Abstract
A total of 55 patients with histologically proven glioblastoma multiforme (total gross resection: n=24, subtotal resection: n=20, stereotactic biopsy: n=11) were treated with the combination of dacarbazine (D) (200 mg m−2) and fotemustine (F) (100 mg m−2) and concomitant radiotherapy (2 Gy day−1, 5 days per week using limited fields up to 60 Gy) to assess efficacy and toxicity of this regimen. Survival (median survival, 12-, 18- and 24-month survival rates) and time to progression (median time to progression (TTP), 6-month progression-free survival) were analysed by Kaplan–Meier's method. A total of 268 (range 1–8, median: 5) cycles were administered. Median survival is 14.5+ (range: 0.5–40+) months, and the 12-, 18- and 24-month survival rates are 58, 29 and 23%, respectively. Median TTP from the start of D/F therapy is 9.5+ (range: 0.5–33+) months. The 6-month progression-free survival is 54%. Partial remissions were observed in 3.6%. Main toxicity was thrombocytopenia. Five patients were excluded from further D/F application, four patients because of prolonged thrombocytopenia NCI-CTC grades 3 and 4 and one patient because of whole body erythrodermia. One patient died because of septic fever during thrombocytopenia and leukopenia NCI-CTC grade 4 after the first cycle. No other toxicities of NCI-CTC grade 3 or 4 occurred. The treatment is feasible in a complete outpatient setting and the results of the D/F regimen justify further investigations with these compounds.
Keywords: glioma, chemotherapy, dacarbazine, fotemustine, glioblastoma multiforme, long-term survival
Generally, the clinical course of patients with newly diagnosed glioblastoma multiforme (GBM) is rapid and fatal with a median survival of less than 1 year.
Standard therapy consists of surgical tumour reduction when feasible, followed by radiotherapy up to a total dose of 60 Gy. Both strategies are associated with a significant survival benefit (Walker et al, 1980; Simpson et al, 1993; Hess, 1999). Although a significant increase in survival has been noted in two meta-analysis (Fine et al, 1993; Glioma Meta-analysis Trialists Group, 2002), the effect of chemotherapy onto survival and progression has been discussed controversially until the recent presentation of the meta-analysis of the Glioma Meta-analysis Trialists Group (Glioma Meta-analysis Trialists (GMT) Group, 2002). Therein, a significant increase in 1-year survival from 40 to 46% is demonstrated. In addition, chemotherapy consistently increases the proportion of long-term survivors from less than 5% approximately 15–20% (Walker et al, 1980; Chang et al, 1983; Green et al, 1983; Fine et al, 1993; Salcman et al, 1994; De Angelis et al, 1998).
The chemotherapeutic agent best studied in GBM has been carmustine (Levin et al, 1990; Prados, 2000). To date, no other drug has been proved to be superior. In comparison with carmustine, the combination of procarbazine/lomustine/vincristine failed to increase survival but resulted in more acute toxicity (Prados et al, 1999).
However, improved treatment paradigms for GBM are continually being sought. Currently, the substance investigated most eagerly is temozolomide. An ongoing international, randomised trial will reveal whether temozolomide produces a significant survival advantage in newly diagnosed GBM (Stupp and Newlands, 2001), after it has shown promise in several adjuvant trials (Newlands et al, 1992,1996; Friedman et al, 1998; Stupp et al, 2000).
In our present study, we concentrated on a more traditional imidazotetrazine derivate, dacarbazine (D), in combination with fotemustine (F), based on the following rationale.
D is a well-tolerated imidazotetrazine derivate (a synthetic analogue of the naturally occurring purine precursor E-amino-1H-imidazole-4-carboxamide) with proven efficacy in recurrent gliomas (Mahaley, 1991). Furthermore, a synergistic effect among the combination of D and F was observed in melanoma cell lines, in patients with disseminated malignant melanoma and recurrent GBM (Fischel et al, 1990; Aamdal et al, 1992; Avril et al, 1990). F, (diethyl 1-(3-(2 chloroethyl) 3 nitrosoureido) ethyl phosphonate) is an alkylating agent characterised by the grafting of a phosphonoalanine group onto the nitrosourea radical with consequent high lipophilia and a high brain permeability coefficient. The improved diffusion through the cell membrane and the blood–brain barrier implies favourable tissue distribution on cerebral tumour lesions (Kayat et al, 1987). F yielded a 26% response rate in recurrent malignant gliomas (Frenay et al, 1991) and response rates between 25 and 28% in patients with cerebral metastases of malignant melanoma (Jacquillat et al, 1990a,1990b). In patients with unresectable GBM, a response rate of 27% could be achieved by combining F with cisplatin and etoposid (Frenay et al, 2000). An own second-line study provides data of the D/F combination in recurrent, nitrosourea-pretreated and previously irradiated GBM, yielding a median survival of 45+ (range: 11–142+) weeks (Fazeny-Dörner et al, 2000).
These encouraging results prompted us to investigate the combination of dacarbazine and fotemustine (D/F) with concomitant radiotherapy into first-line treatment with the aim to assess efficacy and toxicity in newly diagnosed GBM.
PATIENTS AND METHODS
Eligibility criteria
Patients with newly diagnosed, histologically proven GBM, based on the WHO classification (Kleihues and Sobin, 2000) after total gross resection, subtotal resection or after stereotactic biopsy, were eligible for inclusion into this study. For the evaluation of any residual disease a cranial computed tomographic (CT) scan or magnetic resonance imaging (MRI) had to be performed within 72 h after neurosurgical procedure. Patients had to be aged between 18 and 70 years, had to have a Karnofsky performance score (KPS) ⩾60% and a life expectancy of >8 weeks; patients were not allowed to be under cytotoxic chemotherapy because of concurrent malignancy. Other contraindications included any known psychiatric disorder and pregnant or nursing women. Adequate contraception was mandatory.
Radio- and chemotherapy had to be started within 10–14 days after neurosurgical intervention in case of controlled wound healing and missing signs of recent infection (white blood cell counts, C-reactive protein and fibrinogen had to be within normal range according to institutional standard).
Patients had to be on a stable dose of glucocorticoids (or no glucocorticoids) for at least 1 week prior to study entry. Furthermore, patients were required to have adequate liver function (SGOT, SGPT and alkaline phosphatase levels less than two times of institutional normal and bilirubin levels <1.5 mg dl−1), renal (blood urea nitrogen or creatinine levels <1.5 times of institutional normal) and bone marrow function (leukocyte count >3000 μl–1 and a platelet count >100 000 μl−1) before start of D/F therapy. All patients provided written informed consent before study entry.
Study endpoints
The study end points were efficacy of the D/F regimen concomitant to radiotherapy defined as response to chemotherapy, time to progression (TTP), 6-month progression-free survival, median survival, and 12-, 18- and 24-month survival rates.
Therapeutic protocol
Chemotherapy consisted of D in a dosage of 200 mg m−2 (diluted in 250 ml normal saline) and F in a dosage of 100 mg m−2 (diluted in 250 ml glucose 5%). Both solutions were protected from light and were given intravenously in an outpatient setting. D was administered over 30 min, to avoid burning sensations during D infusion, and 500 ml normal saline was concomitantly administered. At 30 min after termination of D infusion, F was given for over 60 min. Cycles were repeated every 3 weeks. Treatment was continued for a maximum of eight cycles, unless there was progression of disease, unmanageable toxicity, fulfilled off-study criteria or withdrawal of consent.
Modification of the doses or the dose interval of D/F was made for haematologic toxicity based on the platelet and leukocyte count on the day of the planned treatment. D/F treatment was postponed up to a maximum of three consecutive weeks in case of thrombocytopenia from the National Cancer Institue (NCI) common toxicity criteria (CTC) (NCI, 1988) grade 1 and/or leukopenia NCI-CTC grade 2 with weekly monitoring of blood cell counts; otherwise the doses of D/F were decreased by 25% each. Patients were excluded from further D/F treatment in the case of thrombocytopenia NCI-CTC grade 2 lasting longer than 3 weeks or immediately after thrombocytopenia or leukopenia NCI-CTC grade ⩾3. After exclusion from study patients were allowed to be treated individually with previously unemployed drugs. Repeat surgery was not considered routinely.
Antiemetics were administered to all patients before and after chemotherapy application according to the institutional standard (granisetron 5 mg orally once a day from days 1–3 after D/F therapy).
Doses of glucocorticoids (dexamethasone) were adjusted according to the patients' clinical status and were given in the lowest dose necessary for neurologic stability. If the dosage was increased to offset marked clinical deterioration, this was considered when evaluating response, using the criteria of MacDonald et al. (1990). Concomitantly ranititidin 300 mg orally was given once daily. Anticonvulsants were used as medically indicated.
Toxicity evaluation
Toxicity was evaluated according to the NCI's common CTC (NCI, 1988) during routine controls in three-weekly intervals or, if clinically indicated, in weekly intervals.
Monitoring of serum chemistry and blood cell counts was performed prior to each cycle of therapy in three-weekly intervals. In case of haematotoxicity necessitating a delay of chemotherapy application, blood counts were performed in weekly intervals.
Patients were monitored with either cranial CT or MRI scan after two, four, six and eight cycles of therapy in case of clinical and neurological stability, and immediately when disease progression was suspected clinically.
Response evaluation
Response evaluation was based on MacDonald's criteria (MacDonald et al, 1990): complete response (CR) was defined as the disappearance of all measurable disease with improved neurology in the absence of corticoid therapy. Partial response (PR) was a ⩾50% decrease in tumour size with an improved or stable neurology on stable or decreased dexamethasone dose. Stable disease (SD) was a less than 50% decrease, or less than 25% increase of the tumour size with an improved or stable neurology on stable or decreased dexamethasone dose. Progressive disease (PD) was a greater than 25% increase in tumour size or the appearance of new lesions. Tumour evaluation was based on the product of the two largest perpendicular diameters of the contrasting lesion. If the tumour did not enhance, the diameters of the hyperintense signal on T-2-weighted MRI images or of the hypodense region on CT scans were used.
Off-study criteria
Patients were excluded from further D/F treatment if one of the following was noted: (1) disease progression or recurrence as documented by CT or MRI anytime after the completion of at least one cycle of therapy as defined above; (2) severe and/or prolonged haematotoxicity as defined above; (3) in case of a deteriorated and unacceptable neurologic status; (4) in case of withdrawal of consent.
Statistical analysis
All analyses were done by intention to treat. The reference point for median survival, 12-, 18- and 24-month survival, was the date of neuropathologic diagnosis, and the end point was survival until death, including deaths from causes not related to the disease.
Time to progression (TTP) was estimated from the first day of D/F application to the first unfavourable event (e.g. radiographically documented tumour recurrence or progression or death). If a patient died without a scan to document disease status, the TTP was measured until documented clinical worsening or until the date of death.
Survival curves and TTP curves were constructed using the Kaplan–Meier's nonparametric method, medians (and their respective 95% confidence intervals) were calculated from the Kaplan–Meier estimates (Kaplan and Meier, 1958; Young et al, 1999). Statistical evaluations were performed with SPSS version 10.0.7 program package. Data were analysed as of 31 January 2002.
RESULTS
A total of 55 patients (female/male: 16/39) with newly diagnosed, histologically proven GBM were treated with D/F chemotherapy between October 1998 and December 2001. In total, 24 patients had gross total resection, 20 patients had a subtotal tumour resection and in 11 patients stereotactic biopsy was performed. Concomitant to chemotherapy all patients received radiotherapy up to a total dose of 60 Gy (30×2 Gy single dose).
Median age was 44 (range: 18–68) years, the median KPS 1 week after neurosurgical intervention was 90% (range: 60–100%).
Survival
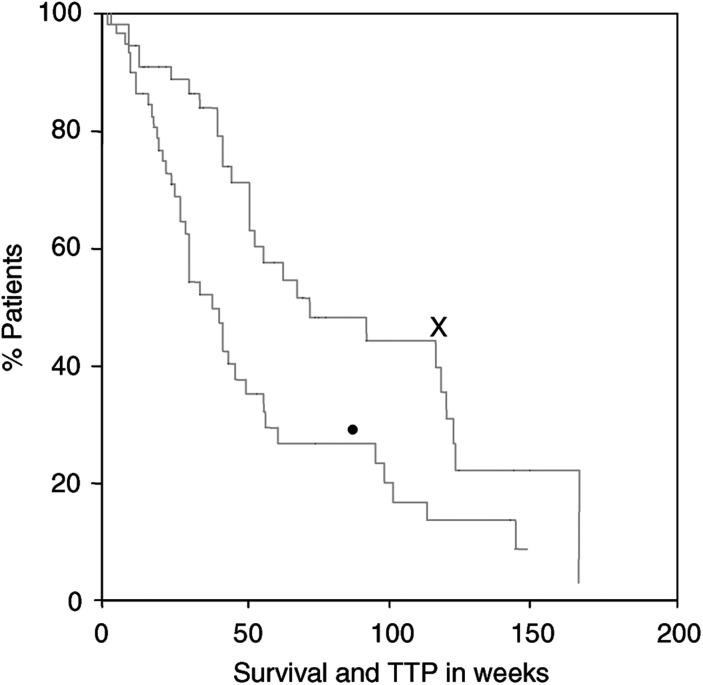
At study evaluation 23 out of 55 (42%) patients are alive. Median survival of all patients (Figure 1) is 72+ (range: 3–156) weeks (=14.5 months in median) with a 95% confidence interval of 28–116 weeks. The 12-, 18- and 24-month survival rates after diagnosis are 58, 29 and 23%, respectively.
Figure 1.
Survival and TTP of patients with GBM after first-line therapy with D and F: ×, survival; K, •, time to progression (TTP).
Time to progression
The median TTP of all patients was 41+ (range: 3–144+) weeks (=9.5 months) (Figure 1).
Response to chemotherapy
A partial remission could be achieved in two patients (3.6%) with subtotal resection, lasting for 12 weeks each. SD or no evidence of disease lasting from 10 to 144+ weeks (median: 29 weeks) could be achieved in 45 (81%) of the patients. Thereof, 24 patients had gross total resection, 14 patients had subtotal resection and seven patients had stereotactic biopsy.
SD or no evidence of disease lasting longer than 12 months was observed in 12 (22%) patients. In these patients, glucocorticoids could be tapered towards the end of radiotherapy and usually stopped within 3 weeks after radiotherapy. Additionally, in these patients the KPS improved at least by 10%.
Toxicity
In all, 55 patients received a total of 268 (range 1–8; median: 5) cycles of D/F.
Major toxicity was thrombocytopenia. Four patients were excluded from further D/F treatment, three because of thrombocytopenia NCI-CTC grade 3 (after the second, fourth and sixth cycle, respectively) and the other patient because of thrombo-cytopenia of NCI-CTC grade 4 (after the eigth cycle of D/F), which was accompanied by anaemia NCI-CTC grade 3, necessitating substitution of platelets and two units of packed red cells.
One patient died owing to septic fever during combined thrombocytopenia and leukopenia NCI-CTC grade 4 after the first D/F application despite inward care, intravenous administration of antibiotics, granulocyte colony-stimulating factors and substitution of platelets. Cranial CT before death showed stable disease of the unresectable tumour.
Thrombocytopenia NCI-CTC grade 1 was observed in 11 cycles (therapy was postponed for 1 week in seven cycles and for 2 weeks in four cycles) and thrombocytopenia NCI-CTC grade 2 occurred in 13 cycles (therapy was postponed for 1 week in five cycles, for 2 weeks in five cycles, and for 3 weeks in three cycles resulting in a 25% dose reduction of D/F). Leukopenia from NCI-CTC grade 1 occurred in six cycles (three cycles had to be postponed for 1 week) and from CTC grade 2 in six cycles, resulting in treatment delay for 1 week in three cycles, for 2 weeks in one cycle and in a 3 weeks delay in two cycles, thus leading to a 25% dose reduction of D/F.
A fifth patient was excluded from further therapy because of a whole body erythordermia within 24 h after administration of the second cycle of D/F.
Alopecia was evaluable in 55 patients. The most severe degree was NCI-CTC grade 2 (n=18).
Neither pulmonary toxicity nor any radiation necrosis was observed during a follow-up period of 150 weeks. Under standardised prophylactic antiemetics, patients did not suffer from gastrointestinal toxicity. The main complaints of patients concerned side effects from chronic glucocorticoid intake, primarily the cushingoid appearance, myopathy and the vulnerability of the skin.
DISCUSSION
The adjuvant administration of D/F concomitant to radiotherapy revealed to be feasible in a complete outpatient setting and yielded a median survival of 14.5 months in patients with newly diagnosed GBM. Our survival and TTP results range in the upper level of those achieved in the limited number of available first-line treatment strategies, which are summarized in Table 1 . Therein, the median TTP is 7 (range: 4.2–9.5) months and the median survival is 12 (range: 9–19.2) months (Table 1; Fetell et al, 1997; Brandes et al, 1998; Friedman et al, 1998; Gruber et al, 1998; Beauchesne et al, 1999; Frenay et al, 2000; Grossman et al, 2000; Stupp et al, 2000).
Table 1. Synopsis of first-line chemotherapy trials in patients with newly diagnosed GBM.
| Author | No. of patients with GBM out of all patients | Agent and treatment design | Median TTP (months) | Median survival (months) | Remarks |
|---|---|---|---|---|---|
| Beauchesne et al (1999) | 23/30 | (Etoposide 100 mg m−2 d1–3) q 4 weeks; ×6 | 7 | 12 | TTP + survival analysis for all 30 patients ‘near-concurrent’ radiotherapy |
| 18-month survival: 15.4% | |||||
| 24-month survival: 4% | |||||
| Brandes et al (1998) | 56/56 | (Carboplatin 350 mg m−2 d1,22,43+ | 7.5 | 12.5 | Concurrent radiotherapy (60 Gy) |
| Teniposide 50 mg m−2 d1–3,22–24,43–45 + | 18-month survival: 28% | ||||
| Carmustine 200 mg m−2) q 8 weeks; ×3 | 24-month survival: 20% | ||||
| Fetell et al (1997) | 33/33 | (Paclitaxel 140–230 mg m−2 d1) q 21 days; ×3 | n.e. | 12.5 | Radiotherapy at tumour progression |
| Frenay et al (2000) | 33/33 | (Fotemustine 100 mg m−2 d1+ | n.e. | 10 | Symptomatic unresectable GBM |
| Cisplatin 33 mg m−2 d1–3 + | |||||
| Vepesid 75 mg m−2 d1–3) q 28 days | |||||
| q 28 days | |||||
| Friedmann et al (1998) | 33/33 | (Temozolomide 200 mg m−2 d1–5, p.o.); q 28 days; ×4 | 7 months in responding patients (n=17) | 12 months in responding patients | Residual tumour mass after surgery; |
| 2 months in non-responding patients (n=16) | 6 months in non-responding patients | Chemotherapy before radiotherapy | |||
| Friedman et al (1999) | 14/63 | (Topotecan 2.6 mg m−2 over 72 h) q weekly; ×16 | n.e. | n.e. | Chemotherapy before radiotherapy |
| Partial response in 2/14 patients | |||||
| Grossman et al (2000) | 219/219 | (1) BCNU+cisplatin vs | (1): 5.4 | (1): 10.7 | No advantage from (1) over (2) |
| (2) BCNU+radiotherapy | (2): 4.2 | (2): 11.2 | |||
| Gruber et al (1998) | 25/25 | (carboplatin 600 mg m−2) q 4 weeks; ×4 | 8.4 | 19.2 | Radiotherapy (60 Gy) started 3–4 weeks after chemotherapy; 23/25 patients: KPS:100% |
| Stupp et al (2000) | 45/45 | (Temozolomide 75–200 mg m−2d×5d) q 28 days | n.e | Estimated 1-year survival: 67% | Concomitant radiotherapy |
| This paper | 55/55 | (Dacarbazine 200 mg m−2 d1+ | 9.5+ | 14.5+ | Concomitant radiotherapy (60Gy) |
| Fotemustine 100 mg m−2 d1) | 18-month survival: 29% | ||||
| q 21days; | 24-month survival: 23% | 61% of patients alive at study evaluation |
In particular, our study, administering the traditional imidazotetrazinederivate, dacarbazine, is comparable with Stupp's preliminary study, administering the imidazotetrazinederivate temozolomide (Stupp et al, 2000). Both imidazotertazinederivate studies are similar concerning study design, extent of surgery and the median Karnofsky performance score. Although the measurable partial response of 3.6% is discouraging, the definitive 12-month survival achieved in our study is 58%, whereas the estimated 12-month survival for the preliminary temozolomide study has been reported to be 67%. It might be speculated whether the younger median age of our patients might have contributed to the favourable outcome. However, the recent meta-analysis of the Glioma Meta-analysis Trialists Group (Glioma Meta-analysis Trialists (GMT) Group, 2002) demonstrated that the relative effect of chemotherapy on 1-year survival does not differ between patients ⩾40 years and those ⩾60 years.
The 18-month survival is the usual marker for beneficial influence of adjuvant chemotherapy (De Angelis et al, 1998). It is documented in two studies of the synopsis summarised in Table 1 and ranges between 15.4 and 28% and is 29% in our study (Brandes et al, 1998; Beauchesne et al, 1999). Concerning median survival, Gruber et al (1998) achieved 19.2 months with the single agent carboplatinum. However, 18- and 24-month survival rates are not reported in their study and a study populat-ion smaller than 30 patients is known to preclude strong statistical power.
An explanation for the efficacy of the D/F combination might be the O6-alkyl synergy of D/F, and the augmentation thereof by the excellent tissue distribution of F in cerebral tumour lesions on the other hand (Jacquillat et al, 1990a,1990b; Lee et al, 1991; Aamdal et al, 1992; Frenay et al, 2000).
One case of fatal neutropenic fever after the first cycle of therapy was unexpected. Neither advanced age nor a dismal pretherapeutic KPS could explain this adverse event (Grant et al, 1995; Raymond et al, 1996). No other case of serious toxicity than uncomplicated leuco- and/or thrombocytopenia from NCI-CTC grades 3 and 4 (9%) occurred during the application of these 268 D/F cycles as well as in further 100 D/F applications in patients with recurrent and lomustine pretreated GBM (Fazeny-Dörner et al, 2000). Otherwise, the toxicity of D/F was similar to the previously reported experience with these compounds, thus making the regimen suitable for completely outward administration (Jacquillat et al, 1990a,1990b; Aamdal et al, 1992; Fazeny-Dörner et al, 2000; Raymond et al, 1996).
The results of the D/F regimen justify further investigations with these compounds.
References
- Aamdal St, Gerard B, Bohman T, D'Incalci M (1992) Sequential administration of dacarbazine and fotemustine in pts with disseminated malignant glioma – an effective combination with unexpected toxicity. Eur J Cancer 28: 447–450 [DOI] [PubMed] [Google Scholar]
- Avril MF, Bonneterre J, Dalaunay M (1990) Combination of chemotherapy of dacarbazine and fotemustine in disseminated malignant melanoma. Cancer Chemother Pharmacol 27: 81–84 [DOI] [PubMed] [Google Scholar]
- Beauchesne P, Soler C, Rusch P, Fosto MJ, Duthel R, Schmitt T, Brunon J (1999) Phase II study of a radiotherapy/etoposide combination for patients with newly diagnosed malignant gilomas. Cancer Chemother Pharmacol 44: 210–216 [DOI] [PubMed] [Google Scholar]
- Brandes AA, Rigon A, Zampieri P, Ermani M, Carollo C, Altavilla G, Turazzi S, Chierichetti F, Fiorentino MV (1998) Carboplatin and teniposide concurrent with radiotherapy in patients with glioblastoma multiforme. A phase II study. Cancer 82: 355–361 [DOI] [PubMed] [Google Scholar]
- Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson JS, Tsukada Y (1983) Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. Cancer 52: 997–1007 [DOI] [PubMed] [Google Scholar]
- De Angelis LM, Burger PC, Green SB, Cairncross JG (1998) Malignant glioma: who benefits from adjuvant chemotherapy? Ann Neurol 44: 691–659 [DOI] [PubMed] [Google Scholar]
- Fazeny-Dörner B, Wenzel C, Killer M, Rössler K, Ungersböck K, Dieckmann K, Piribauer M, Brodowicz T, Marosi C (2000) Second-line chemotherapy with dacarbazine and fotemustine in nitrosourea-pretreated patients with recurrent glioblastoma multiforme – results of a pilot study and review of the literature. Neuro-Oncology 2(Suppl 1): 516 (Abstr. 84) [DOI] [PubMed] [Google Scholar]
- Fetell MR, Grossman SA, Fisher JD, Erlanger B, Rowinsky E, Stockel J, Piantadosi S for the New Approaches to Brain Tumour Therapy Central Nervous System Consortium (1997). Preirradiation paclitaxel in glioblastoma multiforme: efficacy, pharmacology, and drug interactions. J Clin Oncol 15: 3121–3128 [DOI] [PubMed] [Google Scholar]
- Fine H, Dear KBG, Loeffler JS, Black PM, Canellos GP (1993) Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adult. Cancer 71: 2585–2597 [DOI] [PubMed] [Google Scholar]
- Fischel JL, Formento P, Etienne MC, Gioanni J, Deloffre P, Bizzari JP, Milano G (1990) In vitro chemosensitivity testing of fotemustine (S 10036), a new antitumour nitrosourea. Cancer Chemother Pharmacol 25: 337–341 [DOI] [PubMed] [Google Scholar]
- Frenay M, Giroux B, Khoury S (1991) Phase II study of fotemustine in recurrent supratentorial malignant gliomas. Eur J Cancer 27: 852–856 [DOI] [PubMed] [Google Scholar]
- Frenay M, Lebrun C, Lonjon M, Bondiau PY, Chatel M (2000) Up-front chemotherapy with fotemustine (F)/cisplatin (CDDP)/etoposide (VP16) regimen in the treatment of 33 non-removeable glioblastomas. Eur J Cancer 36: 1026–1031 [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kerby T, Fields S, Zilisch JE, Graden D, McLendon RE, Houghton PJ, Arbuck S, Cokgor I, Friedman AH (1999) Topotecan treatment of adults with primary malignant glioma. Cancer 85: 1160–1165 [PubMed] [Google Scholar]
- Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, Henry AJ, Ashley DM, Krischer J, Lovell S, Rasheed K, Marchev F, Seman AJ, Cokgor I, Rich J, Stewart E, Colvin OM, Provenzale JM, Bigner DD, Haglund MM, Friedman AH, Modrich PL (1998) DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to temodal in newly diagnosed malignant glioma. J Clin Oncol 16: 3851–3857 [DOI] [PubMed] [Google Scholar]
- Glioma Meta-analysis Trialists (GMT) Group (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359: 1011–1018 [DOI] [PubMed] [Google Scholar]
- Grant R, Liang BC, Page MA, Crane DL, Greenberg HS, Junck L (1995) Age influences chemotherapy response in astrocytomas. Neurology 45: 929–933 [DOI] [PubMed] [Google Scholar]
- Green SB, Byar DP, Walker MD, Pistenmaa DA, Alexander Jr E, Batzdorf U, Brooks WH, Hunt WE, Mealey Jr J, Odom GL, Paletti P, Ransohoff II J, Robertson JT, Selker RG, Shapiro WR, Smith Jr KR, Wilson CB, Strike TA (1983) Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 67: 121–132 [PubMed] [Google Scholar]
- Grossman SA, O'Neill A, Grunnet M, Mehta M, Pearlman J, Wagner H, Gilbert M, Newton H, Hellman R (2000) Phase III study comparing three cycles of infusional BCNU/cisplatin followed by radiation with radiation and concurrent BCNU for patients with newly diagnosed supratentorial glioblastoma multiforme (ECOG 2394–SWOG 9508). Proc Am Soc Clin Oncol 19: 158a (Abstr. 612) [DOI] [PubMed] [Google Scholar]
- Gruber ML, Glass J, Choudhri H, Nirenberg A (1998) Carboplatin chemotherapy before irradiation in newly diagnosed glioblastoma multiforme. Am J Clin Oncol 21 (4): 338–340 [DOI] [PubMed] [Google Scholar]
- Hess KR (1999) Extent of resection as a prognostic variable in the treatment of gliomas. J Neurooncol 42: 227–231 [DOI] [PubMed] [Google Scholar]
- Jacquillat C, Khayat D, Banzet P (1990a) Chemotherapy by fotemustine in cerebral metastases of disseminated malignant melanoma. Cancer Chemother Pharmacol 25: 263–266 [DOI] [PubMed] [Google Scholar]
- Jacquillat C, Khayat D, Banzet P, Weil M, Fumoleau P, Avril MF, Namer M, Bonneterre J, Kerbrat P, Bonerandi JJ, Bugat R, Montcuquet P, Cupissol D, Lauvin R, Vilmer C, Prache C, Bizarri JP (1990b) Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer 66: 1873–1878 [DOI] [PubMed] [Google Scholar]
- Kayat D, Lokiec F, Bizzari JP, Weil M, Meeus L, Jacquillat C (1987) Phase I clinical study of the new amino acid-linked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res 7: 6782–6785 [PubMed] [Google Scholar]
- Kaplan EL, Meier P (1958) Nonparametric estimation for incomplete observation. J Am Stat Assoc 53: 457–481 [Google Scholar]
- Kleihues P, Sobin LH (2000) World Health Organization classification of tumours. Cancer 88: 2887–2993 [DOI] [PubMed] [Google Scholar]
- Lee SM, Thatcher N, Margison GP (1991) O6-alkylguanine-DNA alkytransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res 51: 614–623 [PubMed] [Google Scholar]
- Levin VA, Silver P, Hannigan J (1990) Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PVC) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys 18: 321–324 [DOI] [PubMed] [Google Scholar]
- MacDonald DR, Cascino TL, Schold SC, Cairncross G (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 7: 1277–1280 [DOI] [PubMed] [Google Scholar]
- Mahaley MS (1991) Neuro-oncology index and review. J Neuro-Oncol 11: 85–148 [DOI] [PubMed] [Google Scholar]
- NCI (1988) Common Toxicity Criteria. Bethesda, MD: National Cancer Institute, Division of Cancer Treatment [Google Scholar]
- Newlands ES, Blackledge GR, Slack JA (1992) Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer 65: 287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands ES, O'Reilly SM, Glaser MG (1996) The Charing Cross Hospital experience with temozolomide in patients with gliomas. Eur J Cancer 32A: 2236–2241 [DOI] [PubMed] [Google Scholar]
- Prados MD (2000) Future directions in the treatment of malignant gliomas with temozolomide. Semin Oncol 3(Suppl. 6): 41–46 [PubMed] [Google Scholar]
- Prados MD, Scott C, Curran Jr WJ, Nelson DF, Leibel S, Kramer S (1999) Procarbazine, lomustine, and vincristine (PVC) chemotherapy for anaplastic astrozytoma: a retrospective review of Radiation Therapy Oncology Group protocols comparing survival with carmustine or PVC adjuvant chemotherapy. J Clin Oncol 17: 3389–3395 [DOI] [PubMed] [Google Scholar]
- Raymond E, Haon C, Boaziz C, Coste M (1996) Logistic regression model of fotemustine toxicity combining independent phase II studies. Cancer 78: 1980–1987 [PubMed] [Google Scholar]
- Salcman M, Scholtz H, Kaplan RS (1994) Long-term survival in patients with malignant astrocytoma. Neurosurgery 34: 213–220 [DOI] [PubMed] [Google Scholar]
- Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS, Weinstein AS, Nelson DF (1993) Influence of location and extent of surgical resection on survival of patients with gioblastoma multiforme: results of three consecutive radiation therapy oncology group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26: 230–244 [DOI] [PubMed] [Google Scholar]
- Stupp R, Newlands E (2001) New approaches for temozolomide therapy: use in newly diagnosed glioma. Semin Oncol 4:19–23 [DOI] [PubMed] [Google Scholar]
- Stupp R, Ostermann S, Kraljevic, Dietrich P, Pica A, Maillard I, Maeder P, Mirabell R, Collao C, Villemure J, De Tribolet N, Mirimanoff RO, Leyvarz S (2000) Promising survival with concomitant and adjuvant temozolamide for newly diagnosed glioblastoma multiforme. Proc Am Soc Clin Oncol 19: 163a (Abstr. 632) [Google Scholar]
- Walker MD, Green SB, Byar DP, Alexander Jr E, Batzdorf U, Brooks WH, Hunt WE, McCarty CS, Mahaley Jr MS, Mealey Jr J, Owens G, Ranshoff II J, Robertson JT, Shapiro WR, Smith Jr KR, Wilson CB, Strike TA (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303: 1323–1329 [DOI] [PubMed] [Google Scholar]
- Young KD, Menegazzi JJ, Lewis RJ (1999) Statistical methodology: IX. Survival analysis. Acad Emergency Med 6: 244–249 [DOI] [PubMed] [Google Scholar]