Abstract
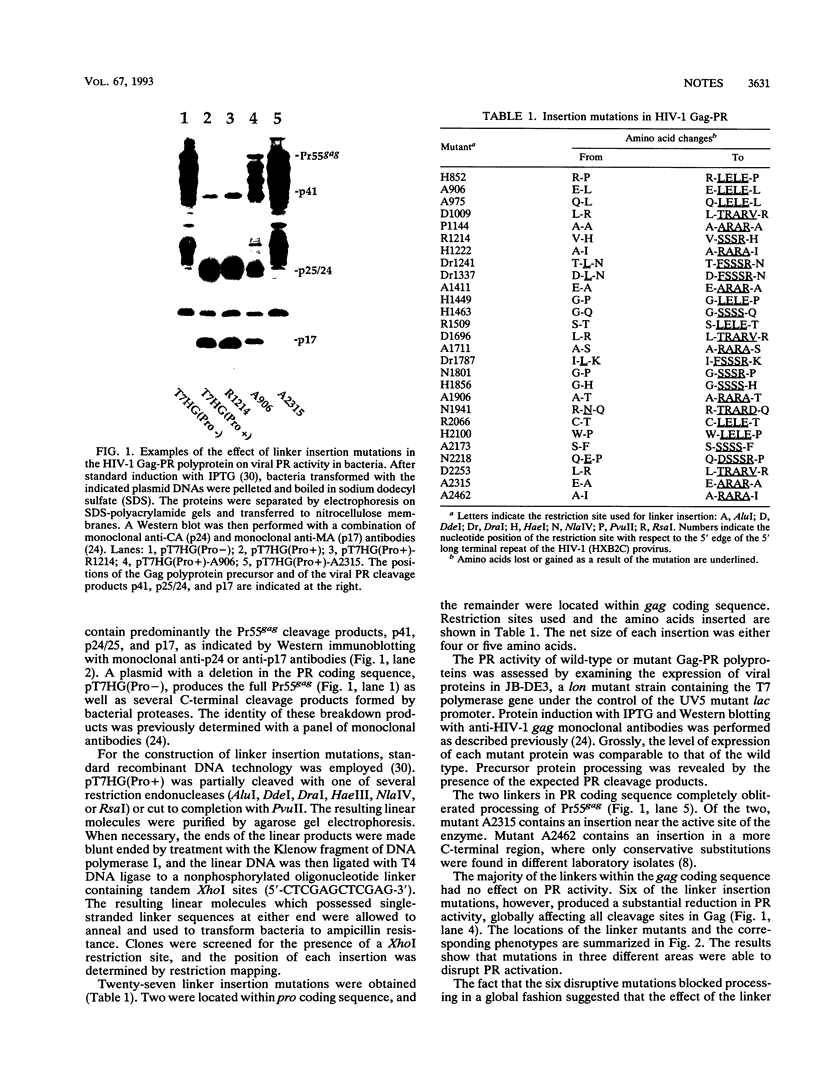
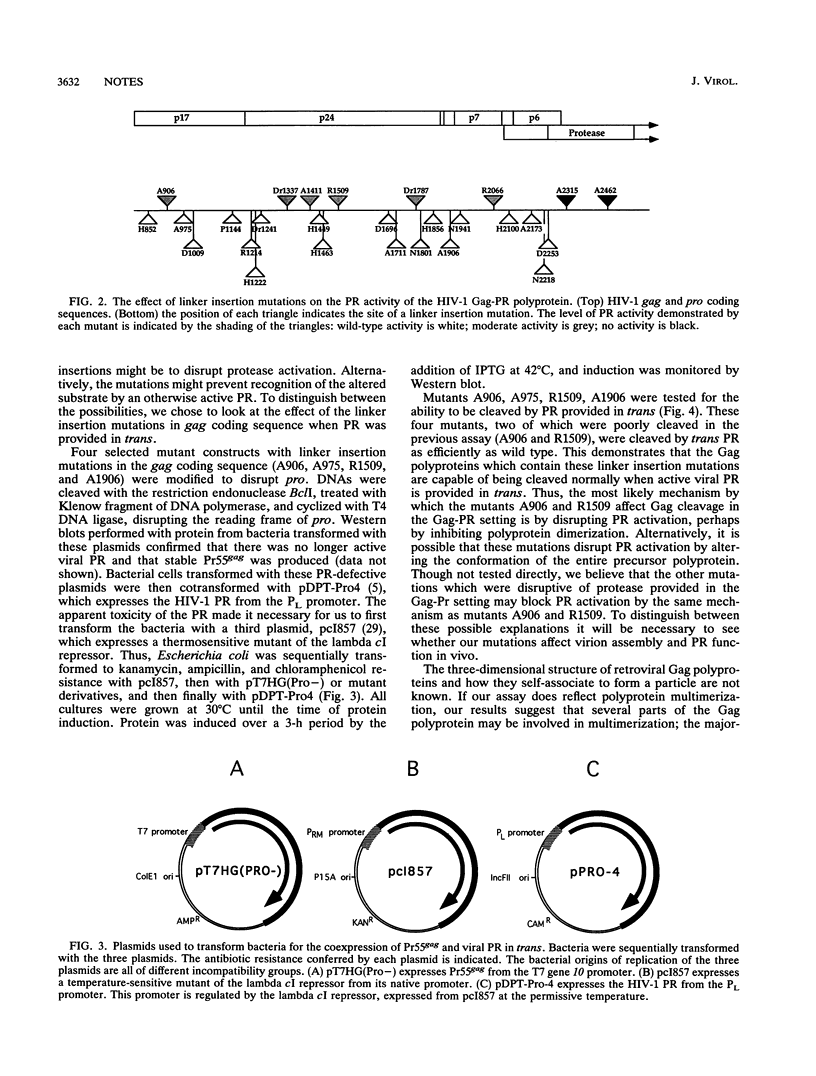
We have expressed the human immunodeficiency virus type 1 (HIV-1) protease (PR) in bacteria as a Gag-PR polyprotein (J. Luban and S.P. Goff, J. Virol. 65:3203-3212, 1991). The protein displays enzymatic activity, cleaving the Gag polyprotein precursor Pr55gag to the expected products. The PR enzyme is only active as a dimer, and we hypothesized that PR activation might be used as an indicator of polyprotein multimerization. We constructed 25 linker insertion mutations throughout gag and assessed the PR activity of mutant Gag-PR polyproteins by the appearance of Gag cleavage products in bacterial lysates. All mutant constructs produced stable protein in bacteria. PR activity of the majority of the Gag-PR mutants was indistinguishable from that of the wild type. Six mutants, one with an insertion in the matrix (MA), four with insertions in the capsid (CA), and one with insertions in the nucleocapsid (NC), globally disrupted polyprotein processing. When PR was provided in trans on a separate plasmid, the Gag proteins were cleaved with wild-type efficiency. These results suggest that the gag mutations identified as disruptive of polyprotein processing did not conceal the scissile bonds of the polyprotein. Rather, the mutations prevented PR activation in the context of a Gag-PR polyprotein, perhaps by preventing polyprotein dimerization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein H., Bizub D., Kotler M., Schatz G., Vogt V. M., Skalka A. M. Processing of avian retroviral gag polyprotein precursors is blocked by a mutation at the NC-PR cleavage site. J Virol. 1992 Mar;66(3):1781–1785. doi: 10.1128/jvi.66.3.1781-1785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L. S., Krausslich H. G., Wimmer E., Carter C. A. Expression in Escherichia coli and purification of human immunodeficiency virus type 1 capsid protein (p24). AIDS Res Hum Retroviruses. 1990 Oct;6(10):1169–1175. doi: 10.1089/aid.1990.6.1169. [DOI] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res Hum Retroviruses. 1989 Dec;5(6):577–591. doi: 10.1089/aid.1989.5.577. [DOI] [PubMed] [Google Scholar]
- Fontenot G., Johnston K., Cohen J. C., Gallaher W. R., Robinson J., Luftig R. B. PCR amplification of HIV-1 proteinase sequences directly from lab isolates allows determination of five conserved domains. Virology. 1992 Sep;190(1):1–10. doi: 10.1016/0042-6822(92)91186-x. [DOI] [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Engleman E. G. Identification of protein intermediates in the processing of the p55 HIV-1 gag precursor in cells infected with recombinant vaccinia virus. J Biol Chem. 1989 May 25;264(15):8459–8462. [PubMed] [Google Scholar]
- Graves M. C., Lim J. J., Heimer E. P., Kramer R. A. An 11-kDa form of human immunodeficiency virus protease expressed in Escherichia coli is sufficient for enzymatic activity. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2449–2453. doi: 10.1073/pnas.85.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Katoh I., Ikawa Y., Yoshinaka Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J Virol. 1989 May;63(5):2226–2232. doi: 10.1128/jvi.63.5.2226-2232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M., Arad G., Hughes S. H. Human immunodeficiency virus type 1 gag-protease fusion proteins are enzymatically active. J Virol. 1992 Nov;66(11):6781–6783. doi: 10.1128/jvi.66.11.6781-6783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M., Katz R. A., Skalka A. M. Activity of avian retroviral protease expressed in Escherichia coli. J Virol. 1988 Aug;62(8):2696–2700. doi: 10.1128/jvi.62.8.2696-2700.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Ingraham R. H., Skoog M. T., Wimmer E., Pallai P. V., Carter C. A. Activity of purified biosynthetic proteinase of human immunodeficiency virus on natural substrates and synthetic peptides. Proc Natl Acad Sci U S A. 1989 Feb;86(3):807–811. doi: 10.1073/pnas.86.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Mills J., Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988 Aug;7(8):2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. D., Hutchison C. A., 3rd, Edgell M. H., Farmerie W. G., Swanstrom R. Mutational analysis of human immunodeficiency virus type 1 protease suggests functional homology with aspartic proteinases. J Virol. 1989 Jan;63(1):111–121. doi: 10.1128/jvi.63.1.111-121.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Zalutsky D. L., Kaback E., Miranda A. F., Silverstein S. C. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J. M., Wondrak E. M., Copeland T. D., Smith C. A., Mora P. T., Oroszlan S. Chemical synthesis and expression of the HIV-1 protease gene in E. coli. Biochem Biophys Res Commun. 1989 Feb 28;159(1):87–94. doi: 10.1016/0006-291x(89)92408-x. [DOI] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991 Jun;65(6):3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Pettit S. C., Simsic J., Loeb D. D., Everitt L., Hutchison C. A., 3rd, Swanstrom R. Analysis of retroviral protease cleavage sites reveals two types of cleavage sites and the structural requirements of the P1 amino acid. J Biol Chem. 1991 Aug 5;266(22):14539–14547. [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Miller M., Jaskólski M., Leis J., Skalka A. M., Wlodawer A. Molecular modeling of the HIV-1 protease and its substrate binding site. Science. 1989 Feb 17;243(4893):928–931. doi: 10.1126/science.2537531. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]