Abstract
The monkey’s lateral intraparietal area (LIP) has been associated with attention and saccades. LIP neurons have visual on-responses to objects abruptly appearing in their receptive fields (RFs) and sustained activity preceding saccades to the RF. We studied the relationship between the on-responses and delay activity in LIP using a ‘stable-array’ task. Monkeys viewed eight distinct, continuously illuminated objects, arranged in a circle with at least one object in the RF. A cue flashed instructing the monkey to make a saccade, after a delay, to the stable object physically matching the cue. The location of the cue was fixed in trial blocks, either in or out of the RF. If the cue was outside the RF, neurons developed delay-period activity tuned for the direction of the saccade target at ~190 ms after cue onset. If the cue appeared in the RF, neurons initially responded to cue onset and developed tuning for saccade direction only toward the end of the delay period, 390 ms after cue onset. The cue- and saccade-target responses coexisted throughout a significant portion of the delay period. The results show that visual-on responses and delay-period activity in LIP are functionally separable, and that, although highly selective, the salience representation in LIP can contain more than one object at a time.
Keywords: attention, lateral intraparietal area, parallel representation, parietal cortex, saccades
Introduction
The monkey’s lateral intraparietal area (LIP) lies at the junction of the visual and oculomotor systems. It has strong anatomical connections with the dorsal and ventral visual streams as well as with the frontal eye field and the superior colliculus, two structures involved in the planning of saccadic eye movements. The LIP has been proposed to participate in covert orienting of attention and in the planning of saccades, but its exact contributions to each function remain a subject of investigation (Colby and Goldberg, 1999; Snyder et al., 2000; Goldberg et al., 2002).
When tested with oculomotor tasks LIP neurons show two major response types: transient, fast on-responses for visual stimuli that appear abruptly in their receptive field, and sustained delay-period activity preceding the preparation of saccades toward their receptive field (Barash et al., 1991a,b). The visual on-responses do not reflect saccade preparation as they remain robust in tasks requiring different motor planning (Powell and Goldberg, 2000; Colby et al., 1996; Snyder et al., 1997), and are in some cases enhanced if monkeys choose not to look at the visual stimulus than if they plan a saccade to it (Gottlieb and Goldberg, 1999; Bisley and Goldberg, 2003). However, these responses are sensitive to the physical salience of the stimulus eliciting them: LIP neurons respond strongly to a salient, sudden-onset stimulus but remain relatively unresponsive when the same object is stable in its environment and enters the receptive field by virtue of the monkey’s saccade (Gottlieb et al., 1998). In contrast to the visual-on response, delay-period activity shows a higher degree of motor specificity as it is stronger if the monkey makes a saccade to, rather than reaching toward a receptive field stimulus (Snyder et al., 1997). For a given saccade, however, delay-period activity also varies with contextual variables, including reward expectation or saccade probability (Platt and Glimcher, 1999; Sugrue et al., 2004), the presence or absence of a visible saccade target (Gottlieb et al., 1998, Gottlieb and Goldberg, 1999) and the difficulty of the visual discrimination that leads to saccade target selection (Shadlen and Newsome, 2001).
One interpretation of these findings is that the LIP provides a selective spatial representation of objects that are likely to attract attention either automatically, by virtue of their physical salience, or voluntarily, by virtue of their task relevance. Consistent with this, the time course of neural responses in LIP predicts the time course of an attentional shift from an abrupt-onset distractor toward a saccade target (Bisley and Goldberg, 2003). Likewise, delay-period activity appears to reflect the process of saccade-target selection (see also Wardak and Duhamel, 2002), which is known to rely on mechanisms that are closely coupled — perhaps identical — to visual attention (Kowler et al., 1995). Thus the variable driving LIP responses may be the propensity of a stimulus to attract attention, as determined by both endogenous and exogenous factors.
In research on LIP neurons, on-responses and delay-period activity have always been confounded. This is because in traditional behavioral tasks saccade targets appear abruptly on each trial, thereby always eliciting an on-response. However, in the world in which primates evolved, abruptly appearing stimuli are extremely rare, and saccade targets are typically selected from among stable, relatively inconspicuous objects. To understand the neural mechanisms of saccade target selection and attention, we therefore must examine responses in more naturalistic settings, in which saccade target selection proceeds independently of visual transients. We have recently addressed this issue by using a stable-array task in which an array of several objects remained stably on the screen for blocks of trials; the monkey selected one of these stable objects and made a saccade to it according to the instruction conveyed, on each trial, by a flashed cue (Gottlieb et al., 1998). We found that LIP neurons responded both to the flashed cue and to the saccade target on this task, showing that these two responses are relatively independent of each other. In the present paper we analyze the spatial and temporal properties of the target-selective activity in LIP on the stable-array task, and the interactions of this target-selection signal with the visual on-response. We show that, in the absence of an on-response, target-selective activity arises gradually in LIP during the delay period. This response interacts synergistically or antagonistically with the visual on-response to the cue, depending on the spatial relationship between the cue and saccade target.
Materials and Methods
General Experimental Methods
Two male rhesus monkeys were prepared for single-neuron recordings using sterile surgery under ketamine and isofluorane anesthesia. Magnetic resonance imaging was used to aid in the placement of the recording chamber and to verify the location of electrode tracks. General behavioral and physiological methods were as described (Colby et al., 1996; Gottlieb et al., 1998), with behavioral monitoring and data collection controlled by a 486 PC running the REX software (Hays et al., 1982), and visual stimuli projected upon a tangent screen by an Electrohome Video Projector driven by a second personal computer. All experimental protocols were approved by the NEI Animal Care and Use Committee as complying with the guidelines established in the Public Health Service Guide for the Care and Use of Laboratory Animals.
Behavioral Tasks
In the memory-guided saccade task the monkey initiated each trial by maintaining fixation of a central fixation point (a 0.5° red square) for 300–500 ms. A cue (a white annulus, 2° in diameter) was then flashed for 200 ms and was followed by a variable delay period (range 400–900 ms) with no eccentric visual stimulation. Cue location was selected pseudo-randomly on each trial from a set of eight standard locations directed at 0° (to the right), 45°, 90° (straight up), 135°, 180°, 225°, 270° and 315° relative to fixation at a constant eccentricity matching the estimated center of each neuron’s receptive field. After the delay the fixation point disappeared and the monkey was rewarded for making a saccade to the remembered location of the cue. Saccades were rewarded and scored as correct if they began within 100–400 ms after fixation point disappearance, and if the distance between their endpoint and the target did not exceed 25% of target eccentricity.
In the stable-array task a circular visual search display consisting of eight distinct visual stimuli appeared once and remained stably on the screen, without flashing on or off, for the duration of data collection (Fig. 1). The stable objects each subtended 2° horizontally and vertically, differed in color and shape, were approximately equated for luminance, and had the same eight standard directions as the cues on the memory-guided saccade task. The radius of the stable array was adjusted to match the eccentricity of each neuron’s receptive field and was equal to the eccentricity of targets in the memory-guided saccade task. The monkey initiated each trial by maintaining central fixation for 300–500 ms. A cue, physically identical to a randomly selected stable object, was then flashed for 200 ms. Cue location was fixed within a block of trials, either inside the receptive field or at a comparable eccentricity outside the receptive field of the neuron under study (Fig. 1). After a variable delay period (range 400–900 ms) the fixation point disappeared and the monkey was rewarded for making a saccade to the stable object that matched the cue. Criteria for identifying and rewarding correct saccades were as in the memory-guided saccade task. A single stable array, differing only in radius from neuron to neuron but otherwise containing the same objects in the same relative locations, was used throughout training and data collection in both monkeys.
Figure 1.

The stable array task. A circular array of eight distinct, uniformly spaced objects remained continuously lit during the trial and inter-trial intervals for the duration of data collection. The display was centered on a central fixation point (small black square). Stimuli were always positioned at eight standard directions separated by 45° as shown. Array radius was adjusted so that the receptive field of the neuron being recorded (gray oval) fell upon one of the stable array elements as soon as the monkey achieved central fixation. Following presentation of the cue there was a variable delay period, after which the monkey was rewarded for making a saccade to the stable array object that matched the cue. The cue-in and cue-out-of-receptive-field versions are identical except for the location of the cue. The objects shown in this illustration resemble the actual objects that were used in shape, but the actual objects also differed from each other in color. Visual stimuli are not drawn to scale.
Neuron Isolation and Recording
Area LIP was identified physiologically by the presence of visual, delay and presaccadic activity on the memory-guided saccade task. For each monkey, the general recording area was consistent with the anatomical location of area LIP as assessed with structural magnetic resonance imaging. Once a neuron was isolated, the approximate location of its receptive field was estimated in a block of memory-guided saccade trials in which cue position was controlled by an experimenter-manipulated joystick. The neuron was then tested with blocks of trials of the memory-guided saccade task, the stable-array task with the cue positioned in the neuron’s receptive field (cue-in configuration) and the stable-array task with the cue positioned out of the receptive field (cue-out configuration; Fig. 1). Fifty-five neurons were tested with the memory-guided saccade task, 24 with the cue-out of receptive field stable array task, and 24 with the cue-in version of the stable-array task. Of these, 28 were tested with both the memory-guided and the cue-out stable-array tasks, and 14 were tested with both versions of the stable-array task.
Among the 55 neurons tested with the memory-guided saccade task, 53 (96%) had visual responses, 40 (73%) had delay-period activity and 39 (71%) had presaccadic activity. Except for the higher incidence of neurons with visual responses, these proportions are similar to those reported previously for LIP (Barash et al., 1991b). Median receptive field eccentricity was 16° (range 8–24°). In the stable-array task median eccentricity of the cues was 6.8° in the cue-out configuration (range 1.3–12.4°), and 9.8° in the cue-in configuration (range 2.4–15.0°). These two sets of eccentricities were statistically equivalent (P = 0.09, Wilcoxon sign-rank test).
Data Analysis
Directional Tuning — Individual Neurons
We analyzed tuning for saccade direction using a vector method. We represented the neural response on each trial as a two-dimensional ‘trial vector’, r, whose direction corresponded to saccade direction and whose amplitude was proportional to the firing rate after baseline subtraction. We then added all trial vectors to compute a sum vector R which characterized the neuron’s directional tuning (Batschelet, 1981). The direction of R was an estimate of the preferred direction of the response. The amplitude of R, normalized by the scalar sum of all vectors, was an index of tuning width independently of firing rate. (Note that, because the amplitude of each trial vector represented firing rate after baseline subtraction, variations in baseline rates did not influence our estimate of directional tuning.) Tuning indices close to 1 indicate strong, narrow directional tuning while tuning indices close to 0 represented sets of responses in which activity was roughly equivalent at all eight directions. Although a data set in which activity was equivalent at one or more pairs of diametrically opposite directions would also have yielded tuning indices close to 0, we did not encounter such a neuron in our sample.
We used a randomization analysis to determine whether a given set of responses had significant directional tuning. Each trial vector amplitude, |r|, was paired with a direction that was randomly selected, with replacement, from among the eight standard directions, and the tuning index was calculated as before. Neural activity was considered to be directionally tuned if the tuning index derived from the original data was greater than that 95% of the vectors obtained in 1000 iterations of the randomization procedure (equivalent to a one-tailed test with P = 0.05).
To determine the time course of directional tuning, we calculated tuning indices in consecutive 20 ms time bins spanning two epochs: a cue-aligned epoch beginning 200 ms before and ending 600 ms after cue onset, and a saccade-aligned epoch beginning 600 ms before and ending 200 ms after saccade onset (40 bins for each epoch). The time-of-tuning for each neuron was defined as the midpoint of the earliest interval, j, for which (i) TIj, the tuning index at that interval, and either TIj+1 or TIj+2 were statistically significant; and (ii) TIj was statistically significant for at least 50% of the time bins between the jth bin and the end of the bin sequence (the 40th bin). Alternatively, the time-of-tuning was estimated as the midpoint of the earliest time bin, j, for which TIj, TIj+1 and TIj+2 were all statistically significant. This procedure identified a time span in which a neuron showed consistent directional tuning, whether that tuning was sustained or transient (as in a phasic visual response).
We determined a neuron’s preferred direction as the direction of the average vector in the last five bins (100 ms) before saccade onset.
Directional Tuning — Population
We calculated the population tuning index by summing the individual trial vectors from all the neurons in the data set. For each neuron: (i) we determined the target direction closest to the neuron’s preferred direction and then rotated the neuron’s trial vectors so that this target direction was at 180°; (ii) we normalized the amplitude of each trial vector by the largest amplitude found in any bin for that neuron. Finally, we calculated the population vector as the vector sum of all trial vectors. The population tuning index was the amplitude of this sum vector normalized by the scalar sum of all component vectors. We determined statistical significance of tuning in a given response set using the randomization procedure described above. In addition, we determined a confidence interval for each time bin by repeating the vector summation 1000 times in a bootstrap procedure. Two tuning indices were considered statistically different if their 95% confidence intervals did not overlap.
It must be noted that even though the vector method we used here was mathematically equivalent to the population vector analysis, it differed from this analysis in that it calculated tuning separately for different groups of neurons and not for the entire population. Indeed, because there was not a constant relationship between cue and target location in our experiment, our data could not be used to calculate a true population vector (Schwartz and Moran, 2000).
Saccade Parameters
To see if neural activity was related to saccade metrics on a trial-by trial basis, each neuron’s presaccadic responses (frp, average firing rates in the 100 ms preceding saccade onset) on the set of trials with saccades to the preferred target were fit with a linear regression model of the form
where lat, vel and err represent the latency, velocity and endpoint error of the corresponding saccades (the latter is defined as the ratio of the distance between saccade endpoint and target, to the target’s eccentricity). The model was evaluated separately for each of the three tasks. Firing rate was considered significantly related to a variable if the 95% confidence interval for that variable’s coefficient did not include 0.
Results
Sixty-three LIP neurons were analyzed for this report. Of these, 48 were recorded in monkey A and 15 in monkey B. We found no significant differences between the neurons recorded in the two monkeys when we compared raw firing rates, the level and time course of the population tuning index, the presaccadic tuning indices of individual neurons on all three tasks, and the time of tuning of individual neurons on all three tasks. We therefore present the results from the pooled neural sample.
Behavior
Error rates were higher on the stable-array task than on the memory-guided saccade task (Fig. 2A). Median percent correct on the memory-guided saccade, cue-out and cue-in stable-array tasks were, for monkey A, 95%, 75% and 75%, and for monkey B, 93%, 66% and 51% (chance performance on the stable-array task was 12.5%). The vast majority of errors on the stable-array task consisted of saccades to array elements that did not match the cue. These erroneous saccades were equally likely to occur in response to any of the eight cues and to be directed to any of the eight stable objects, revealing no consistent bias toward any particular target or saccade direction. Other errors — late or premature saccades, and saccades to the cue itself — occurred on <8% of trials.
Figure 2.

Behavioral performance. Box plots show the distribution of accuracy (top) and saccade latencies (bottom) across all experimental sessions, for each task and monkey. Each box has horizontal lines at the lower quartile, median and upper quartile values, and vertical lines indicating the entire range of data. Notches are robust estimates of the 95% confidence interval for each median. Only those distributions represented by boxes with non-overlapping notches are statistically different from each other (P < 0.05).
In contrast to error rates, saccade latencies were relatively constant across monkeys and tasks (Fig. 2B). Monkey B had equivalent saccade latencies in all three conditions (median latencies were 211 ms on the memory-guided saccade task, 223 ms on the cue-out task and 207 ms on the cue-in task; P > 0.05 for each pairwise comparison, Wilcoxon rank-sum test). Monkey A had comparable latencies on the cue-out and cue-in stable-array trials (medians of 216 versus 210 ms) but had significantly longer latencies on the memory-guided saccade task (median 236 ms, P < 0.05, Wilcoxon rank-sum test).
Neither success rates nor saccade latencies (nor, indeed, saccade endpoints, as shown below) differed between the cue-out and cue-in trials of the stable-array task. As intended, therefore, the two configurations of the stable-array task appeared to be equivalent from the monkeys’ standpoint.
Neural Responses
On the memory-guided saccade task LIP neurons showed spatially tuned visual, delay-period and presaccadic responses, which are illustrated for an example neuron in Figure 3 (left column). In the stable-array task (middle and right columns) a stable object was present in the neuron’s receptive field at all times; however, the neuron responded selectively if either this object was designated as the next saccade’s target (middle column) or if the cue appeared inside its receptive field (right column; Gottlieb et al., 1998). In cue-out of receptive field trials (middle column) the neuron’s firing rate increased during the delay period only if the monkey was instructed to make an upward saccade, toward the receptive field. This gradual, spatially tuned response represents a signal of saccade target location that is independent of an on-response. In cue-in trials (right column) the visual cue always flashed in the neuron’s receptive field. The neuron had a fast on-response to cue onset, which was initially untuned for saccade direction. Its activity became tuned for saccade direction during the late delay period, remaining high if the monkey planned a saccade to the receptive field but declining otherwise. In this version of the task, therefore, visual-on and target-selective responses were superimposed.
Figure 3.

Activity of a representative LIP neuron on the memory-guided saccade and stable-array tasks. Responses on each task and for each saccade target direction (marked on the left) are shown as raster plots aligned on cue onset and on saccade onset. Each tic mark represents one action potential and each row represents one correctly executed trial. Trials are presented in the order in which they were collected with the first trial on the top. Presaccadic tuning indices for this neuron (average across 100 ms before saccade onset) were 0.61 for the memory-guided saccade task, 0.58 for the cue-out stable array task and 0.52 for the cue-in stable array task.
Figure 4 shows a similar pattern of response in the averaged activity of all neurons. For these population histograms neural responses were grouped according to the direction of the saccade relative to each neuron’s receptive field, so that thick traces correspond roughly to saccades toward the receptive field and thin traces to saccades away, and spatial tuning is indicated by the separation between traces. On the memory-guided saccade task neurons had visual and delay-period activity that was tuned for cue/saccade direction from the very beginning of the trial. On the stable array task when the cue was out of the receptive field activity specifying the location of the target emerged gradually at ~200 ms after cue onset. When the cue was in the receptive field population responses were initially dominated by the on-response to the cue, and tuning for saccade direction emerged even later, toward the end of the delay period.
Figure 4.
Averaged response histograms for the sample of neurons tested on each task. Responses were averaged in a common coordinate frame in which each neuron’s preferred target was rotated to point to 180°. Thick traces represent activity for 135°, 180° and 225° in the common coordinate frame (the center of the receptive field and the two closest flanking locations) and thin traces, represent the remaining directions. The activity of 55 neurons was averaged on the memory-guided saccade task, 33 on the cue-out task and 24 on the cue-in task. Binsize is 20 ms.
As our example neuron illustrates, preferred saccade directions remained constant across the memory-guided and stable-array tasks despite the vast differences between them. Across the population of neurons, preferred saccade directions (measured 100 ms before saccade onset) differed neither between the memory-guided saccade and cue-out tasks (r = 0.953; n = 28) nor between cue-out and cue-in versions of the stable-array task (r = 0.940; n = 14).
Simultaneous Presence of Cue and Saccade-related Activity
The population histograms in Figure 4 show that the cue-evoked activity persisted long after saccade-related activity arose in cue-out trials (compare Fig. 4, middle and bottom panels). This implies that when cue and saccade target were at non-congruent spatial locations, neurons with receptive fields at both these locations were simultaneously active in LIP. To examine this temporal overlap directly, we compared the response representing the cue in the absence of saccade planning (cue in receptive field/saccade opposite receptive field stable-array trials) with the response representing the saccade target in the absence of the cue (cue-out of receptive field/saccade to receptive field center; Fig. 5). Responses to the cue were much higher than baseline (pre-cue) activity from 40 to 440 ms after cue onset (P < 0.0009 for each of 10 bins, Wilcoxon signed-rank test). Responses to the saccade target were well above baseline (P < 0.0003) from 200 to 600 ms (10 bins) after cue onset. Thus robust responses to the cue and to the saccade target were simultaneously present in LIP for ~240 ms, from 200 to 440 ms after cue onset.
Figure 5.

Time course of average cue and saccade-related responses. Average cue-aligned histograms (binsize, 40 ms) from trials in which the cue was in the receptive field and the saccade was directed opposite each neuron’s best target direction (24 neurons), and from trials in which the cue was outside the receptive field and the saccade was directed to the best target (33 neurons). Bins in which activity was significantly greater than baseline are indicated by black dots. The epoch in which both histograms showed significant activity is shown by the thick segments and black horizontal bar.
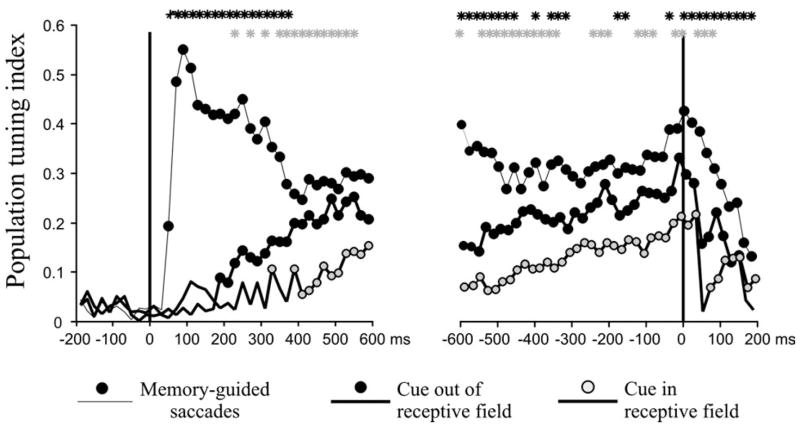
As a result of the long-lasting effect of the cue, directional tuning for the saccade arose much later in neurons that were, relative to those that were not activated by the cue, i.e. in cue-in relative to cue-out trials. To analyze the dynamics of directional tuning we calculated a population tuning index (see Materials and Methods), in non-overlapping 20 ms bins spanning the delay period (Fig. 6). We calculated directional tuning separately for each task configuration. Significant directional tuning (indicated by a tuning index significantly larger than that expected by chance) appeared at 60 ms after cue onset in the memory-guided saccade task, at 190 ms after cue onset in the cue-out stable-array task and at 390 ms after cue onset in the cue-in stable-array task. The neuronal population showed consistent tuning throughout the saccade-aligned interval in all three conditions.
Figure 6.
Dynamics of population tuning in the memory-guided saccade and stable-array task. The population tuning index, computed across all neurons tested in a given task in 40 20 ms bins aligned on cue onset (left) and on saccade onset (right). Circles (black or gray) indicate time bins during which the population vector amplitudes were greater than chance levels as determined by a randomization method (see Materials and Methods). Black stars indicate time bins in which the index on the memory-guided saccade task differed significantly (P < 0.05) from that on the cue-out stable-array task. Gray stars indicate bins in which tuning indices differed significantly (P < 0.05) on the cue-out and cue-in stable-array tasks.
We also estimated the time at which individual neurons developed consistent tuning for saccade direction (see Materials and Methods), and carried out paired comparisons for individual neurons to confirm the conclusions of the population analysis. For the 28 neurons tested in both the memory-guided saccade task and cue-out stable array task, median time of tuning was 90 ms on the former versus 460 ms on the latter (Fig. 7A; P < 10−6, Wilcoxon signed-rank test, n = 18; 10 neurons did not achieve constant tuning by our criteria in the stable-array task during the cue-aligned epoch). For the 14 neurons tested in both cue-out and cue-in stable-array tasks, median time of tuning was 300 ms before the saccade for the former and 190 ms before the saccade for the latter (Fig. 7B; n = 14; P < 0.05, Wilcoxon signed-rank test). [In the cue-aligned interval, median times of tuning were 330 versus 530 ms (P = 0.059, Wilcoxon signed-rank test); however, four neurons did not become tuned during this interval in one or both conditions.]
Figure 7.
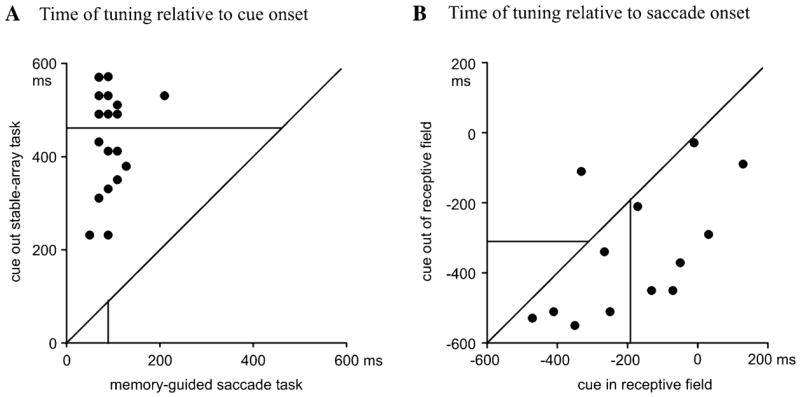
Time of tuning of individual neurons. Each point represents the time bin when an individual neuron became consistently tuned in the task indicated by the axis label. Lines orthogonal to the axes show sample medians.
In addition to its delayed time course, directional tuning also tended to remain broader (lower tuning indices) on the cue-out task than on the memory-guided saccade task (highest tuning indices), and slightly broader still on the cue-in task relative to the cue-out task, up until the time of the saccade. These tendencies remained just shy of statistical significance. In paired comparisons for individual neurons, median tuning indices in the 100 ms before saccade onset were 0.42 on the memory-guided saccade task versus 0.35 on the cue-out stable-array task (n = 28 neurons, P = 0.08, Wilcoxon signed-rank test) and 0.35 on the cue-out task versus 0.28 on the cue-in task (n = 14 neurons, P = 0.08, Wilcoxon signed-rank task). The substrate for both trends can be appreciated in the population histograms (Fig. 4), which show that responses before off-direction saccades tended to be stronger on the stable-array task than on the memory-guided saccade task and slightly stronger on the cue-in task than on the cue-out task.
Relationship to Saccade Metrics
We asked if the simultaneous presence of two loci of activity in LIP in some way affected the monkey’s saccades. If activity at the two loci is averaged according to a population vector hypothesis, we should find that the endpoints of saccades in the stable-array task would shift slightly toward the location of the cue. To determine if this was the case we compared the endpoints of saccades directed to each of the stable targets in cue-out versus cue-in trials. For a given saccade direction, cue-out and cue-in trials were identical except for the location of the cue. If saccades were systematically shifted in the direction of the cue, we would expect to find differences in the landing position or endpoint error between the two conditions. However, we found no such differences. Of 224 pairwise comparisons of the spatial coordinates of saccade endpoints in cue-in and cue-out trials (14 neurons × 8 target locations × 2 endpoint coordinates), only four (1.8%) reached statistical significance (Wilcoxon signed-rank test, P < 0.05), representing a proportion smaller than would be expected by chance. Median saccade endpoint error (the distance between saccade endpoint and target) was 13.4% of target eccentricity in cue-out trials versus 14.1% in cue-in trials (compared with 15.0% the memory-guided saccade task; P > 0.05 for each pairwise comparison, Wilcoxon rank-sum test). The standard deviations of saccade endpoints also did not differ between the two versions of the task (evaluated across all experiments and directions, P = 0.12 and P = 0.25 for x and y coordinates, respectively). To further evaluate this result, we examined specifically saccades made to the target in the center of each neuron’s receptive field. We reasoned that, in cue-in trials, this saccade target was very close to the cue (as they were both in the receptive field), and thus the presence of cue-related activity should cause only a minimal deviation in saccade direction. In cue-out trials, on the other hand, the cue was much farther from this saccade target, and thus a much larger deviation may be found. However, none of the four comparisons that reached statistical significance fell into this class. The standard deviations of saccade endpoints also did not differ between the two versions of the task (evaluated across all neurons for saccades to the receptive field center, P = 0.15 and P = 0.32 for x and y coordinates, respectively).
Finally, we asked whether such a shift in saccade coordinates may have existed earlier in the trial, when the response to the cue was stronger. We therefore repeated our comparison (including all saccade directions) for saccades initiated between 500–750 ms after cue onset. For these saccades, we also found no differences in endpoints between cue-in and cue-out trials (P = 0.12 and 0.18 for x and y coordinates). Thus changing the location of the cue affected neither the spatial distribution nor the variance of saccade endpoints.
As a final test of a possible relation between LIP activity and saccade metrics, each neuron’s presaccadic responses for saccades to the preferred target were fit with a linear regression model with saccade latency, saccade endpoint error and saccade velocity as regressors (see Materials and Methods). Fewer than 5% of neurons tested in each task yielded significant (P < 0.05) regression coefficients for any of these regressors.
Error Trials
Figure 8 shows population tuning indices computed from error trials in which the monkey made a saccade to a stable object other than the one instructed by the cue. In Figure 8A we computed population tuning indices by classifying trials according to the direction of the saccade the monkey actually made (thick traces) and also by classifying trials according to the direction of the saccade that had been required by the cue (thin traces). Significant tuning arose only in the former case, showing that LIP activity largely reflected the direction of the actual, (erroneous) saccade, rather than the direction of the required saccade.
Figure 8.
Population tuning indices for correct and error trials on the stable-array task. (A) Tuning index computed from error trials on the cue-out task by classifying each trial according to the saccade the monkey actually made (thick line, black circles) and according to the saccade required by the cue (thin line, gray circles). (B) Tuning indices on correct and error (actual saccade) trials. Black stars show time bins when tuning indices differed significantly between correct and error trials.
In Figure 8B we compare population tuning for correct versus erroneous saccades in each version of the stable-array task. The final levels of tuning were comparable on correct and error trials on both versions of the task. However, directional tuning arose more slowly on error trials on the cue-out task, with consistent tuning developing only ~400 ms before saccade onset.
Discussion
When faced with a stable visual scene, LIP neurons have only weak responses even when there is ample visual stimulation to their receptive field (Gottlieb et al., 1998). Instead, neurons selectively represent objects that are of immediate behavioral importance — either by virtue of task demands or by virtue of an intrinsic property such as an abrupt onset. In their selectivity LIP neurons appear similar to neurons in neighboring area 7a (Constantinidis and Steinmetz, 2001), suggesting that the construction of salience representations may be a general role of posterior parietal areas.
In this paper we have analyzed the target-selective activity in LIP independently of the visual on-response. We show that the saccade-target response does not require a prior onset transient, but in the absence of such a transient the response develops with a much slower time course: spatial tuning for the saccade target arose in LIP at ~200 ms after cue onset, as opposed to the 40–60 ms latency of the onset transient (Bisley et al., 2004). A second finding is that, although highly selective, the salience representation in LIP is not limited to one object at a time (see also Gottlieb and Goldberg, 1999; Powell and Goldberg, 2000; Bisley and Goldberg, 2003). In the present task, while neurons whose receptive field overlapped only the saccade target were responding robustly to this target, neurons whose receptive field overlapped the cue but not the saccade target were still responding strongly to the cue’s onset. This indicates a certain degree of parallel processing in LIP, and rules out strictly serial schemes, in which a response representing one spatial locus must cease before activity at another locus can arise. Thus LIP does not represent the end result of a winner-take-all selection, but may very well provide the data from which some other area can make the selection.
The coexistence of two interacting loci of activity raises the question of how spatial information is read out from a representation with multiple peaks. A popular hypothesis is that information about spatial location is read using a population vector, which identifies the center of mass of activity across the entire ensemble of tuned neurons (e.g. Schwartz and Moran, 2000). If two loci are simultaneously active, the population vector would point to a location intermediate between the two. However, our results do not support this hypothesis, as we failed to detect any influence of cue location on saccade endpoints in our task. Similarly, in an antisaccade task monkeys do not make erroneous saccades between cue and target location (Gottlieb and Goldberg, 1999), and a distractor flashed during the delay period on a memory-guided saccade task does not affect the accuracy of the ensuing saccade even though the distractor may still evoke significant activity even at the time of saccade onset (Powell and Goldberg, 2000). (We must reiterate here the fact that, while the method we used to compute the tuning index is similar to that used to derive a population vector, our tuning index does not represent a population vector for the entire ensemble of LIP neurons because it was computed separately for different populations of neurons. In fact, because in our task there was not a fixed relationship between the locations of the cue and of the saccade target, we cannot compute a true population vector from this data.) Thus, more sophisticated coding schemes must be used by the brain to allocate attention based on LIP activity. One possibility is that activity on the salience map is read out by a winner-takes-all mechanism, whereby the locus associated with the highest response guides attention at any given time (Bisley and Goldberg, 2003). If this hypothesis is correct, it predicts that in our task, attention in the stable-array task swings from the cue to the saccade target at ~300 ms after cue onset (Fig. 5). Yet another possibility is that attentional allocation depends not only on area LIP but also on other areas carrying information about object identity. While area LIP serves as a relatively undifferentiated salience map for all potentially significant events, information regarding the identity of each event (e.g. whether a salient stimulus is or is not a saccade target) must also be integrated with information from LIP before determining the locus of attention.
Across our tasks, tuning for the saccade target appeared correlated with task difficulty — with tuning appearing fastest and being sharpest on the easiest task — the memory-guided saccade task. The memory-guided saccade task benefited from an unambiguous onset transient which identified the target. Onset transients, however, are rare in the real world, and, as we have previously shown, the entrance of a stable stimulus into a receptive field in LIP by virtue of a saccade is not equivalent to its entrance by an abrupt onset (Gottlieb et al., 1998). The relatively late development of the tuned response in the cue-out task may, in fact, be the norm as the monkey explores its usual environment. Hints as to the functional significance of the saccade-target response can be found from our analysis of error trials. On the cue-out task tuning was significantly delayed on error relative to correct trials. Thus, the earliest signal of saccade target selection, seen in neurons that are not activated by the cue, was absent if the monkey made an error. This suggests that the target-selective response in LIP may be the result of two processes: an early process, operating ~200–400 ms after cue onset in our task, which is in fact critical for saccade target selection, and a later process, which merely reflects the saccade the monkey has committed to whether correctly or in error. Future experiments can address this possibility by selectively interfering with either the early or the late delay period activity in LIP.
Acknowledgments
We are grateful to the staff of the Laboratory of Sensorimotor Research for their help in all phases of this work: T. Ruffner and J. Nichols for machining; L. Jensen for electronics; Dr James Raber for veterinary care; D. Ahrens and Brian Keegan for veterinary technical help; A. Hays for computer programming and maintenance; M. Smith for histology; and J. Steinberg and B. Harvey for facilitating everything. This work was supported by the National Eye Institute.
References
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991a;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties. J Neurophysiol. 1991b;66:1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular statistics in biology. New York: Academic Press; 1981. [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intra-parietal area and spatial attention. Science. 2003;299:54–56. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;23:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel J-R, Goldberg ME. Visual, presaccadic and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple-stimulus displays: I. neurons encode the location of the salient stimulus. Cereb Cortex. 2001;11:581–591. doi: 10.1093/cercor/11.7.581. [DOI] [PubMed] [Google Scholar]
- Goldberg MEBJ, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann NY Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc. 1982;2:1–10. [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol. 2000;84:301–310. doi: 10.1152/jn.2000.84.1.301. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Schwartz AB, Moran DW. Arm trajectory and representation of movement processing in motor cortical activity. Eur J Neurosci. 2000;12:1851–1856. doi: 10.1046/j.1460-9568.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decisin in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304(5678):1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- Wardak C, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]