Abstract
Gelsolin expression is frequently downregulated in lung cancer and several types of different human cancers. To examine the effects of gelsolin restoration on tumorigenicity, we here stably expressed various levels of gelsolin via gene transfer in lung cancer cells (squamous cell carcinoma line, PC10). We observed the alterations in tumorigenicity in vivo when implanted in nude mice, and the changes in growth properties in vitro. As compared to parental cells and control clones, gelsolin transfectants highly reduced tumorigenicity and repressed cell proliferation. Moreover, we investigated bradykinin-induced responses in gelsolin-overexpressing clones, because agonist-stimulated activation of the phospholipases C (PLC)/protein kinase C (PKC) signal transduction pathway is critical for cell growth and tumorigenicity. Bradykinin promotes phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis by PLC and translocation of various PKC isoforms from the cytosolic fraction to the particulate fraction. Bradykinin treatment did not increase inositoltriphosphate (IP3) production and induce the membrane fractions of PKCα and PKCγ in gelsolin tranfectants, while it induced PIP2 hydrolysis and increased the fractions in parental and control clones. These results suggest that gelsolin suppressed the activation of PKCs involved in phospholipid signalling pathways, inhibiting cell proliferation and tumorigenicity.
Keywords: gelsolin, phosphoinositides, PKC, lung cancer, tumour suppression
The long-term accumulation of genetic aberrations plays a major role in the development of various cancers. Molecular abnormalities include expression of oncogenes, such as Ki-ras, c-myc, c-erb-B-2 and bcl-2, and tumour suppressor genes, such as p53, Rb and FHIT (fragile histidine triad) in lung cancer (Otterson et al, 1998; Salgia and Skarin, 1998).
We previously demonstrated that expression of an actin-regulatory protein (Yin and Stossel, 1979), gelsolin, is frequently downregulated in lung cancer (Dosaka-Akita et al, 1998) and several types of different human cancers, such as stomach, bladder and colon (Moriya et al, 1994; Tanaka et al, 1995; Furuuchi et al, 1996). Moreover, we indicated that introducing human cytoplasmic gelsolin cDNA suppresses tumorigenicity in human bladder and colon cancer cell lines (Tanaka et al, 1995; Furuuchi et al, 1996). Gelsolin controls the length of actin polymers in vitro by a variety of mechanisms. At least three different activities are known: severing, capping and nucleating through interaction with both filamentous (F-) and monomeric (G-) actins are responsible for reorganisation of the actin cytoskeleton. These functions are tightly regulated by calcium ions (Ca2+), pH and polyphosphoinositides (Janmey, 1994). Although gelsolin may modulate phospholipid signaling pathways through its high affinity to polyphosphoinositides (Janmey and Stossel, 1987), the mechanism that restored gelsolin expression suppresses tumorigenicity in cancer cells is not well understood.
Protein kinase C (PKC) belongs to a ubiquitous family of serine/threonine kinases that plays a critical role in many signal transduction pathways (Nishizuka, 1984). PKCs are activated by a variety of extracellular stimuli that elicit production of a lipid second messenger diacylglycerol (DAG) and a cofactor phosphatidylserine (PS) (Kishimoto et al, 1980; Nishizuka, 1992; Toker, 1998). Up to 12 different PKC isoforms have been reported in mammalian cells (Dekker and Parker, 1994; Mellor and Parker, 1998). The isoforms have been divided into three distinct subfamilies: the conventional PKCs (cPKCα, βI, II and γ), which are activated by DAG, PS and Ca2+; novel PKCs (nPKCδ, ɛ, η, θ and μ), which are dependent on DAG and PS, but not on Ca2+; and atypical PKCs (aPKCζ, ι/λ), which are activated by PS, but not by DAG and Ca2+ (Toker et al, 1994). Several studies have indicated that the individual PKC isoforms express in their tissue specifically and vary in biochemical properties and intracellular localisation (Nishizuka, 1992).
PKCs are shown to be a target for phorbol ester, such as 12-O-tetradecanoylphorbol-13-acetete (TPA) which is known as a tumour promoter, because TPA contains a diacylglycerol-like structure (Castagna et al, 1982; Gopalakrishna and Barsky, 1988). Several studies showed that overexpression of different PKC isoforms induces high growth rates, cellular saturation densities and enhanced tumorigenicity (Housey et al, 1988; Persons et al, 1988; Cacace et al, 1993; Mischak et al, 1993; Borner et al, 1995). Furthermore, the transfection of antisense PKCα into lung cancer cells repressed cell proliferation and reduced tumorigenicity in nude mice (Wang et al, 1999). However, there are a number of conflicting reports that overexpression of PKC increases or suppresses tumorigenicity in various cells (Janik et al, 1994; Johnson et al, 1999). These observations suggest that various PKCs play various roles in carcinomas and distinctive roles in cell regulation.
In this study, we established lung cancer cells (squamous cell carcinoma, PC10) overexpressing human cytoplasmic gelsolin in various degrees by gene transfer, using retrovirus carrying human cytoplasmic gelsolin cDNA, and examined the effects of restoration of gelsolin expression on tumorigenicity in vivo with nude mice and growth properties in culture in vitro. Moreover, we investigated bradykinin-induced responses, particularly inositoltriphosphate (IP3) production and translocation of various PKC isoforms from the cytosolic fraction to the particulate fraction in gelsolin-overexpressing clones, because agonist-stimulated activation of the phospholipases C (PLC)/PKC signal transduction pathway is critical for cell proliferation and tumorigenicity.
MATERIALS AND METHODS
Materials
Geneticin (G418) and LipofectAMINE were purchased from Life Technologies. Bradykinin and anti-human gelsolin monoclonal antibody (clone: GS-2C4) were from Sigma. An anti-actin monoclonal antibody was from Boehringer Mannheim Biochemica. Monoclonal antibodies to PKC isoforms were from Transduction Laboratories. Chemiluminescence kit (ECL system) was from Amersham Pharmacia Biotech.
Cell culture
Human lung cancer cell PC10 (squamous cell carcinoma) obtained from the Health Science Research Resources Bank of Japan (Osaka, Japan) were grown in RPMI 1640, supplemented with 10% foetal calf serum (FCS; Life Technologies) and 0.03% L-glutamine. Culture was maintained at 37°C in a moist atmosphere of 95% air and 5% CO2.
Retrovirus construction for human cytoplasmic gelsolin and gene transfer
Retroviral vector for the expression of human cytoplasmic gelsolin (pLNChGsn) was constructed as described previously with pLNCX (a generous gift from Dr AD Miller, Fred Hutchinson Cancer Research Center) (Banno et al, 1999). Amphotropic retrovirus containing gelsolin cDNA and empty virus were obtained by transfection of each vector into CAK8 (kindly provided by Dr Karl Riabowol, University of Calgary), a highly efficient virus-packaging cell line, using LipofectAMINE. At 2 days after transfection, virus-containing supernatants were recovered, spun with 1200 r.p.m. and then used for infection with PC10 cells. Infected cells were cultured in a selection medium containing 400 μg ml−1 G418 for 14–20 days and individual colonies were picked randomly and then expanded.
Western blotting and densitometric quantification
Total cell lysates were prepared by extracting the cell pellets with RIPA buffer (50 mM HEPES, 50 mM NaCl, 0.1% SDS, 1% NP-40, 2 mM EDTA, 2 mM EGTA, 1 mM phenylmethylsulphonyl fluoride (PMSF), 5 μg ml−1 leupeptin and 10 μg ml−1 aprotinin). The protein concentration was determined by the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA, USA) with bovine serum albumin (BSA) as a standard. A portion equivalent to 20 μg of protein was separated by SDS–PAGE and electrotransferred to nitrocellulose membrane. After blocking with 5% nonfat dry milk in TBST (Tris-buffered saline: 10 mM Tris-HCl, pH 8.0 and 150 mM NaCl containing 0.05% Tween-20), they were probed with anti-human gelsolin antibody (GS-2C4), 2nd antibody and then visualised with the ECL system. Band images were scanned with a GT-6000 Scanner (Epson-SEIKO) and densitometric analyses were performed using NIH Image, ver. 1.56. Proteins were normalised against the levels of actin protein.
Growth assay in monolayer culture and MTT assay
Cells were seeded at a density of 5×104 cells per well in six-well plates in RPMI 1640 with either 10 or 1% FCS, respectively. This point was designated day 0. Cells number in triplicate wells was determined by counting with a haemocytometer after trypsinisation every 48 h. Flat 96-well culture plates seeded at a density of 5×103 cells per well were used to test growth with 1% FCS medium by MTT assay. MTT was dissolved at 5 mg ml−1 in phosphate-buffered saline solution and used. After 40 h, 10 μl of MTT solution was directly added to the medium and the cells were incubated for an additional 6 h. After removal of the medium, 100 μl of 0.04 N hydrochloric acid in isopropanol was added to each well and the optical density of the plates was measured on a microculture plate reader (Spectra Max 250; Molecular Devices) using a test wavelength of 570 nm and a reference wavelength of 630 nm.
Cell death assay
Cells were treated with staurosporine (STS; Sigma), a potential inducer of apoptotic cell death, at 250 ng ml−1 and incubated for various intervals. Cell death was examined with fluorescence microscopy (TE-300; Nikon) for nuclear morphological changes such as a nuclear condensation after an addition of Hoechst 33342 at a final concentration of 1 μg ml−1. Apoptotic index was determined by counting the number of apoptotic cells with a nuclear condensation divided by the total number of cells (at least 300 cells) in the fields.
Anchorage-independent growth assay
For the assay of colony-forming efficiency in soft agar, 5×103 cells in 1 ml growth medium containing 0.33% Noble agar (DIFCO Laboratories, Detroit, MI, USA) were incubated in a 60 mm dish with glids (Coster Scientific Co. Corning, NY, USA) overlaid on 4 ml of 0.5% agar medium. Cells were incubated at 37°C in a moist atmosphere of 95% air and 5% CO2 and 3 weeks later the number of colonies was counted using an inverted phase microscope. The data are expressed as colony-forming efficiency =[(number of colonies ×100)/(number of cells originally seeded)].
Tumorigenicity in nude mice
Cells were trypsinised from monolayer cultures, counted, and spun down with 1500 r.p.m. for 5 min and resuspended with RPMI 1640. Two BALB/c-nu/nu syngenic 6-week-old male mice (Japan SLC, Inc. Shiruoka, Japan) per clone were injected subcutaneously into the back with 3×106 cells of gelsolin transfectants, neo-control or parental PC10 cells in a final volume of 100 μl of PBS. Tumour size was measured using a hand calipers every 10 days. Volumes were calculated by the formula 1/2×L×W2 where L and W are length and width, respectively, of the tumour measured in two dimensions. The animal experiments were conducted under the guidelines for the use of experimental animals laid down by the Hokkaido University School of Medicine and the United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (Workman et al, 1998).
Assay for phospholipase C (PLC) activity
PLC activity was assessed by the measurement of total inositol phosphates. Cells in six-well plates were prelabelled with 1 μCi ml−1 myo-[3H] inositol for 24 h in inositol-free RPMI 1640 medium containing 0.3% BSA. Cells were then washed twice with the HEPES/Tyrode buffer (10 mM HEPES/NaOH, pH 7.4, 134 mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 0.36 mM NaH2PO4, 1.0mM MgCl2, 1.8 mM CaCl2 1 mg ml−1 BSA and 1 mg ml−1 glucose) containing 20 mM LiCl and further incubated for 15 min at 37°C before stimulation with agonists. Cells were then stimulated with bradykinin at the indicated concentration for 2 min, and the reactions were terminated by the addition of ice-cold 10% perchloric acid. Inositol phosphates were separated using AG 1×8 anion exchange resin (formate form, 200–400 mesh, Bio-Rad) as described previously (Berridge et al, 1983).
Protein kinase C (PKC) translocation assay
Gelsolin transfectants, neo-control and parental PC10 cells were cultured to 70–80% confluence on 150 mm plates in complete medium (RPMI 1640, supplemented with 10% FCS and 0.03% L-glutamine). The cells were starved by serum-free RPMI 1640 medium for 48 h before stimulation. After starvation, the cells were exposed to bradykinin (100 nM) for 5 min at 37°C in a moist atmosphere of 95% air and 5% CO2. The cells were washed with ice-cold phosphate-buffered saline, harvested with scraper, and cell pellets were lysed with homogenisation buffer (20 mM Tris-HCl, pH 7.4, 250 mM sucrose, 2 mM EDTA, 10 mM 2-mercaptoethanol, 1 mM PMSF, 5 μg ml−1 leupeptin and 10 μg ml−1 aprotinin) by Dounce homogenisation (50 strokes). The homogenate was centrifuged at 200 g for 10 min to removed nuclei and unlysed cell, and the resulting supernatant was centrifuged at 100 000×g for 1 h. The supernatants (soluble fraction) were analysed for protein content and prepared for electrophoresis (Rotenburg and Sun et al, 1998). The pellets (particulate fraction) were resuspended with RIPA buffer containing 0.1% Triton X-100, placed at 4°C overnight. Soluble (cytosol) and particulate (membrane) fractions (20 μg per lane) were separated with 10% of polyacrylamide gel and analysed by Western blot analysis with monoclonal antibodies specific for PKC isoforms.
RESULTS
Establishment of lung cancer cell line overexpressing gelsolin
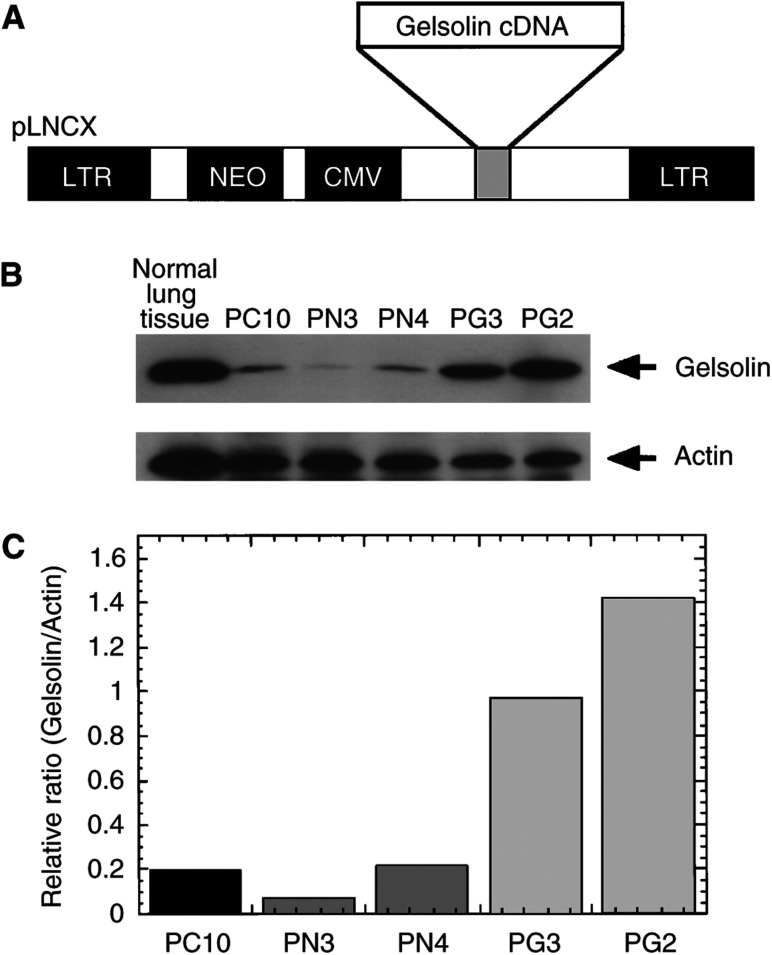
The protein level of gelsolin in PC10 cells was 1/30th in comparison with that of normal lung tissues on Western blot analysis (Dosaka-Akita et al, 1998). To stably transduce gelsolin into lung cancer cells, we obtained amphotropic retrovirus carrying the human cytoplasmic gelsolin cDNA (designated as RGV) through transient transfection of CAK8 packaging cells with pLNChGsn (Figure 1A). An empty vector pLNCX containing only neomycin-resistance gene was generated as control virus (designated as RNV). The lung squamous cell carcinoma PC10 was infected with either RGV or RNV and selected with G418. Initially, six clones infected with RGV were designated as PG and four clones infected with RNV were designated as PN. They were randomly selected and gelsolin expression was confirmed against the parental cell clones on Western blot analysis. Gelsolin expressions in PG clones were higher than those of control cells, PN and PC10 (data not shown). PG2, PG3, PN3 and PN4 clones as well as PC10 were used for this experiment. Gelsolin expressions were verified on Western blot and quantified on densitometric analysis (Figure 1B, C). Gelsolin protein levels were about 5.0- and 7.5-fold higher in PG3 and PG2 cells, respectively, than the parental clone.
Figure 1.
Identification of human lung cancer cells transfected with gelsolin. (A) Construction of gelsolin-overexpressive clones. The human cytoplasmic gelsolin cDNA was cloned into the HindIII–StuI site of pLNCX retoroviral vector as described in Materials and Methods. The resulting construct, pLNChGsn or pLNCX alone was transfected into CAK8, a highly efficient ecotropic virus-packaging cell line. (B) Western blot analysis of the gelsolin expression in stably transfected clones. PC10 cells were transfected with pLNCX or pLNChGsn, and stable transfectants were established after selection with G418 as described in Materials and Methods. Parental (lanes 1), neo-transfectants (lanes 2 and 3) and gelsolin transfectants (lanes 4 and 5) are shown. (C) Densitometric quantification of protein levels in transfectants is shown after normalisation against the levels of actin protein using NIH Image.
Tumorigenicity of gelsolin-overexpressing clones in vitro and in vivo
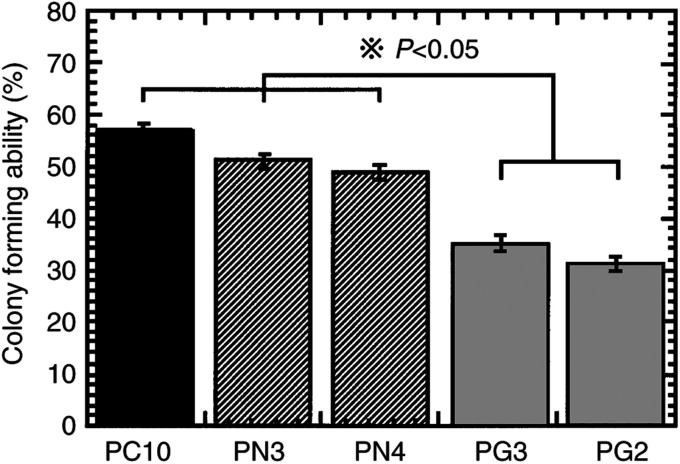
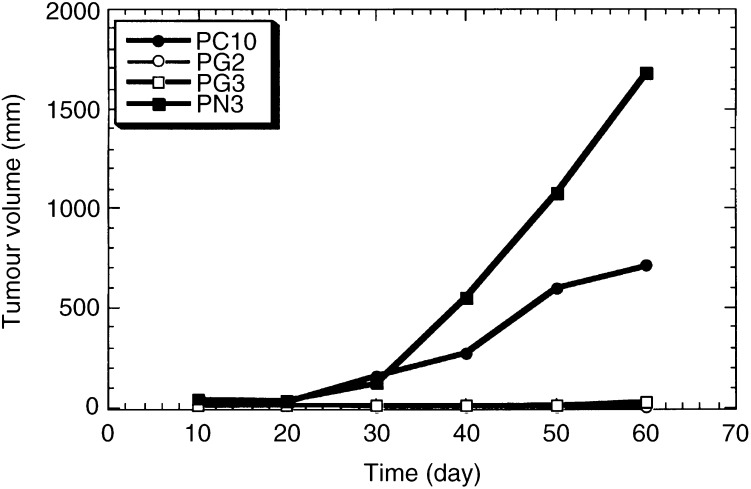
We next examined the effects of restoration of gelsolin expression on tumorigenicity of PC10 in soft agar and in nude mice. As shown in Figure 2, colony formation was significantly reduced by overexpression of gelsolin in PC10 (P<0.05). Next, we tested the tumorigenecity of gelsolin-overexpressing clone PG2 and the neo-control clone PN3 in nude mice. The tumorigenicity of PG2 was suppressed as compared to PN3 in Exp. 1 (Table 1). In Exp. 2, the implanted PG2 and PG3 cells overexpressing gelsolin grew more slowly than PN3 control cells and PC10 parental cells in nude mice, indicating that tumorigenicity of PG2 and PG3 clones was strongly suppressed, in parallel with the amounts of expressed gelsolin (Figure 3). Furthermore, PG2 cells regressed in both mice tested in Exp. 2 (Table 1). These results demonstrated that gelsolin functioned as a tumour suppressor in lung cancer cells, consistent with previous observations in bladder and colon cancers (Tanaka et al, 1995; Furuuchi et al, 1996).
Figure 2.
Effects of gelsolin expression on the colony-forming ability of transfectants in soft agar. Parental PC10, neo-transfectants PN3, PN4 and gelsolin transfectants PG3, PG2 cells were plated in 0.33% agar on 60 mm plates and the rate of colony forming at 3 weeks after incubation was analysed. *P<0.05, compared with parental and neo-transfectant cells by post hoc test.
Table 1. Tumorigenicity in nude mice of human lung cancer cell line (PC10) or infected with gelsolin expression virus (LNChGsn) or neo-control virus (LNCX).
|
Tumour incidence |
Tumour volume at killing (days: 60, mean) (mm3) |
|||
|---|---|---|---|---|
| Cell line | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 |
| PC10 | 2/2 | 710 | ||
| PN3 | 2/2 | 2/2 | 1128 | 1678 |
| PG3 | 2/2 | 22 | ||
| PG2 | 1/2 | 0/2a | 242 | 0 |
The indicated cells were grown as described in Materials and Methods. Two mice per clone were injected subcutaneously into the back with 3×106 cells in a final volume of 100 μl of BS. Tumour size was measured using a hand calipers every 10 days. Volumes were calculated as described in Materials and Methods.
Tumours were regressed at days 24 or 50 later Inoculation.
Figure 3.
Effects of gelsolin expression on tumorigeneseity in nude mice. Cells (3×106) were inoculated subcutaneously into the back of BALB/c-nu/nu syngenic 6-week-old male mice. Tumour size was measured using a hand calipers every 10 days as described in Materials and Methods.
Cell growth and apoptosis in vitro
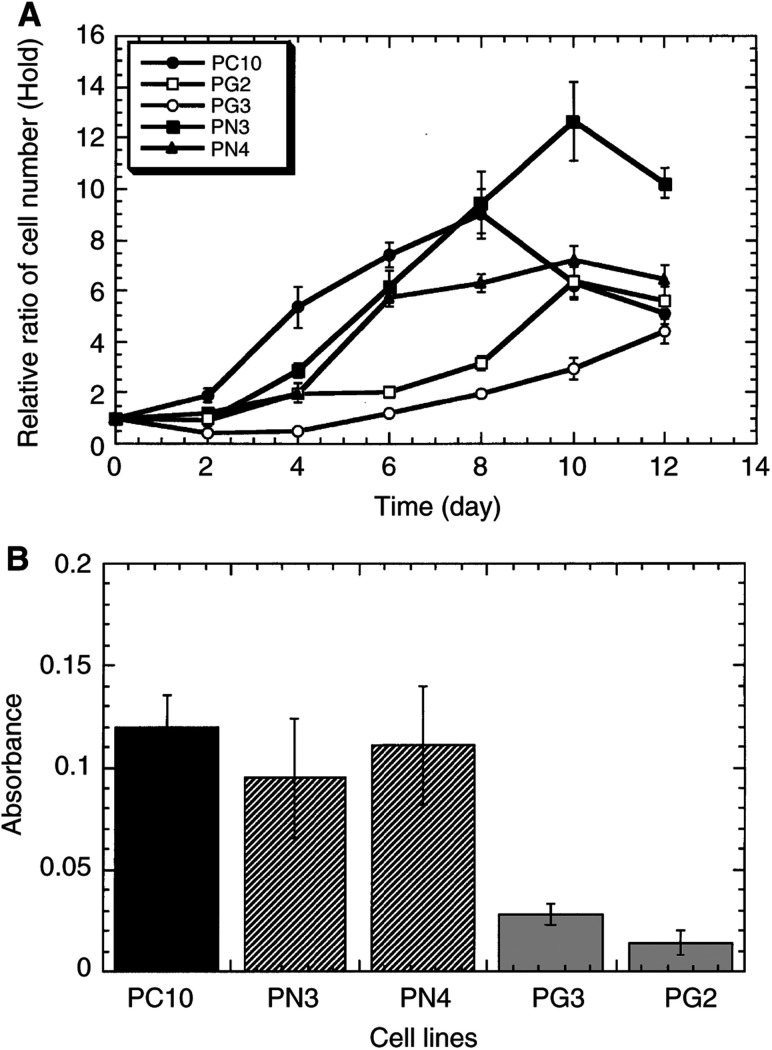
It has been proposed that the balance between cell proliferation and apoptosis determines tumour growth in vivo (Reed, 1999). We next studied in vitro whether gelsolin overexpression led to tumour regression because cell proliferation was restrained or because the apoptotic process was enhanced in PGs or both. The cell growths of the transfectants, control cells and parental cells were examined in a medium containing 10 or 1% FCS. The two clones PG2 and PG3 transfected with gelsolin cDNA grew more slowly than the control cells under 1% FCS condition (Figure 4A). Under 10% FCS condition, there was no difference in the growth rates (data not shown). Similarly, MTT assay indicated that gelsolin transfection subdued cell growth (Figure 4B). In contrast, apoptotic rates assayed by counting cells sensitive to staurosporine were similar among the PGs, PNs and PC10 (data not shown). It was suggested that gelsolin suppressed tumour growth in vivo by affecting the cell-proliferating ability of PC10 rather than by inducing apoptosis.
Figure 4.
Growth properties of wild-type, neo- and gelsolin transfectant cell lines. (A) Growth curve of parent, neo- and gelsolin transfectants. Cells were seeded at a density of 5×104 cells per well in six-well plates in RPMI 1640 with either 10 or 1% FCS as described in Materials and Methods. Cell number in triplicate wells was determined by counting with a haemocytometer after trypsinisation every 24 h. (B) Cell growth was also examined by MTT assay as described in Materials and Methods. Flat 96-well culture plates seeded at a density of 5×103 cells per well were used to test growth with 1% FCS medium. The optical density of the plates was measured on a microculture plate reader using a test wavelength of 570 nm and a reference wavelength of 630 nm.
Inositol triphosphate (IP3) production in response to bradykinin treatment
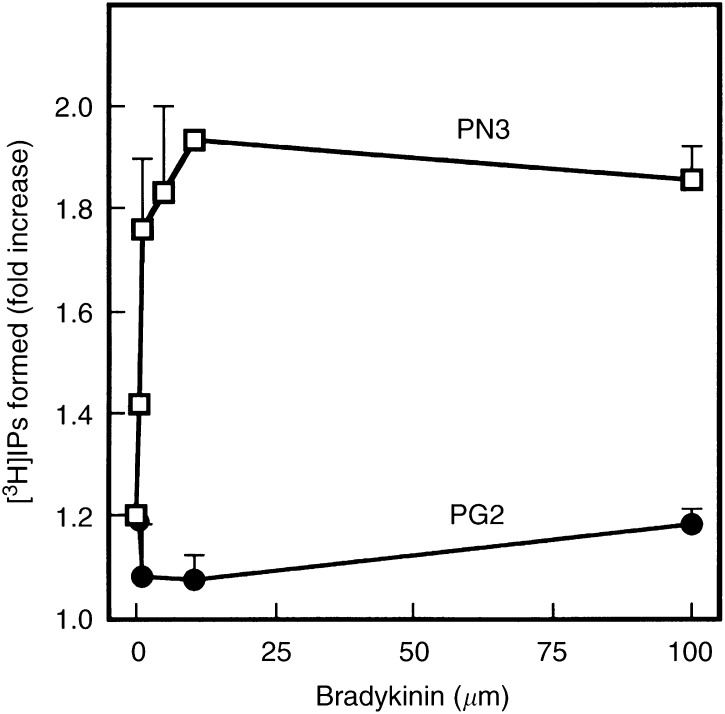
Gelsolin exhibits an ability of binding to phosphatidylinositol 4,5-bisphosphate (PIP2), and inhibits PLC activity by competing with PIP2 (Banno et al, 1992; Sun et al, 1997). Moreover, gelsolin overexpression has recently been reported to suppress bradykinin-induced PLC and phospholipase D (PLD) in NIH3T3 cells (Banno et al, 1999). These results indicate that gelsolin functions as a modulator of lipid metabolism in fibroblast cells. As shown in Figure 5, IP3 production by treatment with bradykinin was markedly inhibited in PG2, overexpressing gelsolin as compared to in the neo-control clone PN3, indicating that PLC activity was also suppressed by overexpression of gelsolin in the epithelial cell system.
Figure 5.
Effects of gelsolin overexpression on PLC activation induced by bradykinin. Vector (PN3)- or gelsolin (PG2)-overexpressing PG10 cells were labelled wtih [3H]inositol in inositol-free RPMI 1640 containing 0.3% BSA for 24 h. [3H]inositol phosphate generation was measured at indicated concentrations of bradykinin for 2 min as described in Materials and Methods. Results represent the mean±s.e. of three independent experiments.
Translocation of PKC isoforms induced by bradykinin
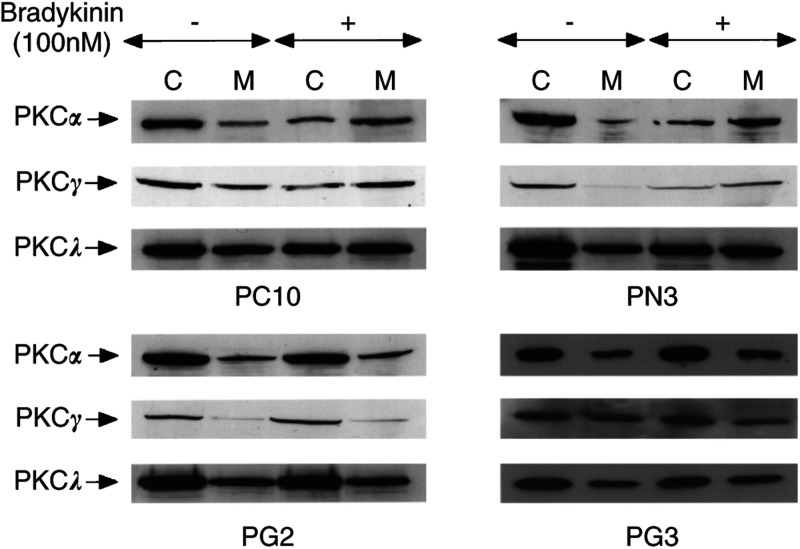
Among the different PKC isoforms, the conventional and the novel PKCs have been demonstrated to be activated by diacylglycerol (DAG) or phorbol esters or both (Toker, 1998). Activation of PKCs is a crucial step in the intracellular signal transduction pathway common to many growth factors (Hunter, 1997). Tumour cells with an increased PKC activity have an enhanced ability to invade and metastasise, indicating PKC roles critical to malignant phenotypes (Korczak et al, 1989; Schwartz et al, 1990). Western blotting of whole cell extracts showed that PC10 cells expressed the PKC isoforms α, γ, ι and λ (data not shown). Gelsolin inhibits the hydrolysis of PIP2 by PLC as described above, and thereby suppresses the generation of DAG. For studying the activation mechanism, we examined the stimulus-induced translocation of PKC isoforms from the cytosolic fraction to the particulate fraction. After treatment with 12-O-tetradecanoylphorbol-13-acetete (TPA) (1000 ng ml−1) for 1 h, the membrane fractions of conventional PKCs, PKCα and PKCγ increased in all cells, indicating no defect of the PKC pathway in transfectants overexpressing gelsolin as well as the neo-control clones and parental PC10 cells. In addition, the translocation of the atypical PKC isoforms (ι and λ) was not observed in all cells as expected (data not shown). As carried out in the other studies of bradykinin-induced translocation of PKC isoforms in different cell lines (Graness et al, 1998), PC10 and transfected cells were stimulated with 100 nM bradykinin for 5 min. The membrane fractions of PKCα and PKCγ increased in PC10 and PN3 cells when treated with bradykinin. On the other hand, in PG2 and PG3 cells treated with bradykinin, PKCα and PKCγ did not change in membrane fractions. Furthermore, the atypical PKC isoforms λ did not translocate in any clones when treated with bradykinin (Figure 6). Our results suggested that gelsolin suppressed the activation of PKC by decreasing the production of DAG. Collectively, our results indicate that overexpression of gelsolin in PC10 cells causes tumour suppression in nude mice through inhibiting the activation of PKCs by sequestering PIP2, which is a substrate of PLC.
Figure 6.
Bradykinin-induced PKCs translocation in transfectants by Western blot analysis. For activation studies, the bradykinin-induced translocation of PKC isoforms from the cytosolic fraction to the membrane fraction was investigated by cell fractionation as described in Materials and Methods. Cytosol and membrane fractions (20 μg per lane) were separated with 10% polyacrylamide gel and analysed by Western blot analysis with monoclonal antibodies specific for PKC isoforms. The PKC proteins are represented by the arrows. C, cytosolic fractions; M, membrane fractions.
DISCUSSION
In this study, we demonstrated that gelsolin suppressed tumorigenicity of PC10 lung cancer cells through inhibiting a PKC signal transduction pathway. Gelsolin is a representative of actin-regulatory proteins with an 82 kDa mass and is present in most vertebrate tissues (Kwiatkowski et al, 1988; Lueck et al, 1998). Its actions are regulated positively by calcium ions or protons, and negatively by polyphosphoinositides, especially by PIP2, suggested by the ability of gelsolin to bind to PIP2 (Janmey and Stossel, 1987; Janmey, 1994). We previously reported that gelsolin expression is reduced or not detected in various lung cancer cell lines and in more than half of the surgically resected tissues (Dosaka-Akita et al, 1998), and demonstrated that ectopic expression of gelsolin cDNA in bladder and colon cancer cell lines results in suppression of tumorigenicity (Tanaka et al, 1995; Furuuchi et al, 1996).
Activation of proto-oncogenes or inactivation of tumour suppressor genes by either mutation, amplification or rearrangement of DNA has been shown to be a critical step for carcinogenesis (Hunter, 1997). Tumour suppressor genes are classified into two categories, class I and class II. In class I tumour suppressor genes, usually isolated by positional cloning strategy, including RB, p53 or APC, loss of their function results from mutation or deletion of DNA. An increasing number of class II tumour suppressor genes have been identified on the basis of their downregulated expressions and of their transformation-inhibitory activity in tumour cells (Sager, 1997; Zhang et al, 1998). All these findings suggest that downregulation of gelsolin is involved in the development of various cancers as one of the early events in carcinogenesis, and that gelsolin may function as a tumour suppressor.
We established stable clones of lung cancer cells overexpressing various levels of gelsolin to investigate the effects of gelsolin restoration on tumorigenicity. In our studies, gelsolin-overexpressing lung cancer cells exhibited a significant growth inhibition under the low-level serum condition, consistent with our previous findings (Ishizaki et al, 1995), decreased the ability of colony formation in soft agar and reduced tumorigenicity in nude mice. PG2, highly expressing gelsolin, reduced and finally lost tumorigenicity, whereas PG3, moderately expressing gelsolin, slightly grew in nude mice. These findings demonstrated that gelsolin played a key role as a tumour suppressor in lung cancer cells and that the effect depended on the levels of gelsolin expression.
Agonist-stimulated activation of PLC β, γ and δ subtypes resulted in hydrolysis of membrane inositol phospholipid PIP2 (Williams, 1999), and led to the generation of DAG and soluble IP3. It is likely that gelsolin may affect the phospholipid signalling pathway after the hydrolysis of PIP2 supported by its high affinity for PIP2. PLC activity is also reported to be modulated in NIH3T3 cells overexpressing wild-type gelsolin or CapG, an actin-capping protein structurally related with gelsolin (Sun et al, 1995; Sun et al, 1997). Our group found that phospholipase D activity is affected by overexpression of human gelsolin cDNA in NIH3T3 cells via PLC/PKC pathway (Banno et al, 1999).
PKC isoforms appear to be localised in the cytoplasm before stimulation. For example, in MCF-7 cells, PKCα is present in the cytosol before stimulation, and 12-O-tetradecanoylphorbol-13-acetete (TPA) treatment induces translocation of PKCα from the soluble fraction to the particulate fraction (Kennedy et al, 1992). To demonstrate activation of PKCs in our experiments, their stimulus-induced translocation from the cytosol to the membrane was investigated. We identified two types of PKC isoforms in PC10 cells: conventional PKC (PKCα and PKCγ) and atypical PKC (PKCι and PKCλ). By TPA treatment, translocation of cPKCs was observed in parental cells, control and gelsolin transfectants. However, aPKC translocation was not detected in any cells by the same stimulation.
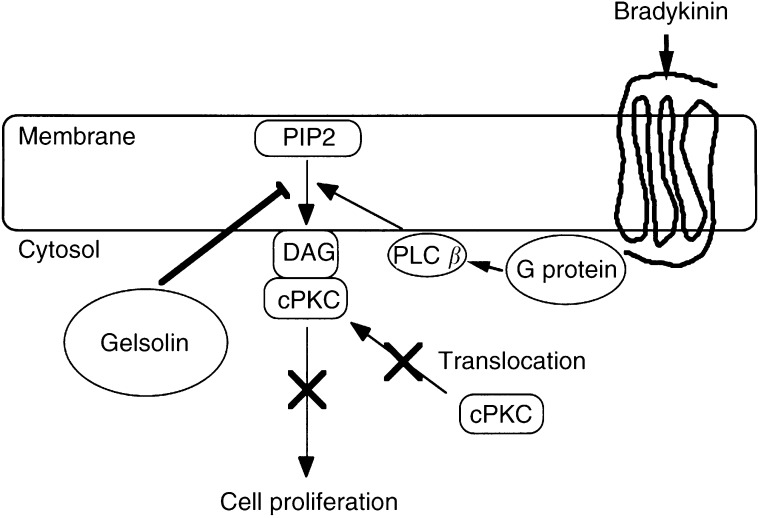
Bradykinin binds to a seven transmembrane domain receptor coupled with G-protein (Burch and Axelrod, 1987), activating PLCβ. PLCβ hydrolyses PIP2, resulting in the generation of DAG, which in turn activates PKC. As shown in Figure 4, translocation of PKCα and γ, but not that of PKCι/λ, was observed in PC10 and PN3 in response to bradykinin treatment, whereas the translocation was hampered in PG2, suggesting that activation of PKCα and γ was inhibited in PG2. We also examined the translocation of PKCs in parental cells, control and gelsolin transfectants by epidermal growth factor (EGF) treatment. PIP2 is hydrolysed to DAG and IP3 by PLCγ, which EGF phosphorylates on its tyrosine residues (Goldschmidt-Clermont et al, 1991). However, we did not detect EGF-induced translocation of PKCs in either clone (data not shown). These results indicated that overexpression of gelsolin inhibited the PLCβ/PKC pathway critical for the growth of PC10 cells, leading to the loss of tumorigenicity. Gelsolin could have a regulatory function in the lipid signalling pathway not only in the fibroblast system but also in epithelial cells. Tumour promotion in PC10 cells mostly depended on the PKC signal transduction pathway mediated by the activation of PLCβ. Consistent with our findings, stable transfection of PKCα antisense was documented to decrease the malignant phenotypes of the parental cells (Wang et al, 1999). This observation supported our findings that gelsolin inhibited the activation of PKC in lung cancer cells and reduced their growth and tumorigenicity (Figure 7).
Figure 7.
Model for the inhibition of bradykinin-induced cPKCs translocation by gelsolin in PC10 cells. Tumour promotion mostly depended on the PKC signal transduction pathway via the route that activated PLCβ stimulated through a G-protein-coupled receptor. Gelsolin sequesters PIP2 and suppresses production of DAG and IP3 by PLCβ, and thereby translocation to membrane fraction and activation of cPKCs is inhibited. As the PLCβ/cPKCs pathway is critical for growth of lung cancer cells, and overexpression of gelsolin in PC10 cells results in the loss of tumorigenicty.
In conclusion, we showed that, in PC10 cells, gelsolin suppressed the activation of PKCs involved in phospholipid signalling pathways and inhibited cell growth and tumorigenicity in nude mice. Further biological researches on gelsolin will certainly be required in order to exploit the tumour-suppressing effect of gelsolin expression in the clinical field. For the present, the p53 gene transfer using an adenovirus vector has been used as a tumour suppressor for gene therapy in nonsmall-cell lung cancers (Roth et al, 1999). The introduction of gelsolin into various cancers might possibly serve as an antitumour gene therapy in the future.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (to HF, HK and NK), Grants from the Uehara Memorial Foundation, Japan, and the Ichiro Kanehara Foundation, Hokkaido Foundation for The Promotion of Scientific and Industrial Technology (to HF). We thank Dr Yoshimasa J Miyazaki (Hokkaido University) for a critical reading of the manuscript.
References
- Banno Y, Fujita H, Ono Y, Nakashima S, Ito Y, Kuzumaki N, Nozawa Y. (1999) Differential phospholipase D activation by bradykinin and sphingosine 1-phosphate in NIH 3T3 fibroblasts overexpressing gelsolin. J Biol Chem 274: 27385–27391 [DOI] [PubMed] [Google Scholar]
- Banno Y, Nakashima T, Kumada T, Ebisawa K, Nonomura Y, Nozawa Y. (1992) Effects of gelsolin on human platelet cytosolic phosphoinositide-phospholipase C isozymes. J Biol Chem 267: 6488–6494 [PubMed] [Google Scholar]
- Berridge MJ, Dawson RMC, Downes P, Heslop JP, Irvine RF (1983) Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J 212: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C, Ueffing M, Jaken S, Parker PJ, Weinstein IB (1995) Two closely related isoforms of protein kinase C produce reciprocal effects on the growth of rat fibroblasts. Possible molecular mechanisms. J Biol Chem 270: 78–86 [DOI] [PubMed] [Google Scholar]
- Burch RM, Axelrod J (1987) Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci USA 84: 6374–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AM, Guadagno SN, Krauss RS, Fabbro D, Weinstein IB (1993) The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene 8: 2095–2104 [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y (1982) Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem, 257: 7847–7851 [PubMed] [Google Scholar]
- Dekker LV, Parker PJ (1994). Protein kinase C–a question of specificity. Trends Biochem Sci 19: 73–77 [DOI] [PubMed] [Google Scholar]
- Dosaka-Akita H, Hommura F, Fujita H, Kinoshita I, Nishi M, Morikawa T, Katoh H, Kawakami Y, Kuzumaki N (1998) Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res 58: 322–327 [PubMed] [Google Scholar]
- Furuuchi K, Fujita H, Tanaka M, Shinohara N, Senmaru N, Ogiso Y, Moriya S, Hamada M, Kato H, Kuzumaki N (1996) Gelsolin as a suppressor of malignant phenotype in human colon cancer. Tumor Target 2: 277–283 [Google Scholar]
- Goldschmidt-Clermont PJ, Kim JW, Machesky LM, Rhee SG, Pollard TD (1991) Regulation of phospholipase C-γ1 by profilin and tyrosine phosphorylation. Science 251: 1231–1233 [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Barsky SH (1988) Tumor promoter-induced membrane-bound protein kinase C regulates hematogenous metastasis. Proc Natl Acad Sci USA 85: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graness A, Adomeit A, Heinze R, Wetzker R, Liebmann C (1998) A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase beta, and protein kinase C epsilon. J Biol Chem 273: 32016–32022 [DOI] [PubMed] [Google Scholar]
- Housey GM, Johnson MD, Hsiao WL, O'Brian CA, Murphy JP, Kirschmeier P, Weinstein IB (1988) Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell 52: 343–354 [DOI] [PubMed] [Google Scholar]
- Hunter T (1997) Oncoprotein networks. Cell 88: 333–346 [DOI] [PubMed] [Google Scholar]
- Ishizaki A, Fujita H, Kuzumaki N (1995) Growth-inhibitory functions of a mutated gelsolin (His321) in NIH/3T3 mouse fibroblasts. Exp Cell Res 217: 448–452 [DOI] [PubMed] [Google Scholar]
- Janik P, Szaniawska B, Kowalczyk D, Miloszewska J (1994) The effect of phorbol ester treatment on migration of C3H 10T1/2 and BT5C glioma cells: possible application to carcinogenesis. J Cancer Res Clin Oncol 120: 156–158 [DOI] [PubMed] [Google Scholar]
- Janmey PA (1994) Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol 56: 169–191 [DOI] [PubMed] [Google Scholar]
- Janmey PA, Stossel TP (1987) Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325: 362–364 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Torri JA, Lippman ME, Dickson RB (1999) Regulation of motility and protease expression in PKC-mediated induction of MCF-7 breast cancer cell invasiveness. Exp Cell Res 247: 105–113 [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Prestigiacomo LJ, Tyler G, May WS, Davidson NE (1992) Differential effects of bryostatin 1 and phorbol ester on human breast cancer cell lines. Cancer Res 52: 1278–1283 [PubMed] [Google Scholar]
- Kishimoto A, Takai Y, Mori T, Kikkawa U, Nishizuka Y (1980) Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem 255: 2273–2276 [PubMed] [Google Scholar]
- Korczak B, Whale C, Kerbel RS (1989) Inhibition of invasion of invasive human bladder carcinoma cells by protein kinase C inhibitor staurosporine. Cancer Res 49: 2597–2602 [PubMed] [Google Scholar]
- Kwiatkowski DJ, Mehl R, Izumo S, Nadal GB, Yin HL (1988) Muscle is the major source of plasma gelsolin. J Biol Chem 263: 8239–8243 [PubMed] [Google Scholar]
- Lueck A, Brown D, Kwiatkowski DJ (1998) The actin-binding proteins adseverin and gelsolin are both highly expressed but differentially localized in kidney and intestine. J Cell Sci 111: 3633–3643 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF (1993) Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem 268: 6090–6096 [PubMed] [Google Scholar]
- Moriya S, Yanagihara K, Fujita H, Kuzumaki N (1994) Differential expression of hsp90, gelsolin and GST-π in human gastric carcinoma cell lines. Int J Oncol 5: 1347–1351 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308: 693–698 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614 [DOI] [PubMed] [Google Scholar]
- Otterson GA, Xiao GH, Geradts J, Jin F, Chen WD, Niklinska W, Kaye FJ, Yeung RS (1998) Protein expression and functional analysis of the FHIT gene in human tumor cells. J Natl Cancer Inst 90: 426–432 [DOI] [PubMed] [Google Scholar]
- Persons DA, Wilkison WO, Bell RM, Finn OJ (1988) Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell 52: 447–458 [DOI] [PubMed] [Google Scholar]
- Reed JC (1999) Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol 11: 68–75 [DOI] [PubMed] [Google Scholar]
- Rotenberg SA, Sun XG (1998) Photoinduced inactivation of protein kinase C by dequalinium identifies the RACK-1-binding domain as a recognition site. J Biol Chem 273: 2390–2395 [DOI] [PubMed] [Google Scholar]
- Roth JA, Swisher SG, Meyn RE (1999) p53 tumor suppressor gene therapy for cancer. Oncology (Huntingt) 13: 148–154 [PubMed] [Google Scholar]
- Sager R (1997) Expression genetics in cancer: shifting the focus from DNA to RNA. Proc Natl Acad Sci, USA 94: 952–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R, Skarin AT (1998) Molecular abnormalities in lung cancer. J Clin Oncol 16: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Redwood SM, Ohnuma T, Holland JF, Droller MJ, Liu BC (1990) Possible involvement of Ca2+ mobilization and protein kinase C activation in the induction of spontaneous metastasis by mouse mammary adenocarcinoma cells. J Natl Cancer Inst 82: 1753–17562231770 [Google Scholar]
- Sun H, Lin K, Yin HL (1997) Gelsolin modulates phospholipase C activity in vivo through phospholipid binding. J Cell Biol 138: 811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HQ, Kwiatkowska K, Wooten DC, Yin HL (1995) Effects of CapG overexpression on agonist-induced motility and second messenger generation. J Cell Biol 129: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Müllauer L, Ogiso Y, Fujita H, Moriya S, Furuuchi K, Harabayashi T, Shinohara N, Koyanagi T, Kuzumaki N (1995) Gelsolin: a candidate for suppressor of human bladder cancer. Cancer Res 55: 3228–3232 [PubMed] [Google Scholar]
- Toker A (1998) Signaling through protein kinase C. Front Biosci 3: D1134–1147 [DOI] [PubMed] [Google Scholar]
- Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC (1994) Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem 269: 32358–32367 [PubMed] [Google Scholar]
- Wang XY, Repasky E, Liu HT (1999) Antisense inhibition of protein kinase C-alpha reverses the transformed phenotype in human lung carcinoma cells. Exp Cell Res 250: 253–263 [DOI] [PubMed] [Google Scholar]
- Williams RL (1999) Mammalian phosphoinositide-specific phospholipase C. Biochim Biophys Acta 1441: 255–267 [DOI] [PubMed] [Google Scholar]
- Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia. Br J Cancer 77: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HL, Stossel TP (1979) Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281: 583–586 [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin KJ, Sheng S, Sager R (1998) Expression genetics: a different approach to cancer diagnosis and prognosis. Trends Biotech 16: 66–71 [DOI] [PubMed] [Google Scholar]