carcinoma binding A33 single chain antibody with cytosine deaminase displayed specific antigen binding and enzyme activity in surface plasmon resonance and is catalytic activity assay. In vitro, it selectively increased the toxicity of 5-FC to A33 antigen-positive cells by 300-fold, demonstrating the potency of this ADEPT strategy.
Keywords: tumour targeting, A33 antibody, antibody directed enzyme-producing therapy (ADEPT), colon carcinoma, recombinant fusion proteins
Antibody-directed enzyme-prodrug therapy (ADEPT) utilises antibody–enzyme constructs for targeted enzyme delivery to tumours and subsequent localised activation of a prodrug. Its potential has been demonstrated in phase I studies (Webley et al, 2001).
Monoclonal antibody A33 recognises a cell-surface antigen that is expressed on ∼95% of colon cancers (Garin-Chesa et al, 1996). In clinical trials, radiolabelled A33 localised specifically to colon cancer cells, where it was retained for several weeks while clearing within days from normal colon (Welt et al, 1996).
Cytosine deaminase (CD) converts 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU) and has been empolyed in ADEPT (Wallace et al, 1994).
Recombinant fusion constructs should overcome the problems of chemical antibody–enzyme conjugation including inhomogeneous products and large protein size. Several recombinant constructs based on F(ab) and F(ab′)2 fragments have been described. Constructs based on single-chain variable fragments (scFv) may have favourable diffusion characteristics in solid tumours, but few descriptions of this approach have been published (Bhatia et al, 2000). Here, we report on a new ADEPT concept based on the A33 antigen and recombinant scFv–CD constructs.
MATERIALS AND METHODS
A33scFv (Rader et al, 2000) and CD (Austin and Huber, 1993) cDNA were PCR-amplified. Primers based on the published sequences were designed to remove start or stop codons and to add flanking restriction sites so that the DNA could be inserted into the pET 25 expression vector (Novagen, Madison, WI, USA) both directly and downstream of the inserted A33scFv DNA, so that the orientation of the fusion protein was 5′-A33scFv-CD-3′ (vector map available upon request).
A33scFv, CD, and A33scFv-CD and the control construct A33scFv-GFP were expressed by a T7-RNA polymerase-controlled bacterial system using BL21 Escherichia coli λDE3 lysogens (Novagen, Madison, WI, USA) at 37°C with IPTG induction at an OD600 nm of 0.5–0.7. Inclusion bodies were retrieved from cell pellets and solubilised using BugBuster™ reagent with 0.3 μl ml−1 Benzonase and Novagen Refolding Kit (both: Novagen, Madison, WI, USA) according to the manufacturer's instructions. Utilizing a C-terminal histidin tag, the protein was purified on sepharose-bound cobalt (Clontech) with imidazole elution.
Plasmon surface resonance assays were performed as described (Catimel et al, 1997) with A33 antigen-coated Biosensor chips. After 400 s, sample flow was replaced by buffer solution. The relative refraction at 600 s was compared with buffer flow and positive controls.
Catalytic activity of cytosine deaminase was determined as described (Austin and Huber, 1993).
For cytotoxicity assays, LIM 1215 or HT 29 tumour cells (Ludwig Institute for Cancer Research cell bank) were incubated on 96-well plates to reach 25–33% surface density. Fusion protein or control was added for 60 min, preceded by 90 min of incubation with “A33scFv-GFP” or hu3S193 IgG (1 mg ml−1) in blocking experiments. After washing, cells were incubated with prodrug or control for 48 h, washed and grown in medium for 72 h, followed by 3 h in 0.5 mg ml−1 MTT-solution, DMSO-lysis and photometry at 595 nm.
RESULTS
Protein expression and activity
Fusion proteins were expressed as inclusion bodies with a final culture yield of about 100 μg l−1. With metal affinity chromatography purity was >95% by SDS–PAGE.
Calculated from the molar extinction coefficient of 1.038 mM−1 for 5-FU, the catalytic activity was 2.5 μM min−1 for recombinant CD and 0.8 μMmin−1 for the A33scFv–CD fusion protein.
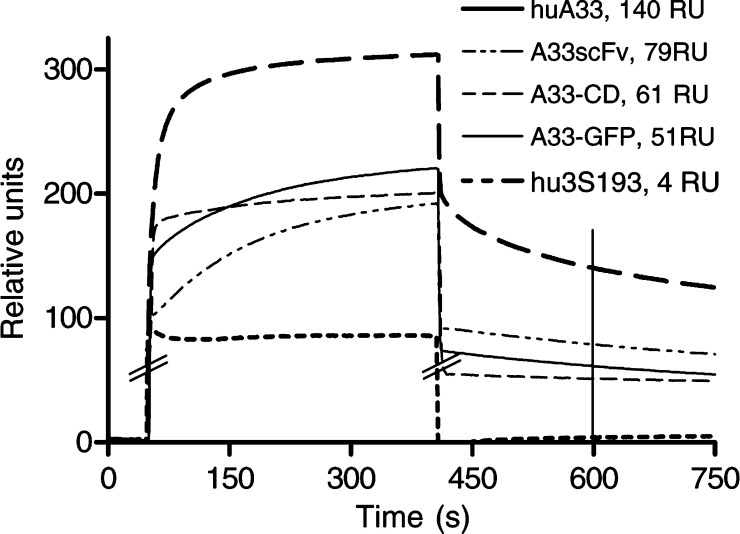
In surface plasmon resonance, all A33 preparations, but not the 3S193 control, displayed typical association and dissociation curves. Univalent A33scFv showed about half the binding activity of divalent of huA33 IgG (Figure 1), and A33scFv–CD had slightly less binding activity than A33scFv.
Figure 1.
Surface Plasmon resonance. Association and dissociation curves of A33 antibody preparations on an A33 antigen-coated biochip. The chip was exposed to either the complete huA33 IgG antibody (huA33 IgG), the rabbit-derived single chain fragment (A33scFv) or inclusion body preparations of the fusion proteins of A33scFv with either cytosine deaminase (A33scFv–CD IB) or green fluorescent protein (A33scFv–GFP IB). At 400 s, antibody flow was stopped and the chip rinsed with buffer solution. Protein binding is measured by the refraction of a light beam and expressed in relative units (RU) over time. The 600 s time point and the relative units at this point are indicated as approximate correlates of affinity.
ADEPT system in vitro
The antigen binding and enzymatic activity of the A33scFv–CD fusion protein was assessed in cytotoxicity assays using complete ADEPT system. The cytotoxicities of 5-FC and 5-FU showed no significant differences between the colon cancer cell lines LIM1215 (A33+) and HT29 (A33–) with an IC50 of about 30 mM for 5-FC and 0.3–0.03 mM for 5-FU (P<0.05 for 5-FC vs 5-FU, no significant difference between cell lines).
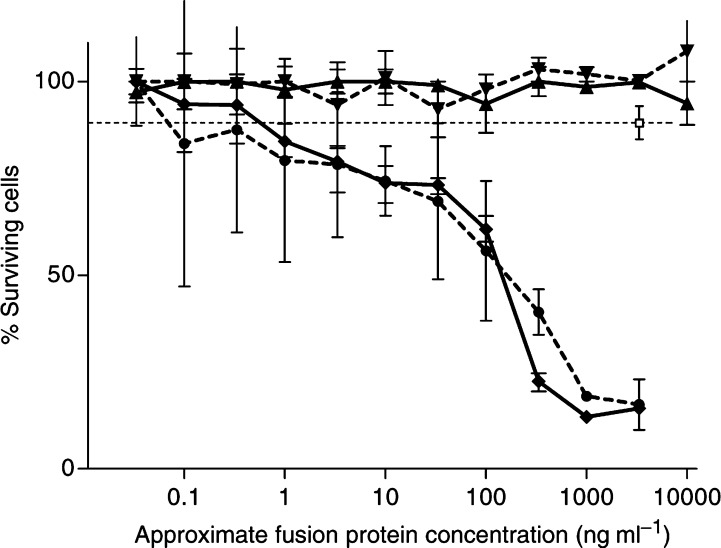
The complete ADEPT system was tested by incubating these two cell lines first with a serial dilution of A33scFv–CD and then, after washing, with the 5-FC prodrug at a fixed concentration. In this assay, crude and purified A33scFv–CD had a dose-dependant cytotoxic effect on A33-positive LIM1215 cells (IC50∼150 ng ml−1), but not on A33-negative cells (P=0.001 in Wilcoxon rank test). No cytotoxicity was observed with the A33scFv–GFP control (Figure 2).
Figure 2.
A33scFv–CD-mediated cytotoxicity on A33 antigen-positive vs negative cells: LIM1215 cells and HT29 cells were incubated with a dilution series of A33scFv–CD fusion protein and, after washing, with the 5-FC prodrug. Survival was measured by the MTT method as described. A33scFv–CD fusion protein from two different preparations was used on HT29 cells (▴ and ▾) and on LIM1215 cells (• and ⧫). As a control, a single, high concentration of A33scFv–GFP (□) was used instead of A33scFv–CD. Mean and s.d. of triplicate samples.
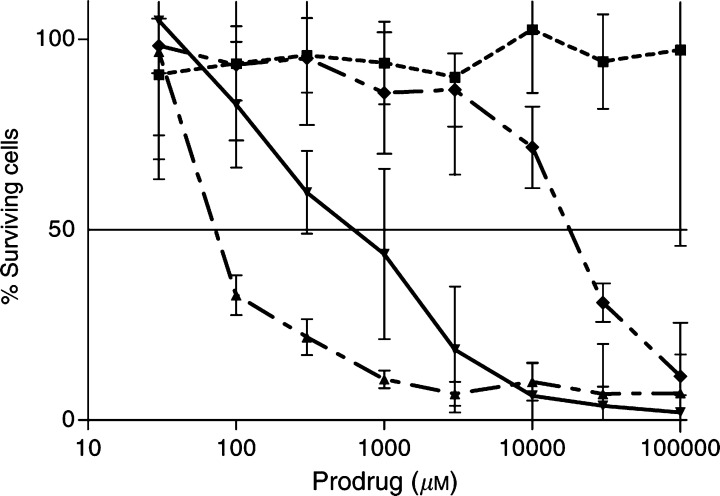
Without subsequent prodrug incubation, even the highest concentration of fusion protein tested had no cytotoxic effect on A33-positive LIM1215 cells (Figure 3). When binding of A33scFv–CD was blocked by preincubation with “A33scFv-GFP”, subsequent 5-FC incubation showed reduced cytotoxicity (IC50, ∼30 mM) compared to wells containing the irrelevant isotype control antibody hu3S193 (IC50, <1 mM, P<0.01).
Figure 3.
MTT cytotoxicity blocking assay. As a negative control, A33scFv–CD was used without subsequent prodrug incubation (▪), and 5-FU alone served as positive control (▴). In the complete ADEPT assay with subsequent 5-FC incubation as described in the text, cells were preincubated either with the “A33scFv-GFP” antibody (⧫) or with hu3S193 as an isotypic control antibody (▾) for 1 h before the fusion protein was added. Mean and s.d. of triplicate samples.
DISCUSSION
Two major obstacles have hampered the progress of ADEPT: the needs for specific, accessible antigens and for chemically stable and defined antibody–enzyme constructs of suitable molecular size. The ADEPT system introduced here is novel regarding the targeted antigen and the use of a recombinant scFv-based CD construct.
Incubation of A33-positive tumour cells with this construct increased 5-FC toxicity by about 300-fold, which was selectively blocked by preincubation with “A33scFv-GFP”, demonstrating antigen specificity. Neither A33scFv–CD without 5-FC nor a control construct with 5-FC inhibited cell growth, showing that specific enzymatic conversion was necessary for cytotoxicity. Together, these results demonstrate dual (i.e antibody and enzyme) specificity of the construct and functioning of this ADEPT system in vitro.
For ADEPT, it is important that CD does not naturally occur in mammalians, making the enzyme construct the exclusive source of prodrug activation, while allogenic immunogenicity can be addressed by polyethylene-glycol conjugation with preserved A33 binding (Deckert et al, 2000).
Only recently has the homohexameric structure of bacterial CD been resolved (Ireton et al, 2002). When the described construct showed effective dual function, either its monomer has catalytic activity, or it can form oligomers in solution or after antigen binding. While the published structure supports monomer activity, both hypotheses would explain the lower catalytic activity of A33scFv–CD compared to enzyme alone.
Acknowledgments
The authors thank Dr Christoph Rader and Dr Carlos F. Barbas III of the Scripps Institute, La Jolla, California, for providing the A33scFv plasmid for their excellent advice in realizing this project and for critical review of the manuscript. This work has been sponsored by National Cancer Institute Grants No. CA-33049 and CA-08748 to SW, and by Deutsche Forschungsgemeinschaft Grant No. De602/1-1 and the US Army Breast Cancer Research Program Grant No. DAMD17-99-1-9370 to PMD.
References
- Austin EA, Huber BE (1993) A first step in the development of gene therapy for colorectal carcinoma: cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol Pharmacol 43: 380–387 [PubMed] [Google Scholar]
- Bhatia J, Sharma SK, Chester KA, Pedley RB, Boden RW, Read DA, Boxer GM, Michael NP, Begent RH (2000) Catalytic activity of an in vivo tumor targeted anti-CEA scFv::carboxypeptidase G2 fusion protein. Int J Cancer 85: 571–577 [PubMed] [Google Scholar]
- Catimel B, Nerrie M, Lee FT, Scott AM, Ritter G, Welt S, Old LJ, Burgess AW, Nice EC (1997) Kinetic analysis of the interaction between the monoclonal antibody A33 and its colonic epithelial antigen by the use of an optical biosensor. A comparison of immobilisation strategies. J Chromatogr A 776: 15–30 [DOI] [PubMed] [Google Scholar]
- Deckert PM, Jungbluth A, Montalto N, Clark MA, Finn RD, Williams JC, Richards EC, Panageas KS, Old LJ, Welt S (2000) Pharmacokinetics and microdistribution of polyethylene glycol-modified humanized A33 antibody targeting colon cancer xenografts. Int J Cancer 87: 382–390 [PubMed] [Google Scholar]
- Garin-Chesa P, Sakamoto J, Welt S, Real FX, Rettig WJ, Old LJ (1996) Organ-specific expression of the colon cancer antigen A33, a cell surface target for antibody-based therapy. Int J Oncol 9: 465–471 [DOI] [PubMed] [Google Scholar]
- Ireton GC, McDermott G, Black ME, Stoddard BL (2002) The structure of Escherichia coli cytosine deaminase. J Mol Biol 315: 687–697 [DOI] [PubMed] [Google Scholar]
- Rader C, Ritter G, Nathan S, Elia M, Gout I, Jungbluth AA, Cohen LS, Welt S, Old LJ, Barbas III CF (2000) The rabbit antibody repertoire as a novel source for the generation of therapeutic human antibodies. J Biol Chem 275: 13668–13676 [DOI] [PubMed] [Google Scholar]
- Wallace PM, MacMaster JF, Smith VF, Kerr DE, Senter PD, Cosand WL (1994) Intratumoral generation of 5-fluorouracil mediated by an antibody – cytosine deaminase conjugate in combination with 5-fluorocytosine. Cancer Res 54: 2719–2723 [PubMed] [Google Scholar]
- Webley SD, Francis RJ, Pedley RB, Sharma SK, Begent RH, Hartley JA, Hochhauser D (2001) Measurement of the critical DNA lesions produced by antibody-directed enzyme prodrug therapy (ADEPT) in vitro, in vivo and in clinical material. Br J Cancer 84: 1671–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt S, Scott AM, Divgi CR, Kemeny NE, Finn RD, Daghighian F, Germain JS, Richards EC, Larson SM, Old LJ (1996) Phase I/II study of iodine 125-labeled monoclonal antibody A33 in patients with advanced colon cancer. J Clin Oncol 14: 1787–1797 [DOI] [PubMed] [Google Scholar]