Abstract
p55 is a common component of many chromatin-modifying complexes and has been shown to bind to histones. Here, we present a crystal structure of Drosophila p55 bound to a histone H4 peptide. p55, a predicted WD40 repeat protein, recognizes the first helix of histone H4 via a binding pocket located on the side of a β-propeller structure. The pocket cannot accommodate the histone fold of H4, which must be altered to allow p55 binding. Reconstitution experiments show that the binding pocket is important to the function of p55-containing complexes. These data demonstrate that WD40 repeat proteins use various surfaces to direct the modification of histones.
Keywords: p55, Nurf55, RbAp48, histone, chromatin, WD40
In eukaryotic cells, DNA is hierarchically packaged into higher order structures called chromatin. The basic unit of chromatin is the nucleosome, which is formed from 146 base pairs of DNA wrapped around a histone octamer. Chromatin is a dynamic structure and can be modified in several ways (Li et al. 2007). First, chromatin is actively assembled and disassembled by histone chaperone complexes. These assembly and disassembly processes are tightly coupled with DNA replication and gene expression (Groth et al. 2007). Second, chromatin can be covalently modified; N- or C-terminal tails of histones can be methylated, acetylated, phosphorylated, adenylated, or ubiquitylated. These modifications can be utilized as marks for recruiting effector proteins and might also directly alter chromatin folding. Third, chromatin structure is altered by ATP-dependent chromatin remodeling complexes, which can alter DNA accessibility by disrupting DNA–histone contacts.
p55 (p55 or Nurf55 in fly, RbAp48/46 in human, and MSI1 in plants) is highly conserved from plants to human. RbAp48/46 was initially identified as a retinoblastoma-associated protein (Huang et al. 1991; Qian et al. 1993). Subsequent studies showed that p55 is a common component of many different chromatin-modifying complexes with a variety of functions. p55 is the smallest subunit in the Chromatin Assembly Factor 1 (CAF1) complex as well as a component of the ATP-dependent chromatin remodeling complexes—Nucleosome Remodeling Factor (NURF), and Nucleosome Remodeling and Deacetylase (NuRD) (Smith and Stillman 1989; Tyler et al. 1996; Verreault et al. 1996; Martinez-Balbas et al. 1998; Wade et al. 1998; Zhang et al. 1998). p55 is a component of acetyltransferase (Hat1), histone deacetylase (HDAC1), and his-tone methyltransferase (Polycomb-Repressive Complex2, PRC2) complexes, and it has been shown that p55 is critical for the function of these complexes (Parthun et al. 1996; Taunton et al. 1996; Hassig et al. 1997; Zhang et al. 1997; Verreault et al. 1998; Czermin et al. 2002; Kuzmichev et al. 2002; Muller et al. 2002). Furthermore p55 copurifies with additional complexes involved in gene regulation (Zhang et al. 1997; Korenjak et al. 2004).
Depleting p55 causes a variety of epigenetic defects (Lu and Horvitz 1998; Hayashi et al. 2004; Taylor-Harding et al. 2004; Guitton and Berger 2005).
In Caenorhabditis elegans, LIN53, the ortholog of p55, shows a multivulva (Muv) phenotype resulting from misexpression of vulval cell fates (Lu and Horvitz 1998). The Arabidopsis ortholog, MIS1, has been extensively characterized, and loss of function mutants cause defects in ovule development and dysregulation of flowering time (Hennig et al. 2003; Kohler et al. 2003; Bouveret et al. 2006). Although the developmental role of p55 in vertebrates and insects is not clear, high expression of RbAp48/46 in the ovary and testis might reflect a function in mammalian gametogenesis (Qian and Lee 1995). Since p55 is required for the function of several essential complexes, it is difficult to define the action of p55 in vertebrates and insects using standard genetics. However, definition of the precise structural interactions of p55 might eventually lead to the design of specific mutations that disrupt distinct functions.
p55 contains WD40 repeats that are predicted to form a β-propeller structure (Ach et al. 1997). The WD40 repeat is a well-known protein–protein interaction domain, and WD40-containing proteins are involved in signal transduction, protein degradation, and gene regulation. Previous in vitro pull-down experiments demonstrated that in the context of Hat1, p55 interacts with the first helix of histone H4, which is buried in the canonical nucleosome structure (Verreault et al. 1998). This is in contrast to the way in which another WD40 repeat protein,WDR5, binds to histone H3. In that complex, it is the exposed tail of histone H3 that is recognized by the surface of the β-propeller structure of WDR5 (Wysocka et al. 2005; Couture et al. 2006; Han et al. 2006; Ruthenburg et al. 2006; Schuetz et al. 2006).
p55 has remarkable sequence conservation and is involved in diverse epigenetic processes, suggesting that it might serve an essential biological role in each complex in which it is found. However, the molecular and structural basis of histone recognition by p55 remains undefined. Moreover, it is not known what functional role(s) p55 plays in p55-containing complexes or how one protein can be integral in so many distinct complexes. Here we present the crystal structures of free p55 and of p55 bound to a histone H4 peptide. These structures reveal that p55 recognizes histone H4 via a binding pocket located on the side of a β-propeller structure. p55 appears to be preorganized for histone binding, as the free and histone H4-bound structures are superimposable. However, the structure shows that the histone fold of H4 has to be altered substantially upon p55 binding. In addition, the H4-binding pocket is critical for the activity of p55-containing complexes and may be used in different ways in different complexes. These and other data suggest that the WD40 repeat domain can utilize various surfaces for recognizing histones and that p55 may serve as a multifunctional protein interaction platform for the complexes.
Results and Discussion
Crystal structure of p55
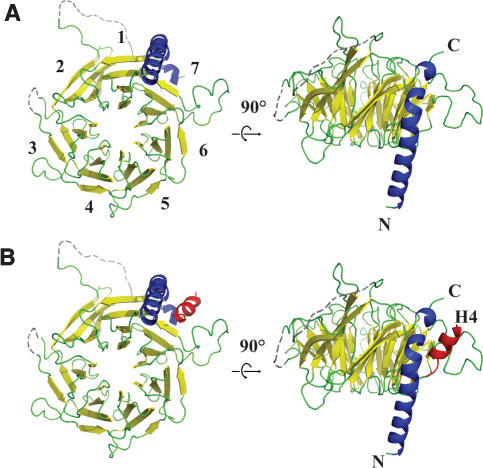
The crystal structure of isolated Drosophila p55 was determined to 2.9 Å using Single Anomalous Dispersion (SAD) (Supplemental Table 1). There was one molecule per asymmetric unit with a relatively high solvent content (81%). The crystal lattice was stabilized by protein–metal (Cd2+) interactions. The structure of p55 encompasses seven WD40 repeats forming a β-propeller structure with an additional α-helix at the N terminus (Fig. 1A). The β-propeller structure of p55 is similar to other known β-propeller structures. In fact, the presence of an N-terminal α-helix is highly reminiscent of the β subunit of the G protein (Sondek et al. 1996). The N-terminal helix of p55 is situated between the first and seventh blades of the β-propeller structure, spans ∼40 Å, and is nearly parallel to the sevenfold symmetric axis of the WD40 repeats. Interestingly, a C-terminal one turn-helix is positioned on top of the N-terminal α-helix, forming an extended helix.
Figure 1.
Crystal structures of p55 and a complex with histone H4 peptide. (A) Ribbon diagram of the p55 crystal structure. p55 contains WD40 repeats forming a seven-bladed β-propeller structure (yellow) with an additional α-helix at the N terminus (blue). Disordered loops were connected and are shown in dashed lines. (B) Ribbon diagram of the p55 bound with histone H4 peptide. Histone H4 (31KPAIRRLARRG41, shown in red) is bound at the side of the β-propeller structure and between the N-terminal α-helix (blue) and the binding loop coming from the seventh blade of the structure.
An interesting feature of the p55 structure is the presence of long connecting loops, which extend from the β-strands and protrude far from the core before connecting back to the β-propeller structure. The loops in the second and seventh blades are particularly long. Unfortunately, the 27-amino-acid-long connecting loop in the second blade is disordered in the structure. However, the loop in the seventh blade is composed of 16 amino acids, and is well ordered. This loop protrudes from the side of the β-propeller structure and forms one side of a pocket, with the other side formed from the N-terminal α-helix. Thus, in addition to the predicted canonical β-propeller structure, p55 contains two unusual features: the N-terminal helix and the long connecting loops.
Crystal structure of p55 bound to histone H4 peptide and histone recognition
Previous work demonstrated that p55 binds to histone H4 (Verreault et al. 1998). To gain insight into how p55 recognizes histone H4, p55 was cocrystallized with a histone H415–41 peptide. The p55–H415–41 complex structure was determined to 3.2 Å by molecular replacement using the p55 structure as the search model (Supplemental Table 1). Of the 27 amino acids in the histone peptide, only 11 amino acids (Lys31 to Gly41) are ordered in the structure (Fig. 1B). This region corresponds to the first helix of the histone fold of histone H4 and agrees well with previous binding studies (Verreault et al. 1998). The histone H4 peptide is nestled into a “binding pocket” formed by the N-terminal α-helix and the “binding loop” emerging from the seventh blade. The structure of p55 bound to histone H4 is almost identical to that of free p55, and the binding loop is well ordered in both structures, suggesting that the binding pocket is preformed.
p55 appears to use a unique mechanism to recognize and bind histone H4. In this structure of the p55–H415–41 complex, the histone H4 peptide is bound in the binding pocket on the side of the β-propeller structure of p55. This pocket is entirely composed of regions of the protein that are specific to p55, the N-terminal α-helix and the loop extruding from the seventh blade of the β-propeller. These additional features may begin to explain the difference in binding modes between p55 and another known histone-binding β-propeller protein, WDR5. Both surfaces of the binding pocket are absent in WDR5. In WDR5, histone H3 peptides are bound on the top of the WD40 repeat β-propeller structure (Couture et al. 2006; Han et al. 2006; Ruthenburg et al. 2006; Schuetz et al. 2006), which is the most common binding mode for WD40 repeat domains.
Given the high sequence conservation of this protein, the surface charge of p55 is likely to be maintained across species (Supplemental Fig. 1). This charge distribution might be key for p55 to be in many different chromatin-modifying complexes. The electrostatic potential representation of p55 (Supplemental Fig. 2) reveals a negatively charged surface with a hole at the top and hydrophobic surface with a larger hole at the bottom of the p55. These surfaces might be used to interact with other subunits in the different complexes. Furthermore, WD40 repeat-containing proteins are known to utilize surfaces other than the one seen in this structure to recognize histones, possibly allowing p55 to act as a multifunctional protein-binding platform.
The interaction between p55 and histone H4
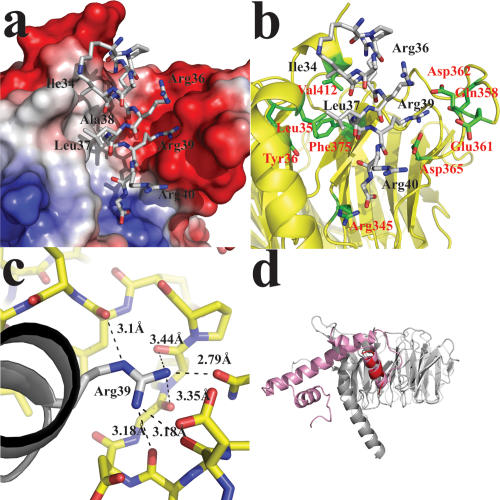
An electrostatic potential surface representation of the histone-binding pocket of p55 shows that the binding pocket consists of two distinct charged surfaces (Fig. 2a). The surface on the binding loop from the seventh blade is highly negatively charged. The other surface formed by the N-terminal α-helix and the side of the seventh blade is hydrophobic. Binding of the histone H4 helix by p55 in this pocket is consistent with an in vivo study of a temperature sensitive mutant (Y41H) of Msi16, a yeast homolog of p55. This mutant showed defects in cell proliferation and histone deposition (Hayashi et al. 2004). Tyr41 in Msi16 corresponds to Tyr36 in p55, which is located beneath the hydrophobic patch in the binding pocket (Fig. 2b). Upon elevated temperature, this mutation might disrupt the binding pocket, suggesting that the binding pocket is functional in vivo.
Figure 2.
p55 recongizes histone H4 via the binding pocket. (a) Electrostatic surface potential representation of the binding pocket with the histone H4 peptide (shown in stick model). The binding pocket is characterized by the high negative charge on the binding loop and the hydrophobic surface on the N-terminal α-helix. (b) Detailed interactions between histone H4 and the p55-binding pocket. Arg39 of histone H4 is inserted in the binding pocket and interacts with the carboxylates of Asp362 and Asp365 in the binding pocket. Leu37 and Ile39 of histone H4 interact with the hydrophobic patch formed on the surface of the N-terminal α-helix. (c) Arg39 of histone H4 forms a hydrogen bond network with carbonyl oxgens of the backbone at the binding pocket. (d) Full-length H4 was superimposed on the p55–H4 complex. The histone fold of histone H4 must be altered to allow p55 binding.
The two surfaces of the pocket match well with the differentially charged surfaces of the first helix of histone H4, where pairs of hydrophobic and basic amino acids (AI–RR–LA–RR) alternate making one side of the helix hydrophobic and the other side positively charged. Specifically, Arg39 of histone H4 forms ionic interactions with the carboxylates of Asp362 and Asp365, and makes extensive hydrogen bonds with carbonyl oxygens from the backbone of the binding loop and the oxygen of the Gln358 side chain (Fig. 2b,c). This extensive hydrogen bond network appears to be a major contributor to histone H4 binding to p55. In contrast to these ionic interactions, the other side of the histone H4 helix makes hydrophobic interactions with the binding pocket. Ile34, Leu37, and Ala38 in histone H4 face a hydrophobic patch composed of Leu35, Phe372, and Val412 from the N-terminal α-helix, the side of the seventh blade, and the C-terminal one-turn α-helix of p55, respectively.
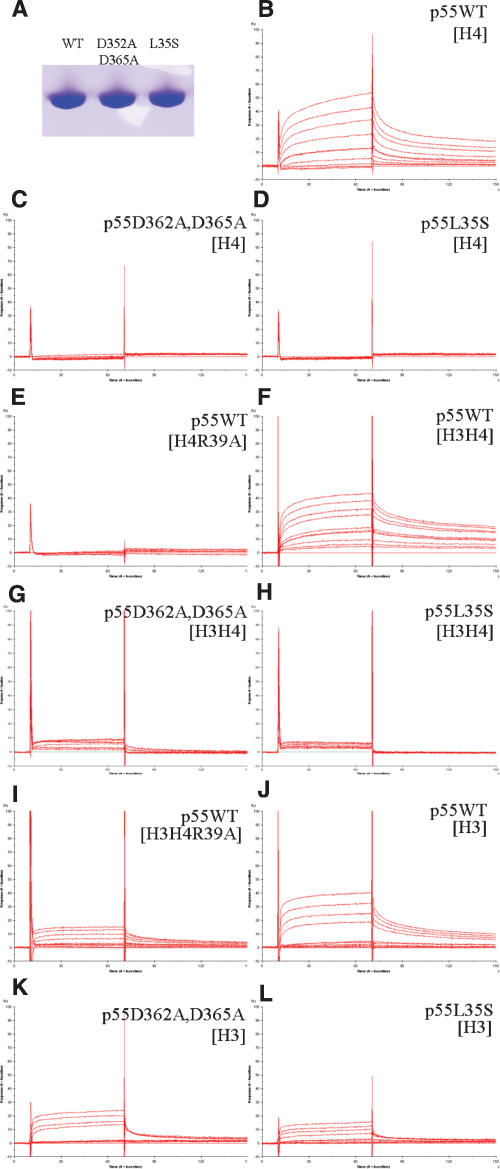
To confirm that the interactions described above are required for histone H4 binding, we generated two p55 mutants (Fig. 3A) and examined their binding properties. In one mutant, we disrupted the ionic interactions between histone H4 and the binding loop by simultaneously replacing Asp362 and Arg365 with alanines. In another mutant, Leu35 was mutated to Ser to disrupt hydrophobic interactions between histone H4 and the N-terminal α-helix. These residues are solvent-exposed and not involved in any stabilizing interaction within p55. Both mutant p55 proteins behaved identically to wild-type protein in size exclusion chromatography (data not shown), indicating that mutating these residues did not significantly alter the folding of the protein.
Figure 3.
Histone-binding properties of p55. (A) Purified wild-type and mutant p55. Wild-type and mutant p55 were used as analytes at the concentrations of 5 nM, 10 nM, 25 nM, 50 nM, 100 nM, 250 nM, 500 nM, 1 μM, and 2 μM on CM5 chips immobilized with full-length histone H4 (B–D); full-length mutant histone H4, H4R39A (E); histone H3-H4 tetramer (F–H); mutant tetramer, H3-H4R39A (I); and full-length histone H3 (J–L) with 520, 430, 177, 180, and 493 RU, respectively.
To examine the binding properties of the mutants, we used Surface Plasmon Resonance (SPR). Full-length histone H4 was immobilized, and p55 wild-type and mutant proteins were used as analytes. Wild-type p55 bound full-length histone H4 tightly with a KD estimated to be in the hundred nanomolar range (Fig. 3B), while we were unable to detect binding of mutant proteins to the immobilized histone H4, even at concentrations up to 2 μM (Fig. 3C,D). This strongly suggests that the interaction observed in the crystal structure is utilized by p55 to bind to histone H4.
In the reverse experiment, we examined the role of Arg39 in histone H4. The crystal structure shows Arg39 of histone H4 inserted into the binding pocket of p55 and forming an extensive hydrogen bond network with p55. To examine if Arg39 of histone H4 is a major contributor to binding, we immobilized full-length mutant (R39A) histone H4 on a Sensor Chip, and wild-type p55 was used as an analyte. The R39A mutation disrupted binding, demonstrating the importance of this residue (Fig. 3E).
Previously, it was shown that p55 interacts with the first helix of histone H4, which is buried in the canonical nucleosome structure (Verreault et al. 1998). That finding is confirmed in the crystal structure reported here, where p55 interacts with most of the surface area of the first helix of histone H4. This indicates that the histone fold of H4 must be altered upon p55 binding even when free histone H4 is the substrate (Fig. 2d). Furthermore, because the first helix of histone H4 is involved in dimerization with histone H3, p55 probably cannot bind to histone H4 in canonical H3–H4 dimer (or tetramer) structure, or in the nucleosome. To examine whether p55 can bind to the H3–H4 dimer (or tetramer), we assembled and immobilized histone H3–H4 tetramer on a Sensor Chip. Interestingly, we were able to detect binding of p55 to the immobilized H3–H4 tetramer (Fig. 3F). This suggests that the first helix of histone H4 might become accessible upon p55 binding even in a tetramer structure. Consistent with our observation, it was shown that hHat1 complex can acetylate H3–H4 tetramer in vitro (Verreault et al. 1996). One possibility, however, is that H3–H4 tetramer falls apart on the surface of the chip during the immobilization step, although it is unlikely due to the stability of histone H3–H4 tetramer. We also were able to detect residual binding of p55 mutants to histone H3–H4 tetramer, although to a significantly lesser extent than wild-type p55 (Fig. 3G,H). Considering that p55 mutants cannot bind histone H4 (see above), this binding might result from interactions with histone H3, an observation that has been made previously (Beisel et al. 2002; Wysocka et al. 2006). Consistent with this, we also observed binding of wild-type p55 to the mutant tetramer H3–H4R39A (Fig. 3I).
To examine whether p55 mutants bind histone H3, we immobilized full-length histone H3, and wild-type and mutant p55 were used as analytes. While the mutant p55 proteins were impaired for binding to H4, these mutants retained similar binding ability for histone H3 as wild-type p55 (Fig. 3J–L). This suggests that p55 utilizes different surfaces for binding to histone H3 and H4, further supporting the hypothesis that p55 serves as a multifunctional protein-binding platform.
The histone H4-binding pocket is important for the function of p55-containing complexes
Since p55 is a common subunit of many complexes, we examined if disrupting the binding pocket in p55 affects the activity of two of these complexes. The human Hat1 complex is composed of the Hat1p catalytic subunit and RbAp48 (the human ortholog of p55), and acetylates free but not nucleosomal histone H4 at Lys5 and Lys12 (Verreault et al. 1998). We compared histone acetyltransferase (HAT) activity with Hat1 complexes containing wildtype or H4-binding pocket mutants of RbAp48. Two mutants of RbAp48, equivalent to the p55 mutants, were constructed based on sequence alignment between RbAp48 and p55 (Supplemental Fig. 1). Leu31 in the N-terminal helix and Asp358 and Asp361 in the binding loop of RbAp48 were mutated to alanines. Hat1 complexes were expressed and copurified with either wild-type or one of the mutant RbAp48 subunits (Fig. 4A). Wild-type and mutant RbAp48 form equivalently stable complexes with the Hat1 subunit, and all complexes behaved similarly during gel filtration chromatography, indicating that these mutations do not disrupt folding or complex formation. The Hat1 complexes containing mutant RbAp48 show significantly less HAT activity than the wild-type Hat1 complex (Fig. 4B). This supports a role for the H4-binding pocket of p55 in the acetyltransferase activity of the Hat1 complex.
Figure 4.
HAT activities of human Hat1 complex. (A) Purified hHat1 subunit alone (N-terminal His-tagged), wild-type human Hat1 complex (His tag on RbAp48), and Hat1 complexes containing mutant RbAp48. (B) Mutations in the binding pocket of RbAp48 decrease the HAT activity. (A,C) Equal amounts of human HAT complexes and full-length H4 were used for HAT assay.
p55 binding to a buried region of histone H4 is consistent with the function of some, but not all, p55-containing complexes. The Hat1 and CAF-1 complexes are involved in histone assembly; the p55 subunit in these complexes most likely binds to free histone H4. p55 is also found in the ATP-dependent chromatin remodeling complexes NURF and NuRD. It is tantalizing to propose that the first helix of histone H4 becomes accessible to p55 during the ATP-dependent chromatin remodeling process where substantial structural changes in the nucleosome might occur (Narlikar et al. 2002). Another p55-containing complex, PRC2, methylates histone H3 at Lys27 in the intact nucleosome where the first helix of histone H4 is buried. It is therefore of interest to examine the function of p55 in this complex.
To examine this, we attempted to form PRC2 complexes that contained wild-type and mutant forms of p55. However, mutant forms of p55 would not stably associate with the remaining subunits of PRC2 (data not shown). This suggests that the binding pocket of p55 might be interacting with other subunits of PRC2 rather than with histone H4 within this complex. This does not appear to be caused by a general defect in the ability of these mutants to form interactions, as these same mutants are able to form a stable complex with hHAT complex and bind to histone H3. This observation implies an interesting function of the histone H4-binding pocket of p55; it might be utilized in different ways depending upon the role for p55 in the complex. Elucidating the functional role for p55 in PRC2 will require further biochemical and structural studies.
The results presented here reveal the molecular basis for histone H4 recognition by p55. The structure of p55 bound to the first helix of histone H4 suggests that the canonical histone fold has to be altered upon p55 binding. Moreover, we have shown that the histone H4-binding pocket of p55 plays a critical role in the function of p55-containing complexes. Together these data suggest that p55 might serve as a multifunctional protein interaction platform within the many p55-containing complexes.
Materials and methods
Protein purification
Full-length Drosophila p55 with an N-terminal His tag was expressed in Sf9 cells infected by Baculovirus. Infected cells were lysed and cleared by centrifugation. The cleared cell lysate was incubated with Ni-NTA beads (Qiagen) in the presence of 20 mM imidazole for 2 h. The Ni-NTA beads were collected and washed with Buffer A containing 20 mM imidazole, and Buffer A containing 1 M NaCl. p55 was eluted with Buffer A containing 100 mM imidazole, and the N-terminal His tag of p55 was removed by TEV protease (Invitrogen). p55 was further purified with Hitrap Q anion exchange (GE Healthcare) and Superdex 200 (GE Healthcare) size exclusion columns. p55 was concentrated up to 10 mg/mL in 50 mM Tris-HCl (pH 8.0) and 150 mM NaCl. Se-Met-substituted protein was expressed in Sf9 cells in methionine-deficient media (Invitrogen) in the presence of 100 mg/L Se-methionine as described in Antipenko et al. (2003). Mutants were generated with QuickChange Site-Directed Mutagenesis Kit (Qiagen) and purified in the same way as the wild-type p55.
Crystallization and structure determination
Initial crystals of p55 were grown by vapor diffusion using the hanging-drop method in a buffer containing 100 mM HEPES (pH 7.5), 1.4 M ammonium sulfate (AMS), and 3% β-octy-glucopyranoside. The optimal crystallization condition was determined as 100 mM HEPES (pH 7.5), 1.4 M AMS, 3% β-octyl-maltoside, 10 mM CdCl2, 3% ethylene glycol, and 50 mM NaCl. p55–H4 peptide complex crystals were obtained in the same condition as p55 alone in the presence of 2 mM H4 peptides. For cryoprotection, crystals were soaked for 1 min in crystallization solution containing 25% glycerol. All data were collected under cryogenic condition (105°K) at beamline X29 or X12B at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (BNL). Data were processed with HKL2000 (http://www.hkl-xray.com). Phases were calculated from the SAD method at the selenium peak. Initial Se sites were found using the program HKL2MAP (Pape and Schneider 2004) and then refined with the program Solve. Density modification was performed using the program Resolve (Terwilliger 2000). Models were built using the program O and crystallographic refinement was done with the program CNS against the native p55 data or p55–H4 data.
Binding studies
All binding studies were performed with the SPR technique using a Biacore T100 (Biacore). The surfaces were activated with an NHS and EDC mixture. Full-length histone H4, H4R39A, H3H4 tetramer, H3H4R39A tetramer, and full-length H3 were immobilized on a Sensor Chip CM5 with 520, 430, 177, 180, and 493 RU, respectively. The surfaces were then blocked with 1 M ethanolamine solution. After immobilization, the surface was stabilized with a regeneration buffer containing 50 mM Tris-HCl (pH 8.0), 1.5 M NaCl, 10 mM imidazole, and 0.05% NP40. Wild-type and mutant p55 (5 nM, 10 nM, 25 nM, 50 nM, 100 nM, 250 nM, 500 nM, 1 μM, and 2 μM) were used as analytes for binding, and the surface was regenerated with the regeneration buffer after each injection. Biacore T100 BiaEvaluation software (GE Healthcare) was used for the data analysis.
HAT activity assay
hHat1 (N-terminal His tag) alone and Hat1 complexes were expressed in Sf9 cells infected by Baculovirus and purified with Ni-affinity and size exclusion chromatography. For the HAT assay, Hat1 complexes (4 pmol) were incubated with tritium-labeled acetyl coenzyme A (1.25 μmol) and free histone H4 (1 μmol) in a 20-μL final volume for 30 min at 37°C. The reactions were stopped with 7 μL of SDS loading buffer and loaded on SDS-PAGE gel. The gel was then transferred to Immobilon membrane (Millipore) and the membrane was exposed to a PhosphorImager screen. In this condition, the reaction is linear within the initial 45 min.
Coordinates
The atomic coordinates and structure factors of the p55 and the histone H4 complex will be deposited in the Protein Data Bank (accession codes 3c9c and 3c99, respectively).
Acknowledgments
We thank Drs. Rebecca Dunn, Ihn Sik Seong, Adam Matthews, Karim Bouazoune, and Jesse Cochrane for critical reading of the manuscript, and Dr. Karim-Jean Armache for helping with data collection and critical reading of the manuscript. We thank Dr. Bruce Stillman for providing human Hat1 clone. We also thank Drs. Alexei Soares and Anand M. Saxena for support with data collection. Data for this study were measured at beamline X12B and X29 of the NSLS at BNL. J.J.S. is a post-doctoral fellowship recipient of the Jane Coffin Childs Memorial Fund. This work was supported by grants from the NIH (R.E.K.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1653308.
References
- Ach R.A., Taranto P., Gruissem W. A conserved family of WD-40 proteins binds to the retinoblastoma protein in both plants and animals. Plant Cell. 1997;9:1595–1606. doi: 10.1105/tpc.9.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antipenko A., Himanen J.P., van Leyen K., Nardi-Dei V., Lesniak J., Barton W.A., Rajashankar K.R., Lu M., Hoemme C., Puschel A.W., et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- Beisel C., Imhof A., Greene J., Kremmer E., Sauer F. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature. 2002;419:857–862. doi: 10.1038/nature01126. [DOI] [PubMed] [Google Scholar]
- Bouveret R., Schonrock N., Gruissem W., Hennig L. Regulation of flowering time by Arabidopsis MSI1. Development. 2006;133:1693–1702. doi: 10.1242/dev.02340. [DOI] [PubMed] [Google Scholar]
- Couture J.F., Collazo E., Trievel R.C. Molecular recognition of histone H3 by the WD40 protein WDR5. Nat. Struct. Mol. Biol. 2006;13:698–703. doi: 10.1038/nsmb1116. [DOI] [PubMed] [Google Scholar]
- Czermin B., Melfi R., McCabe D., Seitz V., Imhof A., Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Groth A., Rocha W., Verreault A., Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Guitton A.E., Berger F. Loss of function of MULTICOPY SUPPRESSOR OF IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Curr. Biol. 2005;15:750–754. doi: 10.1016/j.cub.2005.02.066. [DOI] [PubMed] [Google Scholar]
- Han Z., Guo L., Wang H., Shen Y., Deng X.W., Chai J. Structural basis for the specific recognition of methylated histone H3 lysine 4 by the WD-40 protein WDR5. Mol. Cell. 2006;22:137–144. doi: 10.1016/j.molcel.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Hassig C.A., Fleischer T.C., Billin A.N., Schreiber S.L., Ayer D.E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hennig L., Taranto P., Walser M., Schonrock N., Gruissem W. Arabidopsis MSI1 is required for epigenetic maintenance of reproductive development. Development. 2003;130:2555–2565. doi: 10.1242/dev.00470. [DOI] [PubMed] [Google Scholar]
- Huang S., Lee W.H., Lee E.Y. A cellular protein that competes with SV40 T antigen for binding to the retinoblastoma gene product. Nature. 1991;350:160–162. doi: 10.1038/350160a0. [DOI] [PubMed] [Google Scholar]
- Kohler C., Hennig L., Bouveret R., Gheyselinck J., Grossniklaus U., Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenjak M., Taylor-Harding B., Binne U.K., Satterlee J.S., Stevaux O., Aasland R., White-Cooper H., Dyson N., Brehm A. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell. 2004;119:181–193. doi: 10.1016/j.cell.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes & Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Carey M., Workman J.L. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lu X., Horvitz H.R. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Balbas M.A., Tsukiyama T., Gdula D., Wu C. Drosophila NURF-55, a WD repeat protein involved in histone metabolism. Proc. Natl. Acad. Sci. 1998;95:132–137. doi: 10.1073/pnas.95.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Hart C.M., Francis N.J., Vargas M.L., Sengupta A., Wild B., Miller E.L., O’Connor M.B., Kingston R.E., Simon J.A. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Narlikar G.J., Fan H.Y., Kingston R.E. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Pape T., Schneider T.R. HKL2MAP: A graphical user interface for phasing with SHELX programs. J. Appl. Crystallogr. 2004;37:843–844. [Google Scholar]
- Parthun M.R., Widom J., Gottschling D.E. The major cytoplasmic histone acetyltransferase in yeast: Links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Lee E.Y. Dual retinoblastoma-binding proteins with properties related to a negative regulator of ras in yeast. J. Biol. Chem. 1995;270:25507–25513. doi: 10.1074/jbc.270.43.25507. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Wang Y.C., Hollingsworth R.E., Jones D., Ling N., Lee E.Y. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature. 1993;364:648–652. doi: 10.1038/364648a0. [DOI] [PubMed] [Google Scholar]
- Ruthenburg A.J., Wang W., Graybosch D.M., Li H., Allis C.D., Patel D.J., Verdine G.L. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat. Struct. Mol. Biol. 2006;13:704–712. doi: 10.1038/nsmb1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz A., Allali-Hassani A., Martin F., Loppnau P., Vedadi M., Bochkarev A., Plotnikov A.N., Arrowsmith C.H., Min J. Structural basis for molecular recognition and presentation of histone H3 by WDR5. EMBO J. 2006;25:4245–4252. doi: 10.1038/sj.emboj.7601316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Sondek J., Bohm A., Lambright D.G., Hamm H.E., Sigler P.B. Crystal structure of a G-protein β γ dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- Taunton J., Hassig C.A., Schreiber S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- Taylor-Harding B., Binne U.K., Korenjak M., Brehm A., Dyson N.J. p55, the Drosophila ortholog of RbAp46/RbAp48, is required for the repression of dE2F2/RBF-regulated genes. Mol. Cell. Biol. 2004;24:9124–9136. doi: 10.1128/MCB.24.20.9124-9136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T.C. Maximum-likelihood density modification. Acta Crystallogr. D Biol. Crystallogr. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J.K., Bulger M., Kamakaka R.T., Kobayashi R., Kadonaga J.T. The p55 subunit of Drosophila chromatin assembly factor 1 is homologous to a histone deacetylase-associated protein. Mol. Cell. Biol. 1996;16:6149–6159. doi: 10.1128/mcb.16.11.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault A., Kaufman P.D., Kobayashi R., Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- Verreault A., Kaufman P.D., Kobayashi R., Stillman B. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Curr. Biol. 1998;8:96–108. doi: 10.1016/s0960-9822(98)70040-5. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Jones P.L., Vermaak D., Wolffe A.P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Milne T.A., Dou Y., Zhang X., Burlingame A.L., Roeder R.G., Brivanlou A.H., Allis C.D. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Xiao H., Milne T.A., Kwon S.Y., Landry J., Kauer M., Tackett A.J., Chait B.T., Badenhorst P., et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Iratni R., Erdjument-Bromage H., Tempst P., Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., LeRoy G., Seelig H.P., Lane W.S., Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]