Abstract
Background
Nicotine and methamphetamine are both abused in similar settings, sometimes together. Because there are known interactions between central nicotinic acetylcholine receptors and dopamine receptors, it is of interest to characterize the nature of the interaction of these two compounds in vivo.
Methods
The purpose of this study was to characterize the extent to which these two compounds produce similar discriminative stimulus effects and to identify pharmacological mechanisms for their interaction. Male Sprague-Dawley rats were trained to discriminate methamphetamine or nicotine from saline. First, the ability of methamphetamine and nicotine to cross-substitute in rats trained to the other compound was tested. Subsequently, the ability of a dopamine antagonist (haloperidol) and a centrally-acting nicotinic antagonist (mecamylamine) to block the discriminative stimulus effects of methamphetamine and nicotine were also tested.
Results
Nicotine fully substituted in methamphetamine-trained rats, but methamphetamine only partially substituted in nicotine-trained rats. In nicotine-trained rats, mecamylamine fully antagonized the discriminative stimulus effects of nicotine, but haloperidol had no effect. The partial substitution of methamphetamine was partially attenuated by haloperidol, but not altered by mecamylamine. In methamphetamine-trained rats, mecamylamine failed to antagonize the discriminative stimulus effects of methamphetamine, but haloperidol fully blocked the methamphetamine cue. Mecamylamine blocked the ability of nicotine to substitute for methamphetamine, but haloperidol had no effect.
Conclusions
These results indicate that nicotine and methamphetamine share discriminative stimulus effects in some subjects and that the two compounds do not act at the same site, but produce their interaction indirectly. These findings suggest that these two compounds might be at least partially interchangeable in human users, and that there are potentially interesting pharmacological reasons for the commonly observed co-administration of nicotine and methamphetamine.
Keywords: methamphetamine, nicotine, mecamylamine, haloperidol, nicotinic acetylcholine receptor, dopamine receptor, co-abuse, drug-discrimination, rat
1. Introduction
Methamphetamine and nicotine are commonly used together (Goldsamt et al., 2005; Yen and Chong, 2006), yet the interaction of these two substances has been little studied. There are known interactions between central nicotinic acetylcholine receptors and dopamine receptors in the central nervous system e.g. (e.g., Quarta et al., 2006; Wonnacott et al., 2005), which provide possible mechanisms for the co-abuse of methamphetamine and nicotine. Because of this, it is of interest to characterize the nature of the interaction of these two compounds. Drug discrimination studies provide a useful tool to characterize the abuse liability of compounds, particularly with regard to their subjective effects (Balster, 1991; Stolerman, 1993). The subsequent sections will review the pharmacological mechanisms of the discriminative stimulus effects of methamphetamine and nicotine, and then possible mechanisms by which the two could interact.
1.1. Mechanisms of the discriminative stimulus effects of methamphetamine
Compounds that increase dopamine levels in the synapse (including dopamine releasers, uptake inhibitors, and D1-like and D2-like receptor agonists) all substitute for the discriminative stimulus effects of methamphetamine (Czoty et al., 2004; Munzar et al., 1999a; Munzar and Goldberg, 2000; Tidey and Bergman, 1998). Similarly, D1 and D2 receptor antagonists block the methamphetamine discriminative stimulus (Munzar and Goldberg, 2000; Tidey and Bergman, 1998). In contrast, adrenergic and serotonergic uptake inhibitors do not substitute for methamphetamine (Czoty et al., 2004; Munzar et al., 1999a; Munzar and Goldberg, 1999; Tidey and Bergman, 1998). Beta adrenergic agonists and antagonists fail to substitute or block the stimulus effects of methamphetamine (Munzar and Goldberg, 1999). Alpha-2 adrenergic compounds and various 5-HT agonists (5-HT1A, 5-HT2, 5-HT3) produce at best partial substitution or small leftward-shifts in the dose response for methamphetamine discrimination (Munzar and Goldberg, 1999; Munzar et al., 2002; Munzar et al., 1999b). These findings indicate that the discriminative stimulus effects of methamphetamine are mediated mostly by the dopamine system, as dopaminergic compounds have robust effects in subjects trained to discriminate methamphetamine, whereas adrenergic and serotonergic compounds have only a modest ability to alter the stimulus effects of methamphetamine.
1.2. Mechanism of the discriminative stimulus effects of nicotine
The discriminative stimulus effects of nicotine are primarily mediated by central nicotinic receptors, such that agonists at these receptors substitute in nicotine-trained rats, and mecamylamine blocks the discriminative stimulus effects of nicotine (Miyata et al., 1999; Rosecrans, 1989; Stolerman, 1988). Other receptors also contribute to the stimulus effects of nicotine, dopamine receptors producing the largest effect. In general, dopamine transport blockers and D1-like agonists including cocaine, GBR-12909, bupropion, nomifensine, apomorphine, and SKF-82958 have produced partial substitution for nicotine, as has d-amphetamine (Desai et al., 1999; Gasior et al., 1999; Mansbach et al., 1998). Some exceptions to this pattern of partial substitution have been noted. Cocaine did not substitute at all in squirrel monkeys trained to discriminate intravenous nicotine (Takada et al., 1988) and the dopamine uptake inhibitor bupropion fully substituted for the discriminative stimulus effects of nicotine in two studies (Wiley et al., 2002; Young and Glennon, 2002).
Dopamine receptor antagonists produced mixed results. Flupenthixol blocked the discriminative stimulus effects of nicotine (Desai et al., 2003), although SCH23390 and haloperidol had no effect (Corrigall and Coen, 1994; Mansbach et al., 1998). In contrast, D2 and D3 agonists produced little or no nicotine-appropriate responding (Le Foll et al., 2005; Mansbach et al., 1998) and D2 and D3 antagonists did not block the discriminative stimulus effects of nicotine (Corrigall and Coen, 1994; Le Foll et al., 2005; Mansbach et al., 1998). These findings suggest that increasing levels of dopamine by dopamine releasers or uptake inhibitors may play some sort of role in the discriminative stimulus effects of nicotine, but that nicotine most likely does not act directly at dopamine receptors. This raises the question of how nicotine and methamphetamine might interact if the effects of methamphetamine are primarily dopamine mediated, whereas those of nicotine are primarily mediated by nicotinic acetylcholine receptors. The next section will review evidence for interactions of the behavioral effects of nicotine and methamphetamine as well as possible neural mechanisms for the interaction.
1.3. Interactions between Methamphetamine and Nicotine
Although nicotine and methamphetamine are often co-abused, few behavioral studies on the combination of nicotine and methamphetamine have been conducted. Those few studies have focused on modulation of locomotor activity and self-administration. Repeated administration of methamphetamine and nicotine produced sensitization to their effects in locomotor activity, and produced a symmetrical cross-sensitization in mice (Kuribara, 1999). Chronic treatment with nicotine for 14 days increased locomotor activity and stereotypy induced by methamphetamine (Suemaru et al., 1993). Nicotine attenuated reinstatement of methamphetamine self-administration (Hiranita et al., 2004; Hiranita et al., 2006), and mecamylamine decreased methamphetamine self-administration in rats (Glick et al., 2002). In rats trained to self-administer nicotine, bupropion and methamphetamine increased self-administration of low doses of nicotine and decreased self-administration of high doses of nicotine (Rauhut et al., 2003). These findings indicate a definite interaction between the two compounds; in particular, that the two compounds produce cross-sensitization implies a similar mechanism. The question then arises of what is the mechanism by which a nicotinic agonist and a dopamine releaser can interact. Because both locomotor activity and self-administration are known to be related to mesolimbic and nigrostriatal dopamine systems, it is possible that co-administration of these compounds modulates levels of DA release, particularly as nicotine is well known to modulate dopamine release (Quarta et al., 2006; Wonnacott et al., 2005)
There have indeed been reports that nicotine and methamphetamine modulate each other’s ability to alter dopamine levels. Chronic treatment with nicotine increased release of dopamine in striatal slices (e.g., Takaki, 1995). In an in vivo microdialysis study in rats, nicotine did not alter dopamine levels in nucleus accumbens in naïve rats, but did increase dopamine levels in rats sensitized to nicotine. Methamphetamine produced smaller increases in dopamine levels in rats sensitized to nicotine, but basal levels of dopamine were higher to begin with (Miyata et al., 1996). Not only is there an interaction between presynaptic D2 and nicotinic acetylcholine receptors on their modulation of dopamine release, there is evidence that β2 subunits of the nicotinic acetylcholine receptors physically interact with the D2 receptors, creating a heteromeric receptor complex (Quarta et al., 2006). Taken together, these studies indicate that nicotine and methamphetamine can each modulate the other’s behavioral effects and that there are known interactions between central dopamine and nicotinic acetylcholine receptors that could plausibly serve as mediators of interactions of the behavioral effects of methamphetamine and nicotine.
1.4. Purpose of this study
The present study examined the effects of both nicotine and methamphetamine in rats trained to discriminate nicotine from saline or to discriminate methamphetamine from saline. First, the ability of methamphetamine to substitute in nicotine-trained rats and the ability of nicotine to substitute in methamphetamine-trained rats were tested. Then, the ability of a nicotinic (mecamylamine) and a dopaminergic (haloperidol) antagonist to block the discriminative stimulus effects of nicotine (in nicotine-trained rats) and methamphetamine (in methamphetamine-trained rats) was tested. Finally, the ability of the nicotinic and dopaminergic antagonists to block the ability of nicotine and methamphetamine to cross-substitute was tested. These experiments were conducted first to provide an assessment of the ability of nicotine and methamphetamine to modulate each other’s discriminative stimulus effects. Such an assessment could provide a beginning for understanding why they are often co-abused. Secondly, the experiments provide an assessment of the pharmacological mechanism for the interaction.
2. Methods
2.1. Subjects
Male Sprague-Dawley rats were obtained from Harlan-Sprague Dawley (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 20 g/day, which included the food received during operant sessions. Water was freely available. All housing and procedures were in accordance with the guidelines of the Institute of Laboratory Animal Resources, National Research Council (Institute of Laboratory Animal Resources, 1996) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.2. Discrimination training
Standard operant chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in MED-PC IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Rats were trained to discriminate methamphetamine (1 mg/kg) or nicotine (0.4 mg/kg) from saline using a two-lever choice methodology. Food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer under a fixed ratio 10 schedule when responding occurred on the injection appropriate lever. There was no consequence for incorrect responses. The rats received approximately 60 training sessions before use in any behavioral experiment. Animals were selected for use in experiments when they had met the criteria of emitting 85% of responses on the injection-correct lever for both the first fixed-ratio and during the remainder of the session during their last 10 training sessions.
Training sessions occurred in a double alternating fashion (D-D-S-S-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two saline or two drug training sessions). Rats were tested only if they had achieved 85% drug-lever responding for both first fixed-ratio and total session on the two prior training sessions. Before each session, the rats received an injection of either saline or training drug. Ten minutes later, the rats were placed in an operant chamber. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets.
2.3. Test procedures
For all test sessions, intraperitoneal injections of the antagonists or their vehicle occurred 5 min prior to administration of nicotine or methamphetamine. Intraperitoneal injections of the training dose of methamphetamine occurred 10 min prior to the start of the test session, and subcutaneous injections of nicotine occurred 15 min prior to the start of the session. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained, or for a maximum of 20 min. At least three days elapsed between test sessions. Groups of 9 or 10 rats were tested with each test compound. A repeated measures design was used, such that each rat was tested at all doses in ascending order. Only data from the first reinforcer are depicted. Doses of the test compound for which fewer than four rats completed the first fixed ratio were not considered in the characterization of discriminative stimulus effects.
In nicotine-trained rats, nicotine (0.025 to 0.4 mg/kg) and methamphetamine (0.1 to 2.5 mg/kg) were tested for the ability to substitute for the discriminative stimulus effects of 0.4 mg/kg nicotine. In methamphetamine-trained rats, nicotine (0.05 to 1 mg/kg) and methamphetamine (0.1 to 1 mg/kg) were tested for the ability to substitute for the discriminative stimulus effects of 1 mg/kg methamphetamine. Mecamylamine (0.1–10 mg/kg, i.p.) or haloperidol (0.05–0.5 mg/kg, i.p.) were tested for their ability to antagonize the discriminative stimulus effects of the training dose of methamphetamine (1 mg/kg) or nicotine (0.4 mg/kg). For studies of the antagonism of cross-substitution, haloperidol (0.025 or 0.25 mg/kg) or mecamylamine (0.1 or 0.5 mg/kg) were tested with 0.25 mg/kg nicotine in methamphetamine-trained rats, and haloperidol (0.05 or 0.5 mg/kg) or mecamylamine (0.25, 1 or 10 mg/kg) were tested with 1 mg/kg methamphetamine in nicotine-trained rats.
2.4. Drugs
Nicotine ditartrate and mecamylamine hydrochloride were purchased from Sigma Chemical Co (St. Louis, MO). Haloperidol was donated by Janssen Pharmaceutica (New Brunswick, NJ). (+)-Methamphetamine hydrochloride was obtained from the National Institute on Drug Abuse. Haloperidol was dissolved in 0.16% tartaric acid in deionized water. All remaining drugs were dissolved in 0.9% saline. The pH of the nicotine solution was titrated to approximately neutral with small amounts of 0.1N sodium hydroxide. The doses of nicotine are expressed as weight of base. Nicotine was administered s.c. in a volume of 1 ml/kg, and all remaining drugs were administered i.p. in a volume of 1 ml/kg.
2.5. Data Analysis
Drug discrimination data were expressed as the mean percentage of responses made on the drug-appropriate lever prior to completion of the first fixed ratio. Percent drug-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percent drug-appropriate responding was calculated for a given dose only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding, and partial substitution as ≥40% and <80% drug-appropriate responding. Full antagonism was defined as ≤20% drug-appropriate responding, and partial antagonism as ≤60% and >20% drug-appropriate responding. The potencies of nicotine and methamphetamine were calculated by fitting straight lines to the individual dose-response data for each compound by means of TableCurve 2D (Jandel Scientific, San Rafael, CA). Straight lines were fitted to the linear portion of dose-effect curves, defined by doses producing 20 to 80% of the maximal effect, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. The slopes of the dose effect curves were compared using parallel line procedures (Kenakin, 1997). Comparison of the ED50 values was performed by two-way analysis of variance. Individual differences were compared using the Bonferroni-corrected post hoc test. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data was analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the appropriate control value using a priori contrasts. Criterion for significance was set a priori at p<0.05.
3. Results
3.1. Nicotine-trained rats
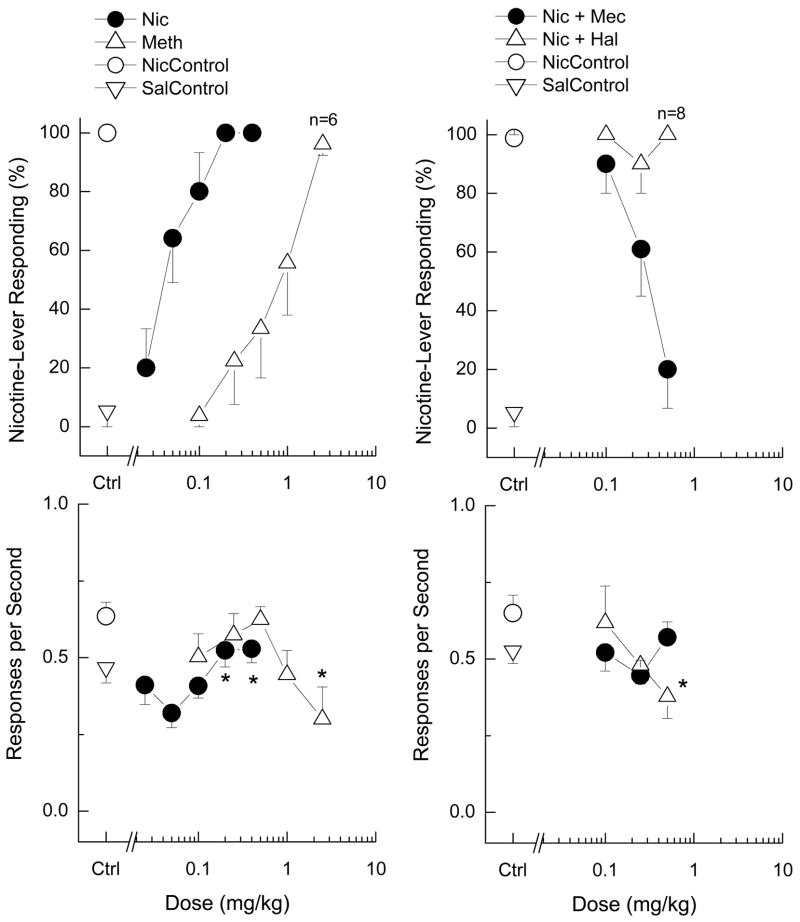
As shown in Figure 1 (left panels), nicotine (N=10) produced dose-dependent increases in nicotine-appropriate responding (ED50 = 0.059±0.008 mg/kg). Doses lower than the training dose of 0.4 mg/kg also produced full substitution (0.1 and 0.2 mg/kg). Nicotine produced an increase in response rate over that of the saline control at the 0.2 and 0.4 mg/kg doses [F(5,45)=2.79; p=0.028]. Methamphetamine (N=9) fully substituted for the training dose of nicotine (ED50 = 0.84±0.17 mg/kg). The full substitution was seen at a dose that produced depression of response rate [F(5,40)=2.73; p=0.032], and 3/9 rats failed to complete the first reinforcer.
Figure 1. Substitution and blockade of the discriminative stimulus effects of nicotine (0.4 mg/kg, i.p.).
Top panels show percent nicotine-lever appropriate responding. Bottom panels show rate of responding (r/s). Nicotine and methamphetamine fully substituted for the discriminative stimulus effects of nicotine (left panels). Nicotine increased response rates, whereas methamphetamine decreased rates. Asterisks show response rates different from vehicle control (p<0.05). Mecamylamine fully blocked the discriminative stimulus effects of nicotine, whereas haloperidol had no effect (right panels). Mecamylamine did not change response rates, whereas haloperidol dose-dependently decreased rates. N=10 except where shown. Asterisks indicate response rates different from nicotine control (p<0.05). Nic=nicotine; Meth=methamphetamine. Hal= haloperidol; Mec=mecamylamine. Ctrl=control.
Mecamylamine (N=10) fully antagonized the discriminative stimulus effects of nicotine (0.4 mg/kg), with the highest dose (0.5 mg/kg) reaching the 20% criterion for full antagonism (Fig. 1, right panels). Response rate was not affected [F(3,27)=0.81; p=0.498]. In contrast, haloperidol (N=10) failed to antagonize the discriminative stimulus effects of nicotine (0.4 mg/kg) and dose-dependently decreased response rates [F(3,27)=3.93; p=0.019]. Higher doses of haloperidol were not tested as they completely suppress response rates.
3.2. Methamphetamine-trained rats
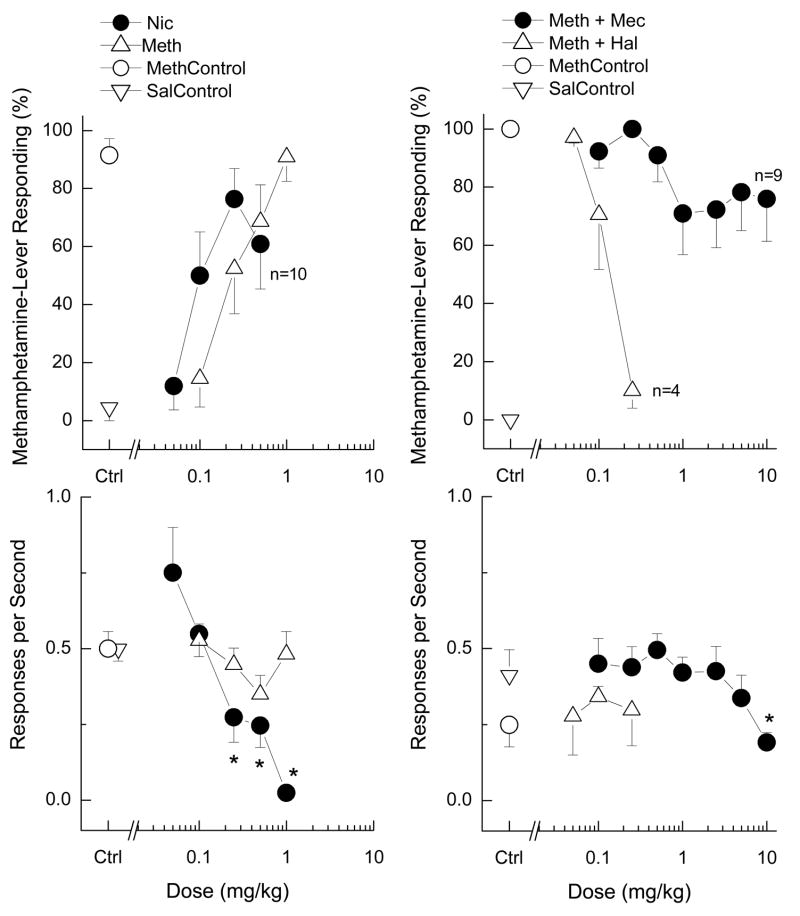
As shown in Figure 2 (left panels), methamphetamine (N=10) produced a dose-dependent increase in methamphetamine-appropriate responding (ED50 = 0.35±0.07 mg/kg), but did not significantly alter response rates [F(4,36)=1.48; p=0.228]. In contrast, nicotine (N=12) only partially substituted, producing a plateau at 60 to 77% methamphetamine-appropriate responding (ED50 = 0.12±0.02 mg/kg). Higher doses were not tested because 1 mg/kg nicotine produced convulsions. The ED50 values for the experiments differed, such that nicotine was more potent than methamphetamine [F(1,28)= 33.93; p<0.001], and each compound was more potent when substituting for itself than when cross-substituting [F(1,28)= 6.22; p=0.019]. Nicotine produced a dose-dependent decrease in response rate [F(5,55)=12.44; p<.001].
Figure 2. Substitution and blockade of the discriminative stimulus effects of methamphetamine (1 mg/kg, i.p.).
Top panels show percent methamphetamine-lever appropriate responding. Bottom panels show rate of responding (r/s). Methamphetamine fully substituted for the discriminative stimulus effects of methamphetamine, but nicotine produced only partial substitution (left panels). Methamphetamine did not alter response rates at the doses tested. Nicotine dose-dependently decreased response rates. Asterisks show response rates different from vehicle control (p<0.05). Haloperidol fully blocked the discriminative stimulus effects of methamphetamine, whereas mecamylamine produced only a small attenuation of drug-appropriate responding (right panels). Haloperidol did not change response rates, whereas mecamylamine decreased rates. N=10 except where shown. Asterisks indicate response rates different from nicotine control (p<0.05). Nic=nicotine; Meth=methamphetamine. Hal= haloperidol; Mec=mecamylamine. Ctrl=control.
Haloperidol (N=6) fully antagonized the discriminative stimulus effects of 1 mg/kg methamphetamine (AD50 = 0.13±0.02 mg/kg). In contrast, mecamylamine (N=10) produced very modest decreases in methamphetamine-appropriate responding (71 to 78%) which were not in the range for partial antagonism (Figure 2, right panels). Haloperidol did not alter response rate at the doses tested [F(3,12)=0.57, p=0.644]. Mecamylamine significantly decreased rates of responding at the highest dose tested to 44% of methamphetamine control [F(7,63)=2.43, p=0.029].
3.3. Antagonism of cross-substitution
3.3.1. Nicotine-trained rats
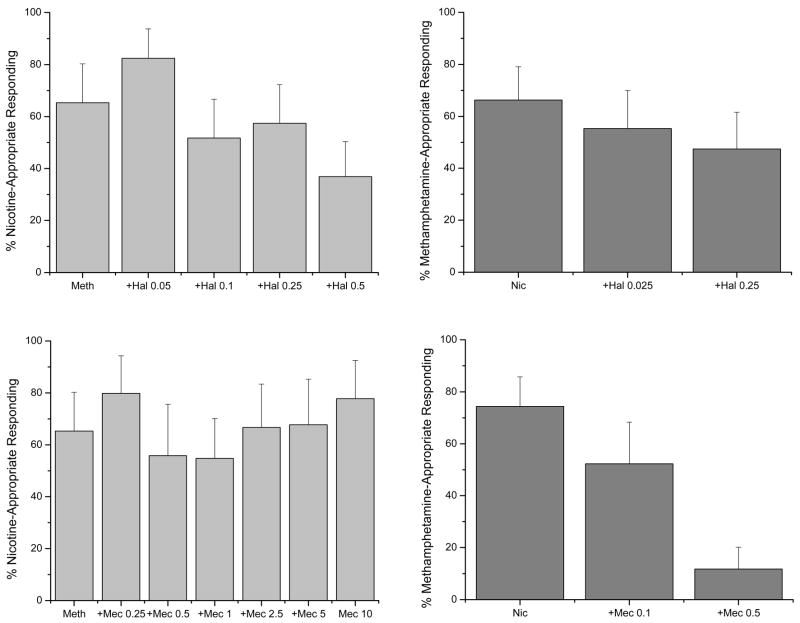
Haloperidol and mecamylamine in combination with the dose of methamphetamine that produced full substitution almost completely suppressed response rates (data not shown). For this reason, antagonism studies were conducted with 1.0 mg/kg methamphetamine (Figure 3, left panels). Methamphetamine (1 mg/kg) produced 65±15% nicotine-appropriate responding. Haloperidol (0.5 mg/kg) decreased nicotine-appropriate responding in the presence of 1 mg/kg methamphetamine to 37±13%. This was only a partial blockade (<60% and >20% nicotine-appropriate responding) of the ability of methamphetamine to substitute for nicotine. Haloperidol did not significantly alter response rates in comparison to methamphetamine control [F(4,36)=2.01; p=0.088]. The effects of mecamylamine (N=9) on methamphetamine substitution was not dose-dependent and nicotine-appropriate responding fluctuated between 56 and 80%. Mecamylamine did not depress response rates below that of methamphetamine alone [F(6,54)=1.36; p=0.246], although some rats did not complete the first fixed ratio at some doses (Figure 3).
Figure 3. Antagonism of cross-substitution.
Left panels show the effects of nicotine (0.25 mg/kg, s.c.) alone and in combination with mecamylamine and haloperidol in methamphetamine-trained rats. Nicotine partially substituted in methamphetamine-trained rats. The top panel shows haloperidol did not alter the effects of nicotine, whereas the bottom panel shows mecamylamine (0.1 and 0.5 mg/kg, i.p.) fully blocked the partial substitution of nicotine for methamphetamine. Right panels show the effects of methamphetamine (1 mg/kg, i.p.) alone and in combination with mecamylamine and haloperidol in nicotine-trained rats. The top panel shows haloperidol modestly attenuated the ability of methamphetamine to partially substitute for nicotine, whereas the bottom panel shows mecamylamine (0.25, 1 and 10 mg/kg, i.p.) did not alter the ability of methamphetamine to partially substitute for nicotine. Nic=nicotine; Meth=methamphetamine. Hal=haloperidol; Mec=mecamylamine.
Mecamylamine (10 mg/kg) when administered alone produced no nicotine-appropriate responding (3.0±3.0% DAR) and decreased response rate to 0.11±0.05 responses/s, which was significantly different from the saline control rate of 0.565±0.06 r/s [t=5.43, p<0.001]. Haloperidol (0.5 mg/kg) also produced no nicotine-appropriate responding (0%) when administered alone, and decreased response rate to 0.16±0.07 r/s, which was significantly different from the saline control rate of 0.42±0.07 r/s [t=2.66, p=0.008].
3.3.2. Methamphetamine-trained rats
As in the nicotine-trained rats, doses of haloperidol and mecamylamine in combination with nicotine (0.4 mg/kg) nearly completely suppressed response rates, so antagonism studies were conducted with 0.25 mg/kg nicotine. Nicotine (0.25 mg/kg) alone produced 74±11% methamphetamine-appropriate responding (Figure 2). Mecamylamine dose-dependently blocked the ability of nicotine to partially substitute for methamphetamine (12±8% following 0.5 mg/kg). In contrast, in a separate group of rats, haloperidol did not alter the level of methamphetamine-appropriate responding produced by 0.25 mg/kg nicotine at either dose (Figure 3). Mecamylamine also reversed the rate-decreasing effects of 0.25 mg/kg nicotine [F(2,18)= 7.98, p=0.003]. Haloperidol did not alter response rates [F(2,18)= 0.48, p=0.625].
Mecamylamine tested at doses from 0.1 to 10 mg/kg did not substitute for the discriminative stimulus effects of methamphetamine. Maximum methamphetamine-appropriate responding (35±16%) was observed following 10 mg/kg. Mecamylamine dose-dependently decreased response rates [F(7,63)=6.81, p<0.001]. The greatest effect (0.20±0.04 responses/s) was at 10 mg/kg, which was significantly different from the saline control rate of 0.49±0.08 r/s (p<0.05).
4. Discussion
4.1. Cross-substitution
In the present study, methamphetamine fully substituted for the discriminative stimulus effects of nicotine. This finding is in agreement with earlier findings that the dopamine uptake inhibitor bupropion also fully substituted for the discriminative stimulus effects of nicotine (Wiley et al., 2002; Young and Glennon, 2002), although other compounds that directly or indirectly activate dopamine receptors only produced partial substitution (Desai et al., 1999; Gasior et al., 1999; Mansbach et al., 1998). In contrast, nicotine only partially substituted for the discriminative stimulus effects of methamphetamine. It is significant to note that this finding was replicated in the present study by three separate groups of rats, one for the cross-substitution study and two for the antagonism experiments. In these experiments, nicotine produced 66 to 74% methamphetamine-appropriate responding. This observation has not been reported in the literature before. However, similar results have been reported for other psychostimulants. Nicotine fully substituted in rats trained to discriminate cocaine from saline (Desai et al., 1999; 2003), and potentiated the discriminative stimulus effects of d-amphetamine (Druhan et al., 1991), whereas monkeys and pigeons showed only partial substitution by nicotine (de la Garza and Johanson, 1983; 1985).
These findings suggest that there is an asymmetrical cross-substitution between methamphetamine and nicotine, such that administration of methamphetamine is sufficient to produce nicotine-like discriminative stimulus effects, whereas administration of nicotine is not sufficient to produce methamphetamine-like discriminative stimulus effects. The case for asymmetry should perhaps not be overstated, as earlier studies have reported both full and partial substitution by dopamine compounds in nicotine-trained subjects (Desai et al., 1999; Gasior et al., 1999; Mansbach et al., 1998) (Wiley et al., 2002; Young and Glennon, 2002) and both full and partial substitution by nicotine in other dopaminergic psychostimulants (de la Garza and Johanson, 1983; 1985; Desai et al., 1999; Desai et al., 2003). It is important to note that the degree of cross-substitution between methamphetamine and nicotine could be determined by training dose, as different patterns of stimulus control can be seen between groups trained to discriminate a high or a low dose of drug (Koek and Slangen, 1982; Stolerman et al., 1984). This effect is more pronounced when there are differences in mechanism or degree of efficacy between the compounds being tested, as is the case for methamphetamine and nicotine. That said, it is possible that methamphetamine is different from the other dopaminergic compounds and that the asymmetric cross-substitution is real. Alternatively, it is possible that the connection between nicotine and the dopaminergic compounds is of moderate strength, and that whether full or partial substitution is observed depends on individual differences among the subjects tested. Regardless of the degree, cross-substitution implies some sort of mechanistic connection between two compounds. The next section addresses possible mechanisms for the shared stimulus effects of nicotine and methamphetamine.
4.2. Mechanisms
In the present study, antagonism experiments were conducted to clarify the mechanism for the cross-substitution of nicotine and methamphetamine. Mecamylamine was used to probe for activity at central nicotinic acetylcholine receptors. Not surprisingly, mecamylamine blocked the discriminative stimulus effects of nicotine in the nicotine-trained rats, in agreement with earlier findings (Miyata et al., 1999; Rosecrans, 1989; Stolerman, 1988). Mecamylamine produced a small attenuation of the discriminative stimulus effects of methamphetamine in the methamphetamine-trained rats, but produced no effect on methamphetamine cross-substitution in the nicotine-trained rats. It should be noted that mecamylamine binds to NMDA receptors at concentrations higher than those necessary to produce nicotinic receptor mediated effects (Reynolds and Miller, 1988), and that NMDA antagonists facilitate the discriminative stimulus effects of methamphetamine (Gatch and Pratt, 2006). These findings indicate that nicotinic acetylcholine receptors do not contribute to the discriminative stimulus effects of methamphetamine as the small attenuation produced by large doses of mecamylamine in the present study were likely mediated by non-nicotinic receptors.
The non-selective dopamine antagonist haloperidol was used to test the contribution of dopamine receptors. Haloperidol blocked the discriminative stimulus effects of methamphetamine in methamphetamine-trained rats in agreement with earlier findings (Munzar and Goldberg, 2000; Tidey and Bergman, 1998). In contrast, the dopamine antagonist haloperidol did not alter the effects of nicotine in either the nicotine- or the methamphetamine-trained rats. These findings are not surprising given earlier studies that reported haloperidol had no effect on the discriminative stimulus effects of nicotine (Corrigall and Coen, 1994; Mansbach et al., 1998). What was unexpected was that haloperidol failed to fully antagonize the cross-substitution of methamphetamine in nicotine-trained rats. Haloperidol dose-dependently decreased nicotine-appropriate responding following methamphetamine from 65% to 37%. This is certainly not full antagonism of methamphetamine as seen with in the methamphetamine-trained rats. There are a number of possibilities of why haloperidol did not fully block the effects of methamphetamine in nicotine-trained rats. It is possible that haloperidol was less potent in the nicotine-trained rats, which may have resulted in the rate-decreasing effects preventing observation of full antagonism. Alternatively, the partial substitution of methamphetamine for nicotine may be mediated by more than just the dopamine system. Again, it should be noted that training dose could be an important factor in determining the degree of sensitivity of the assay, such that increased or decreased degrees of antagonism could be produced by altering training doses. Additional experimentation will be necessary to clarify why haloperidol had such a weak effect.
Taken together, these findings suggest that nicotine produced its discriminative stimulus effects in both the nicotine- and the methamphetamine-trained rats through its actions at central nicotinic acetylcholine receptors and not through any direct action at dopamine receptors. Likewise, methamphetamine produced its discriminative stimulus effects in both the nicotine-and the methamphetamine-trained rats through its effects on dopamine release, and that methamphetamine does not produce its discriminative stimulus effects through actions at central nicotinic acetylcholine receptors. What then mediates the interaction of nicotine and methamphetamine? The most obvious possibility is that nicotinic acetylcholine receptors are located on dopaminergic neurons in the ventral tegmental area which are important in regulating neuronal activity of these dopaminergic neurons (Mameli-Engvall et al., 2006; Ungless and Cragg, 2006). Administration of nicotine modulates dopamine release (Quarta et al., 2006; Wonnacott et al., 2005), and is known to alter behaviors controlled by these dopaminergic pathways including locomotor behavior (Kuribara, 1999; Suemaru et al., 1993) and self-administration of abused compounds, including both nicotine and methamphetamine (Glick et al., 2002; Hiranita et al., 2004; Hiranita et al., 2006; Rauhut et al., 2003). The failure of mecamylamine to block the cross-substitution of methamphetamine in nicotine-trained rats may be related to the finding that perfusion of mecamylamine into the nucleus accumbens did not alter dopamine levels in the ventral tegmental area or the nucleus accumbens (Rahman and McBride, 2002). In addition, there is now evidence that β2 subunits of nicotinic acetylcholine receptors create a heteromeric receptor complex with dopamine D2 receptors (Quarta et al., 2006). This hetereomeric autoreceptor decreases dopamine release, but as yet, the functional significance of this autoreceptor to dopamine-related behaviors has not been investigated. These findings do not confirm that alterations in dopamine levels in the mesocortical pathway mediates the behavioral interaction of nicotine and methamphetamine, but provides a plausible starting place for further research.
4.3. Conclusions
Typically, the effects of single drugs of abuse have been studied, even though they are rarely used singly. Nicotine is commonly used in combination with methamphetamine (Goldsamt et al., 2005; Yen and Chong, 2006), so there is a need for understanding the interactions between the two drugs of abuse. In the present study, nicotine and methamphetamine produced similar discriminative stimulus effects, such that methamphetamine fully substitutes for the discriminative stimulus effects of nicotine, and nicotine partially substitutes for the discriminative stimulus effects of methamphetamine. The mechanism for the cross-substitution between nicotine and methamphetamine is evidently some modulation of dopamine levels, directly by methamphetamine, or indirectly by nicotinic acetylcholine receptors in the case of nicotine. Given that both of these compounds modulate self-administration of the other compound (Glick et al., 2002; Hiranita et al., 2004; Hiranita et al., 2006; Rauhut et al., 2003), it is likely that people co-abuse nicotine and methamphetamine because of modulation of their stimulus and reinforcing effects, not just because both compounds are readily available in the same contexts. It is possible that people take the two compounds together because they modify the effects of each other to produce heightened effects or reduce unpleasant side-effects. Clearly, further clinical and mechanistic studies of the interaction of these two commonly abused substances is warranted.
Acknowledgments
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse (NIH N01DA-2-8822) at whose request the nicotine substitution for methamphetamine-trained rats was tested. The ATDP had no further role in study design; the collection, analysis and interpretation of data; or the writing of the report. They have granted permission for the submission of this data for publication.
Footnotes
Contributors:
Drs. Gatch and Forster, and Ms. Flores designed the study. Ms. Flores and Dr. Gatch collected data and performed statistical analyses. Dr. Gatch managed the literature searches and summaries of previous related work, and wrote the first draft of the manuscript. All authors contributed to have approved the final manuscript.
Conflict of Interest:
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balster RL. Drug abuse potential evaluation in animals. British Journal of Addiction. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacology Biochemistry and Behavior. 1994;48:817–820. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ramanathan CR, Mutschler NH, Makriyannis A, Bergman J. Drug discrimination in methamphetamine-trained monkeys: effects of monoamine transporter inhibitors. J Pharmacol Exp Ther. 2004;311:720–727. doi: 10.1124/jpet.104.071035. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacology Biochemistry and Behavior. 1983;19:145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology. 1985;85:23–30. doi: 10.1007/BF00427317. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behavioural Pharmacology. 1999;10:647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology. 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1053–1073. [PubMed] [Google Scholar]
- Gatch MB, Pratt R. Ethanol modulates the discriminative stimulus effects of methamphetamine. In: Toolaney GH, editor. New Research on Methamphetamine Abuse. Nova Science Publishers, Inc.; Hauppauge, NY: 2006. [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Eur J Pharmacol. Vol. 448. 2002. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors; pp. 185–191. [DOI] [PubMed] [Google Scholar]
- Goldsamt LA, O’Brien J, Clatts MC, McGuire LS. The relationship between club drug use and other drug use: a survey of New York City middle school students. Subst Use Misuse. 2005;40:1539–1555. doi: 10.1081/JA-200066886. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Anggadiredja K, Fujisaki C, Watanabe S, Yamamoto T. Nicotine attenuates relapse to methamphetamine-seeking behavior (craving) in rats. Ann N Y Acad Sci. 2004;1025:504–507. doi: 10.1196/annals.1316.062. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Koek W, Slangen JL. The role of fentanyl training dose and of the alternative stimulus condition in drug generalization. Psychopharmacology. 1982;76:149–156. doi: 10.1007/BF00435269. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Does nicotine modify the psychotoxic effect of methamphetamine? Assessment in terms of locomotor sensitization in mice. J Toxicol Sci. 1999;24:55–62. doi: 10.2131/jts.24.55. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Freedland CS. The role of monoamine neurotransmitter systems in the nicotine discriminative stimulus. Drug and Alcohol Dependence. 1998;52:125–134. doi: 10.1016/s0376-8716(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Miyata H, Ando K, Yanagita T. [Comparison of the effects of nicotine and methamphetamine on extracellular dopamine in the nucleus accumbens of behaviorally sensitized rats] Nihon Shinkei Seishin Yakurigaku Zasshi. 1996;16:41–47. [PubMed] [Google Scholar]
- Miyata H, Ando K, Yanagita T. Medial prefrontal cortex is involved in the discriminative stimulus effects of nicotine in rats. Psychopharmacology. 1999;145:234–236. doi: 10.1007/s002130051054. [DOI] [PubMed] [Google Scholar]
- Munzar P, Baumann MH, Shoaib M, Goldberg SR. Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology. 1999a;141:287–296. doi: 10.1007/s002130050836. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 1999;143:293–301. doi: 10.1007/s002130050950. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Goldberg SR. Differential involvement of 5-HT2A receptors in the discriminative-stimulus effects of cocaine and methamphetamine. European Journal of Pharmacology. 2002;436:75–82. doi: 10.1016/s0014-2999(01)01598-9. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Novakova J, Goldberg SR. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. Journal of Pharmacology and Experimental Therapeutics. 1999b;291:239–250. [PubMed] [Google Scholar]
- Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Franco R, Wise RA, Goldberg SR, Hope BT, Woods AS, Ferre S. Heteromeric Nicotinic Acetylcholine-Dopamine Autoreceptor Complexes Modulate Striatal Dopamine Release. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- Rahman S, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–654. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Miller RJ. [3H]MK801 binding to the N-methyl-D-aspartate receptor reveals drug interactions with the zinc and magnesium binding sites. J Pharmacol Exp Ther. 1988;247:1025–1031. [PubMed] [Google Scholar]
- Rosecrans JA. Nicotine as a discriminative stimulus: a neurobiobehavioral approach to studying central cholinergic mechanisms. Journal of Substance Abuse. 1989;1:287–300. [PubMed] [Google Scholar]
- Stolerman IP. Characterization of central nicotinic receptors by studies on the nicotine cue and conditioned taste aversion in rats. Pharmacology Biochemistry and Behavior. 1988;30:235–242. doi: 10.1016/0091-3057(88)90451-0. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Drug discrimination. In: Van Haaren F, editor. Methods in Behavioral Pharmacology. Elsevier; Amsterdam: 1993. pp. 217–243. [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Gomita Y, Furuno K, Araki Y. Chronic nicotine treatment potentiates behavioral responses to dopaminergic drugs in rats. Pharmacol Biochem Behav. 1993;46:135–139. doi: 10.1016/0091-3057(93)90329-r. [DOI] [PubMed] [Google Scholar]
- Takada K, Hagen TJ, Cook JM, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous nicotine in squirrel monkeys. Pharmacology Biochemistry and Behavior. 1988;30:243–247. doi: 10.1016/0091-3057(88)90452-2. [DOI] [PubMed] [Google Scholar]
- Takaki T. [Chronic treatment with nicotine enhances the sensitivity of dopamine autoreceptors that modulate dopamine release from the rat striatum] Nihon Shinkei Seishin Yakurigaku Zasshi. 1995;15:335–344. [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. Journal of Pharmacology and Experimental Therapeutics. 1998;285:1163–1174. [PubMed] [Google Scholar]
- Ungless MA, Cragg SJ. A choreography of nicotinic receptors directs the dopamine neuron routine. Neuron. 2006;50:815–816. doi: 10.1016/j.neuron.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Martin BR, Damaj MI. Nicotine-like discriminative stimulus effects of bupropion in rats. Experimental and Clinical Psychopharmacology. 2002;10:129–135. doi: 10.1037//1064-1297.10.2.129. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Yen CF, Chong MY. Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr Psychiatry. 2006;47:215–220. doi: 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. European Journal of Pharmacology. 2002;443:113–118. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]