Abstract
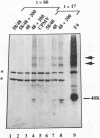
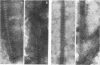
Tubular structures extending from plasmodesmata in cowpea mosaic virus (CPMV)-infected tissue have been implicated to play an important role in cell-to-cell movement of this virus. Using a cauliflower mosaic virus 35S promoter-based transient expression vector, we show that expression of only the CPMV M RNA-encoded 48-kDa protein (48K protein) in cowpea protoplasts is sufficient to induce these structures. Strikingly, expression of the 48K protein in protoplasts from a number of nonhost plant species, such as barley, Arabidopsis thaliana, and carrot, also resulted in tubular structure formation. Thus, it is not likely that the viral 48K protein, though playing a key role in cell-to-cell movement of CPMV, has a role in determining the host range of CPMV.
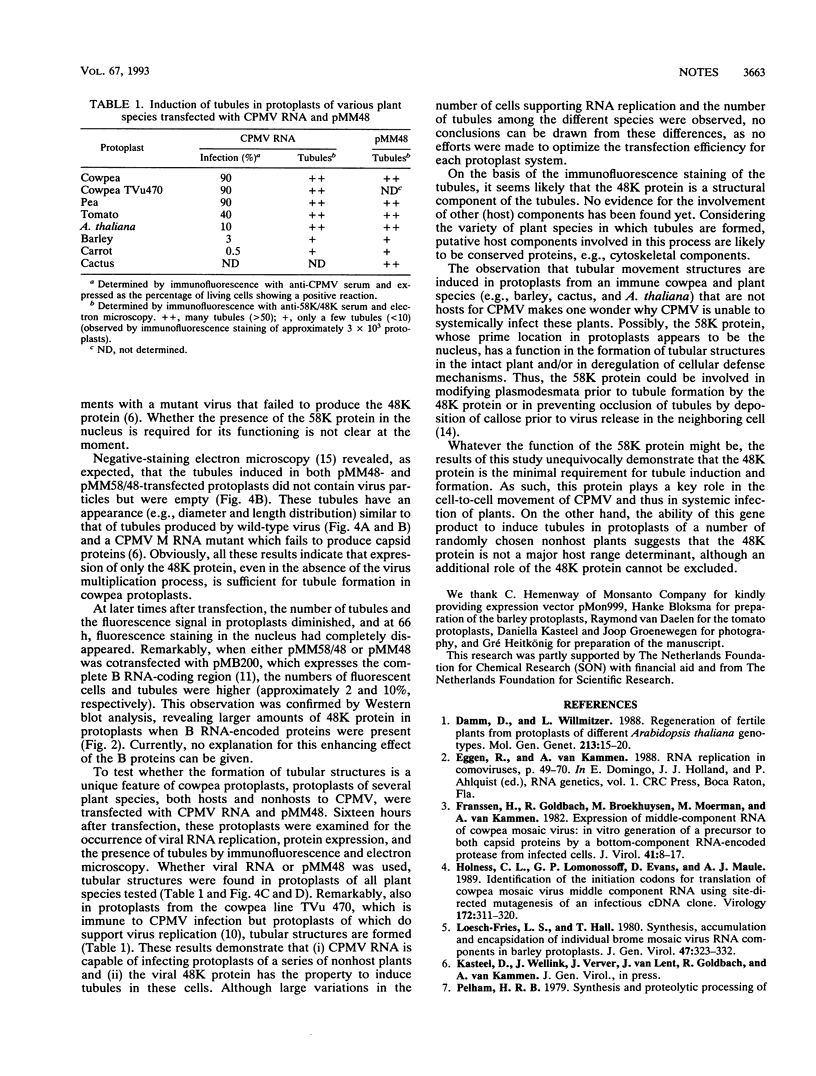
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness C. L., Lomonossoff G. P., Evans D., Maule A. J. Identification of the initiation codons for translation of cowpea mosaic virus middle component RNA using site-directed mutagenesis of an infectious cDNA clone. Virology. 1989 Sep;172(1):311–320. doi: 10.1016/0042-6822(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Rezelman G., van Kammen A. The inhibition of cowpea mosaic virus replication by actinomycin D. Virology. 1979 Jan 30;92(2):299–309. doi: 10.1016/0042-6822(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Vos P., Verver J., van Wezenbeek P., van Kammen A., Goldbach R. Study of the genetic organisation of a plant viral RNA genome by in vitro expression of a full-length DNA copy. EMBO J. 1984 Dec 20;3(13):3049–3053. doi: 10.1002/j.1460-2075.1984.tb02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven H., Wellink J., Usmany M., Vlak J. M., Goldbach R., van Kammen A. Expression of plant virus genes in animal cells: high-level synthesis of cowpea mosaic virus B-RNA-encoded proteins with baculovirus expression vectors. J Gen Virol. 1990 Nov;71(Pt 11):2509–2517. doi: 10.1099/0022-1317-71-11-2509. [DOI] [PubMed] [Google Scholar]
- van Lent J., Storms M., van der Meer F., Wellink J., Goldbach R. Tubular structures involved in movement of cowpea mosaic virus are also formed in infected cowpea protoplasts. J Gen Virol. 1991 Nov;72(Pt 11):2615–2623. doi: 10.1099/0022-1317-72-11-2615. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Scheer C., Groenewegen J. Structure in cells of Vigna unguiculata infected with cowpea mosaic virus. Virology. 1971 Nov;46(2):493–497. doi: 10.1016/0042-6822(71)90051-1. [DOI] [PubMed] [Google Scholar]