Abstract
Control of integrin activation is required for cell adhesion and ligand-induced signaling. Here we report that loss of the focal adhesion protein Kindlin-2 in mice results in peri-implantation lethality caused by severe detachment of the endoderm and epiblast from the basement membrane. We found that Kindlin-2-deficient cells were unable to activate their integrins and that Kindlin-2 is required for talin-induced integrin activation. Furthermore, we demonstrate that Kindlin-2 is required for integrin outside-in signaling to enable firm adhesion and spreading. Our findings provide evidence that Kindlin-2 is a novel and essential element of bidirectional integrin signaling.
Keywords: Integrin activation, Kindlin-2, adhesion, mouse development, embryonic stem cells
The establishment and maintenance of cell–extracellular matrix (ECM) and cell–cell interactions are essential for the development of multicellular organisms. These interactions are mediated via membrane-associated proteins that firmly bind to ECM proteins or cell counterreceptors. The integrins, consisting of at least 24 members, represent the largest and most important family of cell–ECM receptors in vertebrates (Hynes 2002). Integrins are heterodimeric glycoproteins composed of α and β subunits. Each subunit consists of a large extracellular domain, a transmembrane domain, and a short cytoplasmic domain. Integrins are bidirectional signaling molecules. They regulate their affinity for ligand (integrin activation) by direct interactions of the β-subunit cytoplasmic tails with the cytoskeletal protein talin (inside-out signaling) (Calderwood et al. 1999, 2002; Wegener et al. 2007). Following ligand binding, integrins transduce signals into cells (outside-in signaling) by recruiting signaling and adaptor proteins to the cytoplasmic tails of the α and/or β subunits, which results in actin reorganization and modulation of various intracellular signaling pathways (Hynes 2002).
Kindlins are a novel family of adaptor proteins that are recruited to integrin-containing adhesion sites, termed focal adhesions (FAs) (Rogalski et al. 2000; Tu et al. 2003; Weinstein et al. 2003; Ussar et al. 2006). The Kindlin family consists of three members, termed Kindlin-1/Unc-112-Related Protein 1 (URP1), which is expressed in epithelial cells; Kindlin-2/Mig-2, which is ubiquitously expressed; and Kindlin-3/URP2, whose expression is restricted to hematopoietic cells. The structural hallmark of Kindlins is a FERM (Band 4.1/Ezrin/Radixin/Moesin) domain that was shown to associate with the β1-integrin subunit cytoplasmic tails (Kloeker et al. 2004; Shi et al. 2007).
Kindlin-1 is the founding member of the Kindlin family. Mutations in the Kindlin-1 gene lead to Kindler syndrome in humans, which is characterized by skin blistering (Jobard et al. 2003; Siegel et al. 2003). The defect in Kindler patients suggested a role of Kindlin-1 in integrin–ECM adhesion in vivo. This assumption has been corroborated further in siRNA-mediated depletion studies of Kindlins in different cell lines and genetic analyses of the Kindlin-2 ortholog in Caenorhabditis elegans. Knockdown experiments in mammalian cells showed that Kindlin-1 and Kindlin-2 are essential for integrin-mediated cell–ECM adhesion and spreading (Tu et al. 2003; Kloeker et al. 2004). Absent expression of UNC-112, the nematode ortholog of the mammalian Kindlins, gives rise to a PAT (paralyzed, arrested elongation at twofold) phenotype. The embryonic lethality of UNC-112 mutants is caused by an abrogated integrin function leading to pronounced muscle cell rounding and detachment. An identical phenotype is also observed in nematodes lacking the expression of PAT-3/β integrin or PAT-4/ILK and, consistent with this observation, UNC-112/Kindlin was shown to be required for the proper spatial localization of PAT-3/β integrin and PAT-4/ILK to cell–ECM adhesion sites (Rogalski et al. 2000; Mackinnon et al. 2002).
To address the role of Kindlin-2 in vivo, we generated mice, embryonic stem cells (ESCs), and embryoid bodies (EBs) lacking Kindlin-2 expression. We found that Kindlin-2 is required for integrin activation. The consequences of impaired integrin activation in Kindlin-2-deficient mice are severe endoderm and epiblast detachments, which arrest development at the peri-implantation stage.
Results and Discussion
Loss of Kindlin-2 leads to peri-implantation lethality and impaired integrin activation
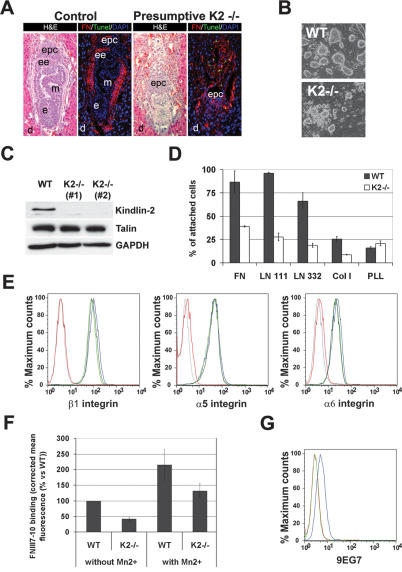
To investigate Kindlin-2 function, we disrupted the Kindlin-2 gene in mice (Supplemental Fig. 1). Mice with a heterozygous Kindlin-2-null mutation (Kindlin-2+/−) were viable and normal (data not shown). Among 166 newborn offspring from Kindlin-2+/− intercrosses, 67% were Kindlin-2+/− and 33% were wild type. Timed mating of Kindlin-2+/− intercrosses revealed that Kindlin-2−/− embryos were missing at embryonic day 7.5 (E7.5), and histology of implantation chambers at E6.5 showed that 74% of the embryos were normally developed (presumptive wild type or Kindlin-2+/−) and 26% were severely misshapen or already partially resorbed (putative Kindlin-2−/−) (Fig. 1A). These results suggest that ablation of Kindlin-2 leads to lethality at the peri-implantation stage.
Figure 1.
Peri-implantation lethality and impaired integrin activation in ESCs in the absence of Kindlin-2 expression. (A) Hematoxilin and eosin staining of an E6.5 control and presumptive Kindlin-2−/− implantation chamber. TUNEL staining (green) and FN staining (red) show apoptosis and disturbed matrix deposition in Kindlin-2−/− embryos. Nuclei are counterstained with DAPI (blue). (B) Bright-field pictures of wild-type and Kindlin-2−/− ESCs lines seeded on feeder cells. (C) Western Blot for Kindlin-2 and talin in wild-type and two independent Kindlin-2−/− ESCs lines. (D) Adhesion assay of wild-type and Kindlin-2−/− ESCs on different ECM substrates. (E) Integrin surface expression of wild-type (blue), Kindlin-2−/− (light and dark green), and β1-integrin−/− (red) ESC lines. A background control is shown in gray. (F) Binding of FNIII7-10 to wild-type and Kindlin-2−/− ESCs in the presence and absence of MnCl2. (G) 9EG7 binding on wild-type (blue) and Kindlin-2−/− (green) ESC lines. A background control is shown in red. (d) Decidua; (epc) ectoplacental cone; (ee) extraembryonic ectoderm; (e) ectoderm; (m) mesoderm.
To analyze the function of Kindlin-2 in vitro, we established wild-type and Kindlin-2−/− ESCs. While Kindlin-2−/− ESCs were readily obtained and showed a normal proliferation rate, their colonies barely adhered to the feeder layer and were less compact than wild-type ESC colonies (Fig. 1B). Western blot analysis revealed that Kindlin-2 is the only Kindlin expressed in ESCs and its expression was lost in Kindlin-2−/− ESCs (Fig. 1C; data not shown). To test whether the poor adhesion of Kindlin-2−/− ESC colonies to the feeder layer is caused by impaired adhesion to ECM proteins deposited by feeder cells, we performed adhesion assays on defined ECM substrates. They revealed that wild-type ESCs readily adhered to laminin-111 (LN111), laminin-332 (LN332), or fibronectin (FN), while Kindlin-2−/− ESCs showed strongly reduced adhesion to these substrates (Fig. 1D).
To exclude impaired integrin expression as the reason for the severe adhesion defects of Kindlin-2−/− ESCs, the expression levels of major ESC integrins were determined by flow cytometry and were found to be similar to wild-type ESCs (Fig. 1E). Next we tested whether diminished integrin-binding affinity (activation) is responsible for the adhesion defect. To this end, we compared the binding of a fluorescently labeled FN fragment containing the central cell-binding domain for αv and α5β1 integrins (FNIII7-10) to wild-type and Kindlin-2−/− ESCs. Interestingly, the binding of FNIII7-10 to Kindlin-2−/− ESCs was strongly reduced when compared with wild-type ESCs (Fig. 1F). The antibody 9EG7, which specifically recognizes activated mouse β1 integrins, also failed to bind Kindlin-2−/− ESCs (Fig. 1G). When cellular activation of integrins was bypassed by the addition of MnCl2, a powerful activator of integrins (Chen et al. 2003), wild-type as well as Kindlin-2−/− ESCs bound significant amounts of FNIII7-10 fragment (Fig. 1F). Collectively, these findings show that loss of Kindlin-2 prevents integrin activation.
Kindlin-2–integrin interaction enhances talin-mediated integrin activation
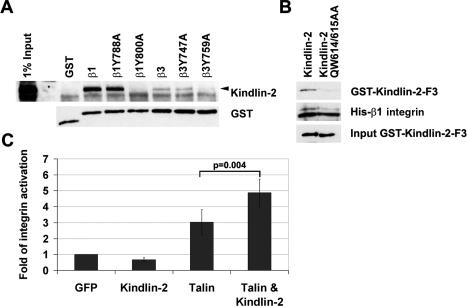
It is currently believed that talin binding to β-integrin tails is necessary and sufficient to trigger the activation of integrins (Calderwood et al. 1999, 2002; Wegener et al. 2007). Talin levels, however, were not decreased in Kindlin-2−/− ESCs (Fig. 1C), indicating that Kindlin-2 does not control integrin activation by regulating talin levels. To test whether Kindlin-2 controls integrin activation by binding to the β tails, we performed pull-down assays with GST-tagged β1- and β3-integrin cytoplasmic tails. We found that Kindlin-2 in wild-type ESC lysates bound the β1- and β3-integrin tails. Moreover, substitutions of the tyrosine (Y) to alanine (A) in the proximal NPxY motifs of the β1 and β3 tails (β1Y788A; β3Y747A), known to abrogate talin binding (Calderwood et al. 2002), did not diminish Kindlin-2 association. However, mutation of the distal NxxY motifs (β1Y800A; β3Y759A), which is known to be dispensable for talin binding but required for integrin activation (Xi et al. 2003), completely abolished the interaction with Kindlin-2 (Fig. 2A). To test whether the interaction between β-integrin tails and Kindlin-2 is direct, pull-down assays were performed with purified GST-tagged Kindlin-2 phosphotyrosine-binding (PTB) domain and recombinant His-tagged β1-integrin cytoplasmic tail. They demonstrated that the Kindlin-2 PTB domain was able to interact with β1-integrin cytoplasmatic tails and that this interaction was eliminated by mutating the PTB domain (QW614/615AA) of Kindlin-2 (Fig. 2B). Together, these results indicate that, despite the sequence similarities between the FERM domains of talin and Kindlin-2, these proteins bind distinct sites within the β1- and β3-integrin tails.
Figure 2.
Kindlin-2 interacts with integrin tails and is required for integrin activation. (A) GST pull-down assay from ESC lysates with wild-type and mutant GST-tagged β1- and β3-integrin cytoplasmic tails. (B) Pull-down assays with the recombinant GST-tagged wild-type and mutant (QW614/615AA) PTB domain of Kindlin-2 and the recombinant His-tagged β1-integrin cytoplasmatic tail. (C) αIIbβ3-integrin activation in CHO cells transfected with EGFP, EGFP-Kindlin-2, EGFP-Talin head, and EGFP-Kindlin-2 together with the Talin head.
Since Kindlin-2 can directly bind β1- and β3-integrin tails and is essential for ESC adhesion and binding to ECM substrates, we hypothesized that a potential role of Kindlin-2 could be to regulate integrin affinity for ligands. To confirm this hypothesis, we overexpressed Kindlin-2 in CHO cells engineered to express the inactive form of the human platelet integrin αIIbβ3 (O’Toole et al. 1994). As shown previously, overexpression of the talin FERM domain is sufficient to activate the αIIbβ3 integrin (Fig. 2C; Calderwood et al. 2002). Interestingly, overexpression of Kindlin-2 did not significantly change integrin activation when compared with EGFP-transfected cells. However, expression of the talin FERM domain together with Kindlin-2 resulted in a synergistic effect on αIIbβ3-integrin activation. These results clearly demonstrate that Kindlin-2 acts in concert with talin to trigger the activation of β3 integrins.
Kindlin-2−/− EBs show severe endoderm and epiblast detachments
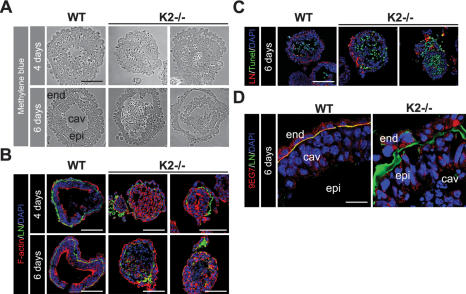
To investigate whether the peri-implantation defect of Kindlin-2−/− embryos is caused by an integrin activation defect, we generated EBs from wildtype and Kindlin-2−/− ESCs (Li et al. 2003; Montanez et al. 2007). When wild-type and Kindlin-2−/− ESCs were cultured in suspension for 2–4 d, they developed into simple EBs consisting of an outer layer of primitive endoderm cells and a core of undifferentiated ESCs. By day 4–6 in suspension, wild-type EBs developed into cystic EBs consisting of an outer layer of cubical-shaped endoderm cells, an inner layer of columnar pseu-dostratified epithelial cells (called epiblast or primitive ectoderm), a thin and continuous basement membrane (BM) between epiblast and endoderm, and a central cavity (Fig. 3A–C). Immunostaining with 9EG7 antibody revealed that endoderm and epiblast cells expressed activated integrins adjacent to their BM (Fig. 3D). In contrast, Kindlin-2−/− EBs were severely abnormal. About half of Kindlin-2−/− simple EBs did not develop further and degenerated. They consisted of a compact aggregate of ESCs covered by a discontinuous, spotted BM and detached endoderm. Despite evidence of scattered apoptotic cells, they lacked discernable cavities. This phenotype is similar to that described in β1-integrin−/− EBs, which arrested their development at the same stage of differentiation, showing defects in LN-α1 secretion and impaired cavitation (Aumailley et al. 2000; Li et al. 2002). The remaining half of the Kindlin-2−/− EBs formed an epiblast layer with poorly developed cavities and BMs and varying degrees of endoderm and epiblast detachment (Fig. 3A–C). The absence of 9EG7 staining at the basal sites of endoderm and epiblast cells indicates that β1 integrins of Kindlin-2−/− EBs were expressed in an inactive conformation (Fig. 3D). The abnormal BM contained all major proteins including LN-α1 (Supplemental Fig. 2A,B), suggesting that loss of Kindlin-2 does not impair expression and secretion of BM proteins. We reported previously that Kindlin-2 colocalizes with E-cadherin at cell–cell junctions (Ussar et al. 2006). Interestingly, however, Kindlin-2−/− EBs showed a normal E-cadherin distribution and developed normal cell–cell junctions (Supplemental Fig. 3; data not shown), indicating that they are not required for the establishment of cell junctional complexes in EBs. Together, these findings show that loss of Kindlin-2 expression impairs the activity of integrins in developing EBs, resulting in severe cell–ECM adhesion defects, abnormal BM depositions, abnormal cavity formation, and impaired cell survival.
Figure 3.
Kindlin-2 EBs show endoderm and epiblast detachment and diminished integrin activation. (A) Micrographs of methylene blue-stained sections of 4- and 6-d-old wild-type and Kindlin-2−/− EBs. (B) Cryosections of wild-type and Kindlin-2−/− EBs were stained with an antibody specific for LN-α1 chain (green) and fluorescently labeled phalloidin to visualize F-actin (red). Nuclei are counterstained with DAPI (blue). (C) TUNEL staining (green), LN-α1 chain (red), and nuclei (blue) of 6-d-old wild-type and Kindlin-2−/− EBs. (D) Six-day-old wild-type and Kindlin-2−/− EBs stained with LN-α1 chain (green) and 9EG7 (red) antibodies. Nuclei are counterstained with DAPI (blue). (epi) Epiblast; (end) endoderm.
Kindlin-2 is required for actin polarization, cell spreading, and ILK localization into FAs
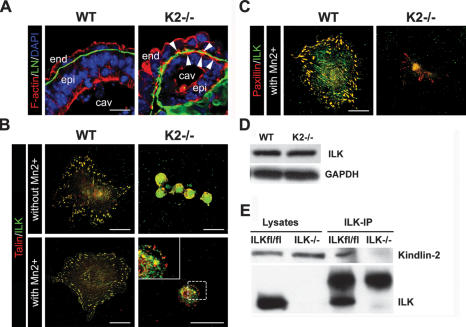
In endoderm and epiblast cells from wild-type EBs, F-actin predominantly accumulated at E-cadherin-positive adherens junctions to form the characteristic apical F-actin belt. In contrast, BM-attached endoderm and epiblast cells of Kindlin-2−/− EBs showed in addition to the apical F-actin belt a prominent F-actin accumulation at their basal sites, indicating that loss of Kindlin-2 affects F-actin polarization (Fig. 4A). To determine the mechanism by which Kindlin-2 depletion affects actin reorganization, we isolated endoderm cells from wild-type and Kindlin-2−/− EBs and plated them on FN. Endoderm cells from wild-type EBs readily adhered to FN, spread, and formed multiple FAs. In contrast, loss of Kindlin-2 expression abrogated adhesion and spreading to FN (Fig. 4B). To determine if this difference in the phenotype was only due to lack of integrin activation, the Kindlin-2−/− endoderm cells were treated with MnCl2 (Cluzel et al. 2005) and then plated on FN for 3 h. Under these conditions, Kindlin-2−/− cells developed only a few FA-like structures containing talin and paxillin and were still incapable of spreading (Fig. 4B). In nematodes, the ortholog of Kindlin called Unc-112 was shown to bind and recruit ILK to integrin adhesion sites (Mackinnon et al. 2002). ILK forms a complex together with the adaptor proteins PINCH and parvin that regulates cell spreading and actin cytoskeleton organization (Legate et al. 2006). Interestingly, ILK was either absent from the FA-like structures in Kindlin-2−/− cells (Fig. 4C) or did not properly colocalize with other FA proteins (Fig. 4B). Western blot analysis revealed normal ILK levels in Kindlin-2−/− endoderm cells, and immunoprecipitation assays with lysates from fibroblasts showed that Kindlin-2 interacts with ILK (Fig. 4D,E). These results indicate that Kindlin-2 is dispensable for ILK stability but required for ILK localization into integrin-containing adhesion sites. This observation together with the impaired FA formation and spreading suggest that Kindlin-2 is required for integrin inside-out as well as for integrin outside-in signaling.
Figure 4.
Impaired cell spreading of Kindlin-2−/− endoderm cells. (A) Cryosections of 6-d-old wild-type and Kindlin-2−/− EBs stained with LN-α1 chain antibody (green) and fluorescently labeled phalloidin to visualize F-actin (red). Nuclei are counterstained with DAPI (blue). (B) Immunofluorescence staining for talin (red) and ILK (green) of wild-type and Kindlin-2−/− endoderm cells seeded of FN in the presence or absence of MnCl2. (C) Immunofluorescence staining for paxillin (red) and ILK (green) of wild-type and Kindlin-2−/− endoderm cells seeded of FN in the presence of MnCl2. (D) Western blot analysis of ILK in wild-type and Kindlin-2−/− endoderm cells. (E) Coimmunoprecipitation of ILK and Kindlin-2 from ILKfl/fl and ILK−/− fibroblasts.
The results of our study show that Kindlin-2 is a novel and essential component for integrin inside-out and outside-in signaling, and its absence results in a peri-implantation lethality in mice. We found that loss of Kindlin-2 severely impairs activation of β1 and β3 integrins and that, following Mn2+ treatment Kindlin-2−/− endoderm cells fail to form multiple FAs and were incapable of spreading.
Bidirectional integrin signaling requires interactions between the cytoplasmatic domains of the integrins and intracellular signaling molecules. It is currently believed that the interaction between the membrane-proximal NPxY motif of the integrin β cytoplasmatic domain and talin is necessary and sufficient for integrin activation (Calderwood et al. 2002). Using conditional gene ablation, it was shown recently that talin is indeed essential for integrin activation in vivo (Nieswandt et al. 2007; Petrich et al. 2007). In contrast to the previous assumptions, however, our findings indicate that talin is not sufficient for triggering the activation of integrins, at least in ESCs, primitive endoderm, and epiblast, which clearly require Kindlin-2 for this process. In line with this observation, loss of Kindlin-3 was also found to compromise integrin activation on platelets, rendering them incapable of binding integrin ligands such as fibrinogen or collagen despite the expression of normal levels of talin (Moser et al. 2008). Future studies are now needed to investigate whether all cell types require Kindlins for integrin activation; whether the entire Kindlin family, including Kindlin-1, can trigger integrin activation; and to what extent the different Kindlins can compensate each other.
Interestingly, it has been shown previously that the membrane-distal NxxY motif of the β3-integrin cytoplasmatic domain is required for bidirectional integrin signaling, although the binding partner for the distal NxxY motif remained elusive (Xi et al. 2003). In our pull-down studies, we found that Kindlin-2 bound specifically the membrane-distal NxxY motif since the substitution of Y759 to an A residue eliminated the interaction of Kindlin-2 with the integrin tail. Therefore, we propose that Kindlin-2 mediates integrin activation (1) through a direct interaction of the PTB site in the F3 subdomain of Kindlin-2 and the distal NxxY motif of the β1- and β3-integrin cytoplasmic domains, and (2) in cooperation with talin, which binds the proximal NPxY motif of integrin tails. The close proximity of the two NP/XxY motifs raises questions about the hierarchy of their binding to the β-integrin tails and the specific roles of Kindlin-2 and talin in integrin activation. Future studies examining how and at which step of integrin activation Kindlin-2 and talin cooperate will be instrumental to our understanding of how these receptors become activated. Finally, we also found that elimination of Kindlin-2 also prevents the formation of multiple FAs, recruitment of ILK to integrin adhesion sites, and cell spreading. Hence, Kindlin-2 also seems to be involved in integrin outside-in signaling. A similar double function has been assigned to talin, since thrombin treatment of talin-deficient platelets failed to trigger their spreading on immobilized fibrinogen (Nieswandt et al. 2007). In analogy to talin, Kindlin-2 fulfils an important requirement of a bidirectional signaling relay for integrins: Upon integrin activation and cell adhesion, Kindlin-2 remains in focal complexes and FAs (Ussar et al. 2006) and is thus optimally positioned to transduce signals from the integrin tail to downstream effectors.
Materials and methods
Generation of Kindlin-2-deficient mice
A 417-base-pair fragment was amplified from mouse genomic DNA using the primers (fwd) TTCTTCAGGGTGTAGCTACTGG and (rev) CACCC CTAGAAAGACAGAAATAG, and was used to screen a PAC (phage artificial chromosome) library (BACPAC Resource Center) and as an external probe for ESC genotyping. The PAC clone 638-M21 Mouse PAC (RPCI21) was used to generate the Kindlin-2 knockout construct. To abolish Kindlin-2 gene function, an IRES-β-galactosidase cassette followed by a PGK neomycin resistance cassette was inserted into exon 2.
Antibodies and TUNEL staining
The following antibodies were used: rat antibody against β1 integrin, clone MB1.2 (Chemicon); rat antibody against β1 integrin, 9EG7 epitope (BD Bioscience); mouse antibody against talin (Sigma); rabbit antibody against LN-α1 chain (a gift from Rupert Timpl); rat antibody against LN-α1 chain (Chemicon); rabbit antibody against GAPDH (Calbiochem); rabbit antibody against ILK (Cell Signaling Technology); mouse antibody against paxillin (Transduction Laboratories); Cy3- and FITC-conjugated antibodies specific for mouse IgG, rabbit IgG, and rat IgG (Jackson Immunochemicals Laboratories, Inc.) were used as secondary antibodies. TRITC-conjugated phalloidin was used to detect F-actin (Molecular Probes). A Kindlin-2-specific polyclonal antibody was produced in rabbits (Ussar et al. 2006).
Apoptosis in embryos and EB sections was analyzed with the In situ Cell Death Detection Kit, POD (TUNEL technology; Roche).
ESC adhesion assay
ESCs were trypsinized and plated on cell tissue culture dishes for 30 min to remove the feeder cells. ESCs were then plated onto 96-well plates coated with FN, LN, or collagen IV at 1 × 105 cells per well. After 45 min of incubation, cells were lysed in a substrate buffer (7.5 mM NPAG [Sigma], 0.1 M Na citrate at pH 5, 0.5% Triton X-100) overnight at 37°C. Stop buffer (50 mM glycine at pH 10.4, 5 mM EDTA) was added and OD 405 was recorded.
FNIII7-10 binding and flow cytometry
FNIII7-10 binding and flow cytometry assays were performed as described previously (Czuchra et al. 2006). The human FNIII7-10 fragment was subcloned into pET15b plasmid (Invitrogen), expressed and purified from Escherichia coli, and directly labeled with Alexa Fluor 647 carboxylic acid via a succinimidyl ester (Invitrogen).
Generation of EBs and isolation of endoderm cells
EBs from wild-type and Kindlin-2−/− ESCs were generated as described (Montanez et al. 2007). To isolate endoderm cells, wild-type and Kindlin-2−/− EBs were collected in a 15-mL conical tube and shaken, and the EBs were allowed to settle down by gravity for 10 min at room temperature. The supernatant containing the detached endoderm cells was carefully aspirated and transferred into a fresh 15-mL conical tube. The process was repeated two times to ensure complete removal of EBs. The resulting suspension with endoderm cells was transferred into a FN-coated (10 μg/mL) tissue culture dish.
SDS-PAGE and immunoblotting
Cells were lysed in lysis buffer (in 150 mM NaCl, 50 mM Tris at pH 7.4, 1 mM EDTA, 1% Triton X-100 supplemented with protein inhibitors [Roche] and phosphatase inhibitors [Sigma]), homogenized in sample buffer, and boiled for 5 min. Cell lysates were resolved by SDS-PAGE gels. Proteins were then electrophoretically transferred from gels onto nitrocellulose membranes, followed by incubation with antibodies. For antibody detection, a blotting ECL detection kit (Millipore Corporation) was used.
GST pull-downs
The β1A- and β3-integrin cytoplasmic domains and their mutant forms were expressed as GST fusion proteins in BL21 cells. ESCs were lysed in lysis buffer (50 mM Tris at pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and proteinase [Roche] and phosphatase inhibitors [Sigma]). Six-hundred micrograms of lysate were incubated overnight with 5 μg of the indicated GST constructs. The complexes were precipitated with GST beads, subsequently washed and resuspended in LDS Sample buffer (Invitrogen), and used for SDS-PAGE.
For direct protein–protein interaction assay, recombinant GST-tagged Kindlin-2-F3 domain (GST-K2F3) and a mutated form (QW614/615AA) were expressed in BL21 cells. His-tagged β1-integrin cytoplasmatic tails were expressed in BL21 cells upon 1 mM IPTG induction and purified under denaturing conditions. Five micrograms of GST-K2F3 or QW614/615AA were incubated with His-tagged β1-integrin cytoplasmatic tails bound to Ni2+-coated beads for 2 h in buffer D (50 mM NaCl, 10 mM PIPES, 150 mM sucrose, 0.1% Triton X-100 at pH 6.8, containing protease inhibitor cocktails [Roche]). After washing in buffer D, bound proteins were analyzed by SDS-PAGE and Western blotting.
αIIbβ3-integrin activation assay
CHO cells stably expressing human αIIbβ3 integrin were transiently transfected with 2 μg of the indicated EGFP-tagged cDNAs using Lipofectamine 2000 following the manufacturer’s instructions. Double-transfected cells were transfected with 2 μg of each plasmid. Twenty-four hours after transfection, cells were trypsinized and incubated with the mouse monoclonal antibody PAC-1 (BD) in Tyrode’s buffer (pH 7.35) for 40 min at room temperature. Cells were washed and stained with a secondary anti-mouse IgM Alexa 647-labeled antibody (Invitrogen) for 20 min on ice. PAC-1 binding was measured with a FACSCalibur (BD), gating for living cells, using propidium iodide exclusion. Data evaluation was performed with the FlowJoe software. Statistical analysis was performed with five independent experiments using the t-test.
Coimmunoprecipitation
ILKfl/fl and ILK−/− fibroblast cells were lysed in lysis buffer, and 1 mg of each cell lysate was incubated with an anti-ILK mouse monoclonal antibody (BD Bioscience) for 1 h on ice. Bound protein complexes were bound to protein A beads (Sigma) for 1 h, subsequently washed in lysis buffer containing 0.1% Triton X-100, resuspended in LDS sample buffer (Invitrogen), and used for SDS-PAGE.
Histological analysis, inmunostaining, and electron microscopy
Immunohistochemistry of embryos and immunofluorescence studies of EBs were performed as described previously (Montanez et al. 2007).
Acknowledgments
We thank Dr. Gerhard Wanner for EM analysis, Mrs. Simone Bach for expert technical assistance, and Drs. Kyle Legate and Sara Wickström for critically reading the manuscript. Roy Zent is supported by a Merit Award from the Department of Veterans Affairs and RO1 DK69921 and P01 DK65123. The work was supported by the BMBF (01GM0301), Austrian Science Foundation (SFB021), and the Max Planck Society.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.469408.
References
- Aumailley M., Pesch M., Tunggal L., Gaill F., Fässler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in β1 integrin-deficient embryoid bodies. J. Cell Sci. 2000;113:259–268. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- Calderwood D.A., Zent R., Grant R., Rees D.J., Hynes R.O., Ginsberg M.H. The Talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Calderwood D.A., Yan B., de Pereda J.M., Alvarez B.G., Fujioka Y., Liddington R.C., Ginsberg M.H. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- Chen J., Salas A., Springer T.A. Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat. Struct. Biol. 2003;10:995–1001. doi: 10.1038/nsb1011. [DOI] [PubMed] [Google Scholar]
- Cluzel C., Saltel F., Lussi J., Paulhe F., Imhof B.A., Wehrle-Haller B. The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J. Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuchra A., Meyer H., Legate K.R., Brakebusch C., Fässler R. Genetic analysis of β1 integrin ‘activation motifs’ in mice. J. Cell Biol. 2006;174:889–899. doi: 10.1083/jcb.200604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jobard F., Bouadjar B., Caux F., Hadj-Rabia S., Has C., Matsuda F., Weissenbach J., Lathrop M., Prud’homme J.F., Fischer J. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum. Mol. Genet. 2003;15:925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- Kloeker S., Major M.B., Calderwood D.A., Ginsberg M.H., Jones D.A., Beckerle M.C. The Kindler syndrome protein is regulated by transforming growth factor-β and involved in integrin-mediated adhesion. J. Biol. Chem. 2004;279:6824–6833. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- Legate K.R., Montañez E., Kudlacek O., Fässler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Li S., Harrison D., Carbonetto S., Fassler R., Smyth N., Edgar D., Yurchenco P.D. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Edgar D., Fässler R., Wadsworth W., Yurchenco P.D. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Mackinnon A.C., Qadota H., Norman K.R., Moerman D.G., Williams B.D. C. elegansPAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- Montanez E., Piwko-Czuchra A., Bauer M., Li S., Yurchenco P.D., Fässler R. Analysis of integrin functions in peri-implantation embryos, hematopoietic system, and skin. Methods Enzymol. 2007;426:239–289. doi: 10.1016/S0076-6879(07)26012-4. [DOI] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Nieswandt B., Moser M., Pleines I., Varga-Szabo D., Monkley S., Critchley D., Fässler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 2007;204:3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole T.E., Katagiri Y., Faull R.J., Peter K., Tamura R., Quaranta V., Loftus J.C., Shattil S.J., Ginsberg M.H. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich B.G., Marchese P., Ruggeri Z.M., Spiess S., Weichert R.A., Ye F., Tiedt R., Skoda R.C., Monkley S.J., Critchley D.R., et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski T.M., Mullen G.P., Gilbert M.M., Williams B.D., Moerman D.G. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J. Cell Biol. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D.H., Ashton G.H., Penagos H.G., Lee J.V., Feiler H.S., Wilhelmsen K.C., South A.P., Smith F.J., Prescott A.R., Wessagowit V., et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin–extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am. J. Hum. Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Ma Y.Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E.F., Wu C. The MIG-2/integrin interaction strengthens cell–matrix adhesion and modulates cell motility. J. Biol. Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- Tu Y., Wu S., Shi X., Chen K., Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Ussar S., Wang H.V., Linder S., Fässler R., Moser M. The Kindlins: Subcellular localization and expression during murine development. Exp. Cell Res. 2006;312:42–51. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Wegener K.L., Partridge A.W., Han J., Pickford A.R., Liddington R.C., Ginsberg M.H., Campbell I.D. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Weinstein E.J., Bourner M., Head R., Zakeri H., Bauer C., Mazzarella R. URP1: A member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Biochim. Biophys. Acta. 2003;1637:207–216. doi: 10.1016/s0925-4439(03)00035-8. [DOI] [PubMed] [Google Scholar]
- Xi X., Bodnar R.J., Li Z., Lam S.C., Du X. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin β3 cytoplasmic domain in inside-out and outside-in signaling. J. Cell Biol. 2003;162:329–339. doi: 10.1083/jcb.200303120. [DOI] [PMC free article] [PubMed] [Google Scholar]