Abstract
Background
To analyse available evidence on the efficacy and safety of anti-TNFα drugs (infliximab, etanercept and adalimumab) for treating rheumatoid arthritis (RA).
Methods
We searched systematically for randomised controlled clinical trials on treatment of RA with anti-TNFα drugs, followed by a systematic review with metaanalysis. Trials were searched from MEDLINE, EMBASE and Cochrane Library databases. The American College of Rheumatology (ACR) efficacy response criteria were used. Safety parameters provided by the trials were also assessed. Positive and undesired effects were estimated using combined relative risks (RR), number needed to treat (NNT) and number needed to harm (NNH). Heterogeneity was evaluated by Cochrane's Q and I2 statistics.
Results
Thirteen trials (7087 patients) met the inclusion criteria. The combined RR to achieve a therapeutic response to treatment with recommended doses of any anti-TNFα drug was 1.81 (95% CI 1.43–2.29) with a NNT of 5 (5–6) for ACR20. NNT for ACR50 [5 (5–6)] and ACR70 [7 (7–9)] were similar. Overall therapeutic effects were also similar regardless of the specific anti-TNFα drug used and when higher than recommended doses were administered. However, lower than recommended doses elicited low ACR70 responses (NNT 15). Comparison of anti-TNFα drugs plus methotrexate (MTX) with MTX alone in patients with insufficient prior responses to MTX showed NNT values of 3 for ACR20, 4 for ACR50 and 8 for ACR70. Comparison of anti-TNFα drugs with placebo showed a similar pattern. Comparisons of anti-TNFα drugs plus MTX with MTX alone in patients with no previous resistance to MTX showed somewhat lower effects. Etanercept and adalimumab administered as monotherapy showed effects similar to those of MTX. Side effects were more common among patients receiving anti-TNFα drugs than controls (overall combined NNH 27). Patients receiving infliximab were more likely to drop out because of side effects (NNH 24) and to suffer severe side effects (NNH 31), infections (NNH 10) and infusion reactions (NNH 9). Patients receiving adalimumab were also more likely to drop out because of side effects (NNH 47) and to suffer injection site reactions (NNH 22). Patients receiving etanercept were less likely to drop out because of side effects (NNH for control versus etanercept 26) but more likely to experience injection site reactions (NNH 5).
Conclusion
Anti-TNFα drugs are effective in RA patients, with apparently similar results irrespective of the drug administered. Doses other than those recommended are also beneficial. The main factor influencing therapeutic efficacy is the prior response to DMARD treatment. The effect of treatment with etanercept or adalimumab does not differ from that obtained with MTX. The published safety profile for etanercept is superior but the fact that no patients are treated with higher than recommended doses requires explanation.
Background
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disease of the joints, which often causes joint destruction, deformity and functional impairment [1]. Early administration of disease-modifying antirheumatic drugs (DMARDs) is crucial and the use of nonsteroidal anti-inflammatory drugs and glucocorticoids remains a fundamental aspect of medical management of RA. The discovery that the macrophage-derived proinflammatory cytokine tumour necrosis factor alpha (TNFα) plays a central role in the pathogenesis of RA [2] led to the introduction of anti-TNFα drugs, a new biological DMARD class. Evidence showing that anti-TNFα drugs are very effective in RA has led to a substantial change in the treatment of this disease [3]. Three such drugs have been commercialized since 1999: infliximab, etanercept and adalimumab. Despite this relative short history, a considerable amount of information has already been accumulated [4-6]. However, many questions about this new class of drugs still remain unanswered: are all available anti-TNFα drugs equally effective; does their efficacy depend upon their being administered together with methotrexate (MTX); does efficacy depend on dose; are they more effective than MTX; are all anti-TNFα drugs equally safe; what is the efficacy/safety profile? To date, no direct "head-to-head" comparative studies of the different anti-TNFα drugs have been published. An alternative approach to answering the foregoing questions is to perform a systematic review with metaanalysis of relevant research. A metaanalysis with emphasis on the risk of cancer and infections has been reported [7]. Also, a study using an indirect comparative approach to the relative efficacies of the three anti-TNFα drugs in the treatment of RA showed no differences among them [8].
In this paper, we conduct a systematic review of randomised controlled clinical trials of anti-TNFα drugs in RA followed by a metaanalysis of the efficacy and safety of different doses of infliximab, etanercept and adalimumab.
Methods
Study selection criteria
We carried out a search of all randomised controlled clinical trials of anti-TNFα drugs (infliximab, etanercept or adalimumab) for treating patients with RA. Patients had to satisfy the American College of Rheumatology (ACR) criteria [9] for diagnosis and to have active disease. Trial duration had to be at least 6 months with efficacy measured by ACR response [10]. Clinical trials were excluded if they either used administration routes other than recommended or included no treatment arm with recommended doses. Only information published in the trial reports was assessed.
Efficacy parameters
We used the ACR responses ACR20, ACR50 and ACR70 (improvements of at least 20, 50 and 70%, respectively, on a series of predetermined measures) as efficacy parameters [10].
Safety parameters
The following safety parameters reported in the selected trials were analyzed: number of patients suffering any adverse event, withdrawals due to adverse events, serious adverse events, infections, serious infections, infusion reactions, injection-site reactions, malignancies and overall mortality.
Search strategy
Trials were searched in scientific journals and congress conference proceedings. Information from the MEDLINE, EMBASE and Cochrane Library databases up to October 2006 was checked using a high-sensitivity strategy. The descriptors used were rheumatoid arthritis, infliximab, etanercept, adalimumab, randomised controlled trial and meta-analysis. The computerised search was completed with a manual search of reference lists from the articles retrieved and from rheumatological journal articles published in 2006 (technical details are available from the authors). There was no language restriction.
Data extraction
Two investigators (AA-R and MC) independently examined each eligible study and extracted data. Trials with information only in abstract format were excluded. Data were extracted using an ad hoc form with key items for each trial: study design, patients' characteristics (sex, age and duration of disease evolution), patient inclusion criteria, drugs and doses used, treatment duration and ACR response and safety parameters. Special attention was paid to both inclusion criteria and clinical features of patients included in each trial, as they were deemed central aspects for assessing heterogeneity. The quality of each individual study was assessed and scored using the Jadad scale [11].
Statistical analysis
For each single trial the relative risk (RR) of attaining an ACR response was obtained as a measure of the effect. Overall efficacy estimates (combined relative risk) for each anti-TNFα drug (as monotherapy or in association with MTX or another DMARD) compared to a control (placebo, MTX or another DMARD) were attained using the ACR20, ACR50, and ACR70 criteria as the main outcome variables. We used DerSimonian-Laird's method to estimate a random-effects model. Heterogeneity was evaluated using Cochrane's Q and I2 statistics and explored via subgroup analysis. The I2 statistic is calculated from Q and can be interpreted as the percentage variability in study results attributable to between-study differences [12]. The number of patients needed for experimental treatment versus control (NNT) to obtain an additional positive ACR response was also estimated [13].
We also used the RR to estimate the risk of adverse effects; and we estimated the number needed to harm (NNH), defined as the number of patients receiving active treatment that would harm one patient compared to controls [13-15].
Publication bias was assessed graphically using a funnel plot [16] and statistically evaluated by the regression symmetry test described by Egger et al. [17] and the adjusted rank correlation test proposed by Begg and Mazumdar [18]. We used the specific software Comprehensive Metaanalysis Version 2.0 for analysis and presentation of main results.
Results
Search results
Of the 46 publications located [19-65], only 15 met the selection criteria and were consequently included in the metaanalysis [19-33]. The remaining 31 were excluded for several reasons [34-64] (Figure 1). The Maini trial [21] was included in the Lipsky et al. trial [19] and the van der Heijde et al. [28] and Klareskog et al. [27] trials were the same (TEMPO trial). We analyzed the entire set of 7087 patients recruited for the 13 trials selected: four using infliximab [19,20,22,23] (2581 patients), four etanercept [24-26,28] (1637 patients) and five adalimumab [29-33] (2869 patients). The methodological quality of the studies was moderate to high (3–5) except Bathon's trial [24], which had a lower Jadad score of 2 because it neither mentioned nor explained whether treatment allocation was based on a random procedure (Table 1).
Figure 1.
Flow chart of the selection process for inclusion of Randomised Controlled Trials (RCT) in the overview.
Table 1.
Summary of trials included in the metaanalysis
| Trial (reference) Comparisons Jadad's scale (J) | Groups and N of patients | Mean age (years) | Mean disease duration (years) | N of swollen joints | N of tender joints | CRP (mg/dl) | HAQ | Mean N of previous DMARDs | Previous response to MTX | |
| Lipsky et al. (19) Infliximab+MTX vs. MTX J [5] | 3 mg/Kg 8 wk * | 86 | 54 | 10 | 22 | 32 | 3.9 | 1.8 | 3.8 | Insufficient |
| 3 mg/Kg 4 wk | 86 | 52 | 9 | 21 | 31 | 3.5 | 1.7 | 2.6 | ||
| 10 mg/Kg 8 wk | 87 | 54 | 11 | 23 | 32 | 3.3 | 1.7 | 2.5 | ||
| 10 mg/Kg 4 wk | 81 | 52 | 12 | 24 | 34 | 4.2 | 1.7 | 2.5 | ||
| MTX | 88 | 51 | 11 | 21 | 31 | 4.0 | 1.7 | 2.5 | ||
| total | 428 | |||||||||
| St. Clair et al. (20) Infliximab+MTX vs. MTX J [3] | 3 mg/Kg 8 wk * | 373 | 51 | 0,8 | 21 | 32 | 2.9 | 1,5 | 71% | Not previously MTX |
| 6 mg/Kg 8 wk | 378 | 50 | 0,9 | 22 | 33 | 3.0 | 1,5 | 68% | ||
| MTX | 298 | 50 | 0,9 | 22 | 34 | 2.6 | 1,5 | 65% | ||
| total | 1049 | DMARDs naive | ||||||||
| Quinn et al. (22) Infliximab+MTX vs. MTX J [3] | 3 mg/Kg 8 wk * | 10 | 51 | 0,6 | NA | NA | 4.7 | 1,3 | Not previously DMARDs | Not previously MTX |
| MTX | 10 | 53 | 0,5 | 3.7 | 1.3 | |||||
| total | 20 | |||||||||
| Westhovens et al. (23) Infliximab+MTX vs. MTX J [4] | 3 mg/Kg 8 wk * | 360 | 53 | 7.8 | 15 | 22 | 1.6 | 1.5 | NA | Insufficient |
| 10 mg/Kg 8 wk * | 361 | 52 | 6.3 | 15 | 22 | 1.6 | 1.5 | |||
| MTX | 363 | 52 | 8.4 | 15 | 22 | 1.2 | 1.5 | |||
| total | 1084 | |||||||||
| Moreland et al. (24) Etanercept vs. placebo J [5] | 25 mg twice weekly * | 78 | 11 | 25 | 33 | 4.7 | 1.6 | 3.3 | Insufficient | |
| 10 mg twice weekly | 76 | 53 | 13 | 25 | 34 | 5.3 | 1.7 | 3.4 | ||
| placebo | 80 | 53 | 12 | 25 | 35 | 4.1 | 1.7 | 3.0 | ||
| total | 234 | 51 | ||||||||
| Weinblatt et al. (25) Etanercept+MTX vs. MTX J [3] | 25 mg twice weekly * | 59 | 13 | 20 | 28 | 2.2 | 1.5 | 2.7 | Insufficient | |
| MTX | 30 | 13 | 17 | 28 | 2.6 | 1.5 | 2.8 | |||
| total | 89 | 48 | ||||||||
| 53 | ||||||||||
| Bathon et al. (26) Etanercept vs. MTX J [2] | 25 mg twice weekly * | 207 | 51 | 1 | 24 | 31 | 3.3 | NA | 0.5 | Not previously MTX |
| 10 mg twice weekly | 208 | 50 | 0.9 | 24 | 31 | 4.4 | 0.5 | |||
| MTX | 217 | 49 | 1 | 24 | 30 | 3.7 | 0.6 | |||
| total | 632 | |||||||||
| van der Heijde et al. (28) (TEMPO) Etanercept+MTX vs. MTX vs. Etanercept J [4] | 25 mg twice weekly +MTX * | 231 | 7 | 22 | 34 | 2.9 | NA | 2.3 | ||
| 25 mg twice weekly * | 223 | 6 | 23 | 35 | 2.2 | 2.3 | ||||
| MTX | 228 | 52 | 7 | 22 | 33 | 3.5 | 2.3 | |||
| total | 682 | 53 | ||||||||
| 53 | ||||||||||
| Weinblatt et al. (29) (ARMADA) Adalimumab+MT X vs. MTX J [3] | 40 mg/2 wk * | 67 | 57 | 12 | 17 | 28 | 2.1 | 1.5 | 2.9 | Insufficient |
| 20 mg/2 wk | 69 | 53 | 13 | 17 | 28 | 2.8 | 1.5 | 3.0 | ||
| 80 mg/2 wk | 73 | 55 | 12 | 17 | 30 | 2.8 | 1.5 | 3.1 | ||
| MTX | 62 | 56 | 11 | 16 | 28 | 3.1 | 1.6 | 3.0 | ||
| total | 271 | |||||||||
| van de Putte et al. (30) Adalimumab vs. Placebo J [5] | 40 mg/2 wk * | 113 | 52 | 10 | 20 | 33 | 5.2 | 1.8 | 3.8 | Insufficient |
| 20 mg/2 wk | 106 | 53 | 9 | 19 | 33 | 5.2 | 1.8 | 3.7 | ||
| 20 mg/wk | 112 | 54 | 11 | 19 | 35 | 4.7 | 1.8 | 3.6 | ||
| 40 mg/wk | 103 | 51 | 11 | 19 | 33 | 4.9 | 1.8 | 3.8 | ||
| Placebo | 110 | 53 | 11 | 19 | 35 | 5.7 | 1.8 | 3.6 | ||
| total | 544 | |||||||||
| Furst et al. (31) (STAR) Adalimumab+ DMARD vs. DMARD J [3] | 40 mg/2 wk * | 318 | 9 | 20 | 27 | 1.5 | 1.3 | 57–60% | Insufficient | |
| DMARD | 318 | 11 | 21 | 27 | 1.5 | 1.4 | 2 or plus | |||
| total | 636 | 55 | ||||||||
| 55 | ||||||||||
| Keystone et al. (32) Adalimumab+MT X vs. MTX J [3] | 40 mg/2 wk * | 207 | 56 | 11 | 19 | 27 | 1.8 | 1.4 | 2.4 | Insufficient |
| 20 mg/wk | 212 | 57 | 11 | 19 | 27 | 1.4 | 1.4 | 2.4 | ||
| MTX | 200 | 56 | 10 | 19 | 28 | 1.8 | 1.4 | 2.4 | ||
| total | 619 | |||||||||
| Breedveld et al. (33) (PREMIER) Adalimumab+MT X vs. MTX vs. Adalimumab J [4] | 40 mg/2 wk + MTX * | 268 | 52 | 0,7 | 21 | 31 | NA | 1.5 | NA | Not previously MTX |
| 40 mg/2 wk | 274 | 52 | 0,7 | 22 | 32 | 1.6 | ||||
| MTX | 257 | 52 | 0,8 | 22 | 32 | 1.5 | ||||
| total | 799 | |||||||||
*groups receiving doses currently recommended
NA: not available
CRP: C-reactive protein
HAQ: health assessment questionnaire
Trial characteristics
Table 1 shows the major characteristic of the 13 trials included in the review. Information on efficacy at 6, 12 and 24 months and the previously-described safety parameters were analyzed.
There are four infliximab trials: Lipsky et al. [19], St. Clair et al. [20], Quinn et al. [22] and Westhovens et al. [23]. Lipsky et al. [19] used a randomised double blind 12-month trial (with information at 6 and 12 months). Four hundred and twenty-eight patients insufficiently responsive to MTX were included. Patients were randomized to 5 arms, four with infliximab plus MTX and a control arm with MTX alone. The purpose of this study was to demonstrate that infliximab was capable of inhibiting the progression of structural joint damage. It was a continuation of the Maini et al. trial [21]. The St. Clair et al. trial [20] compared infliximab plus MTX with MTX alone. The 1049 patients included in this trial had a recent onset of RA (disease duration in the range 3 months to 3 years). They had not previously received MTX, and MTX was administered following a rapid dose-increasing schedule during the trial (7.5 mg/wk at week 0, increasing by 2.5 mg per week to 15 mg/wk at week 5 and 20 mg/wk at week 8). The trial lasted 12 months. The Quinn et al. [22] trial was a small study comparing infliximab plus MTX with MTX alone in recent-onset RA patients. Westhovens et al. [23] conducted a 12-month trial but the double blind efficacy data were analyzed at week 22.
Four trials testing etanercept were analysed. Moreland et al. [24] compared etanercept in monotherapy with a placebo. Weinblatt et al. [25] compared etanercept plus MTX with MTX alone. In the Bathon et al. trial [26], etanercept in monotherapy was compared with MTX. Finally, the TEMPO trial (Trial of Etanercept and Methotrexate with radiographic Patient Outcomes) [28] compared etanercept in monotherapy with both MTX and a combination of etanercept plus MTX. Moreland et al. included 234 patients [24] in a 6 month-trial comparing the response to etanercept with placebo. Patients had previously shown an inadequate response to at least 1 DMARD (80% to MTX). They had received an average of three DMARDs and were therefore defined as refractory to standard treatments. Weinblatt et al. [25] included 89 patients with inadequate responses to MTX. The duration of the trial was 6 months. Bathon et al. [26] recruited 632 patients with recent RA onset (less than 3 years' duration). Patients ought not to have been treated with MTX previously. The trial lasted 1 year (with information at 6 and 12 months) and MTX was administered in a rapid dose-increasing schedule (initial dose 7.5 mg/wk, increased to 15 mg/wk at week 4 and 20 mg/wk at week 8). The TEMPO trial [28] included 682 patients with RA and insufficient DMARD responses. Around 40% had previously received MTX (but patients previously treated with MTX had neither discontinued it owing to toxicity nor been treated with MTX within 6 months of enrolment). The trial lasted 2 years. MTX was administered in a rapid dose-increasing schedule to 20 mg/wk in 8 weeks. The main goal of this study was to demonstrate that etanercept was capable of inhibiting the progression of structural joint damage.
There were five adalimumab trials. The ARMADA trial (The Anti-tumour necrosis factor Research study programme of the Monoclonal Antibody adalimumab in rheumatoiD Arthritis) [29] compared the efficacy of 6 months' treatment with adalimumab plus MTX with MTX alone in 271 patients with insufficient responses to MTX and at least one other DMARD. The van de Putte et al. trial [30] compared the efficacy of adalimumab with a placebo after 6 months treatment. All 544 patients included had inadequate responses to MTX and 3 other DMARDs. The basic purpose of the STAR trial (Safety Trial of Adalimumab in Rheumatoid arthritis) [31] was to study adalimumab safety. It recruited 636 patients with inadequate responses to any DMARD who were subsequently randomised either to continue with their current DMARD alone or to use a combination of the DMARD with adalimumab for 6 months. The Keystone et al. [32] trial had a similar design to ARMADA with a 12-month duration (information at 6 and 12 months), comparing adalimumab plus MTX with MTX alone in 619 patients. The basic purpose of this study was to demonstrate that adalimumab could inhibit the progression of structural joint damage. The PREMIER trial [33] compared the efficacies of adalimumab, adalimumab plus MTX and MTX alone in 799 patients at 24 months without previous treatment with MTX.
For each selected trial we extracted data on major features of the study design and characteristics of the patients included (Table 1). Weekly doses of MTX administered to the patients during the trials averaged 16 mg, except for the St. Clair et al. [20], TEMPO [28]. Bathon et al. [26] and PREMIER trials [33], in which MTX was administered in a rapid dose-increasing schedule to 20 mg/wk. The clinical profile of RA also varied across trials. Patients had a long history of RA (around 10 years) in most trials but a shorter evolution time in four of them: under 3 years in the St. Clair et al. [20], Bathon et al. [26] and Quinn et al. [22] and PREMIER [33] trials and around 6 years in the TEMPO [28] study. Therapeutic use of MTX prior to enrolment in the trial was also considered, because failure of or inadequate response to prior MTX administration entails a low response rate in the control group.
Metaanalysis results
Efficacy of anti-TNFα drugs. Global analysis
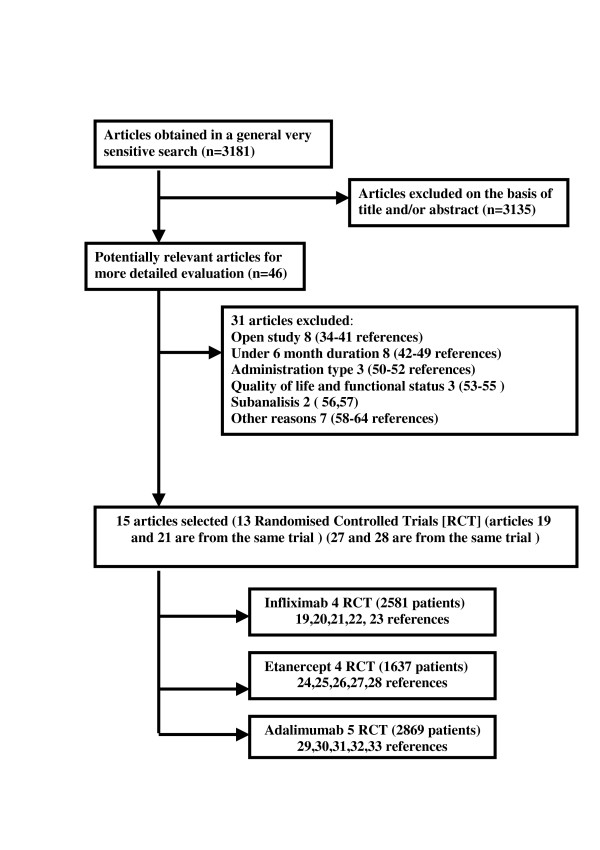
We studied the efficacies of the anti-TNFα drugs in the 13 trials included [19-33] (Table 2). Global comparison of the ACR20 efficacy of any dose of any anti-TNFα drug with any control treatment showed a combined effect of 1.81 (95% CI 1.43–2.29) with an NNT of 6 (5–7). The combined effects were 1.89 (1.30–2.75) for adalimumab trials, 1.71 (1.11–2.63) for etanercept trials and 1.82 (1.19–2.77) for infliximab trials. Further analyses using ACR50 and ACR70 efficacies showed very similar results (Figure 2).
Table 2.
Efficacy of anti-TNFα drugs on ACR20, ACR50 and ACR70 responses
| Trial (reference) Comparisons Duration of trial in months | Groups | N of patients | 6 month ACR20 | 6 month ACR50 | 6 month ACR70 | 12 month ACR20 | 12 month ACR50 | 12 month ACR70 | 24 month ACR20 | 24 month ACR50 | 24 month ACR70 |
| Lipsky et al. (19) Infliximab+MTX vs. MTX 12 months | 3 mg/Kg 8 wk +MTX* | 86 | 22/86 | 7/86 | 36/86 | 18/86 | 9/86 | ||||
| 3 mg/Kg 4 wk +MTX | 86 | 43/86 | 25/86 | 9/86 | 41/86 | 29/86 | 31/86 | ||||
| 10 mg/Kg 8 wk +MTX | 87 | 43/86 | 27/87 | 15/87 | 51/87 | 34/87 | 22/87 | ||||
| 10 mg/Kg 4 wk +MTX | 81 | 45/87 | 21/81 | 9/81 | 48/81 | 31/81 | 15/81 | ||||
| Total Infliximab | 340 | 47/81 | 95/340 | 40/340 | 176/340 | 112/340 | 77/340 | ||||
| MTX | 88 | 178/340 | 4/88 | 0/88 | 15/88 | 7/88 | 2/88 | ||||
| Total | 428 | 18/88 | |||||||||
| St. Clair et al. (20) Infliximab+MTX vs. MTX 12 months | 3 mg/Kg 8 wk +MTX* | 373 | NA | NA | 231/373 | 171/373 | 123/373 | ||||
| 6 mg/Kg 8 wk +MTX | 378 | 249/378 | 189/378 | 140/378 | |||||||
| Total Infliximab | 751 | NA | 480/751 | 360/751 | 263/751 | ||||||
| MTX | 298 | 161/298 | 95/298 | 62/298 | |||||||
| Total | 1049 | ||||||||||
| Quinn et al. (22) Infliximab+MTX vs. MTX 12 months | 3 mg/Kg 8 wk +MTX* | 10 | 8/10 | 8/10 | 7/10 | ||||||
| MTX | 10 | NA | NA | NA | 6/10 | 4/10 | 3/10 | ||||
| Total | 20 | ||||||||||
| Westhovens et al. (23) Infliximab+MTX vs. MTX 6 months | 3 mg/Kg 8 wk +MTX* | 360 | 110/360 | 48/360 | |||||||
| 10 mg/Kg 8 wk +MTX | 361 | 199/360 | 119/361 | 54/341 | |||||||
| Total Infliximab | 721 | 205/361 | 229/721 | 102/721 | |||||||
| MTX | 363 | 404/721 | 33/363 | 16/363 | |||||||
| Total | 1084 | 87/363 | |||||||||
| oreland et al. (24) Etanercept vs. placebo 6 months | 25 mg 2 twice weekly * | 78 | 31/78 | 11/78 | |||||||
| 10 mg 2 twice weekly | 76 | 46/78 | 18/76 | 7/76 | |||||||
| Total Etanercept | 154 | 37/76 | 49/154 | 18/154 | |||||||
| Placebo | 80 | 83/154 | 4/80 | 1/80 | |||||||
| Total | 234 | 9/80 | |||||||||
| Weinblatt et al. (25) Etanercept+MTX vs. MTX 6 months | 25 mg 2 +MTX * | 59 | 42/59 | 23/59 | 9/59 | ||||||
| MTX | 30 | 8/30 | 1/30 | 0/30 | |||||||
| Total | 89 | ||||||||||
| Bathon et al. (26) ** Etanercept vs. MTX 12 months | 25 mg 2 twice weekly * | 207 | 147/207 | NA | NA | 149/207 | 101/207 | 52/207 | |||
| 10 mg 2 twice weekly | 208 | NA | NA | NA | NA | NA | NA | ||||
| Total Etanercept | 415 | 56/217 | NA | NA | 141/217 | 93/217 | 47/217 | ||||
| MTX | 217 | ||||||||||
| Total | 632 | ||||||||||
| van der Heijde et al. (28) (TEMPO) Etanercept+MTX vs. etanercept vs MTX 24 months | 25 mg 2 twice weekly +MTX * | 231 | NA | NA | NA | 196/231 | 159/231 | 99/231 | 199/231 | 164/231 | 113/231 |
| 25 mg 2 twice weekly* | 223 | 169/223 | 107/223 | 54/223 | 167/223 | 120/223 | 60/223 | ||||
| Total Etanercept | 454 | 365/454 | 266/454 | 153/454 | 386/454 | 284/454 | 173/454 | ||||
| MTX | 228 | 171/228 | 98/228 | 43/228 | 162/228 | 96/228 | 4/228 | ||||
| Total | 682 | ||||||||||
| Weinblatt et al. (29) ARMADA) dalimumab+MTX vs. MTX 6 months | 40 mg/2 s+MTX* | 67 | 45/67 | 37/67 | 18/67 | ||||||
| 20 mg/2 s+MTX | 69 | 33/69 | 22/69 | 7/69 | |||||||
| 80 mg/2 s+MTX | 73 | 48/73 | 31/73 | 14/73 | |||||||
| Total Adalimumab | 209 | 126/209 | 90/209 | 39/209 | |||||||
| MTX | 62 | 9/62 | 5/62 | 3/62 | |||||||
| Total | 271 | ||||||||||
| van de Putte et al. (30) Adalimumab vs. Placebo 6 months | 40 mg/2 wk * | 113 | 25/113 | 14/113 | |||||||
| 20 mg/2 wk | 106 | 52/113 | 20/106 | 9/106 | |||||||
| 20 mg/wk | 112 | 38/106 | 23/112 | 11/112 | |||||||
| 40 mg/wk | 103 | 44/112 | 36/103 | 19/103 | |||||||
| Total Adalimumab | 434 | 55/103 | 104/434 | 53/434 | |||||||
| Placebo | 110 | 189/434 | 9/110 | 2/110 | |||||||
| Total | 544 | 21/110 | |||||||||
| Furst et al. (31) (STAR) Adalimumab+DAMAR D vs. DAMARD 6 months | 40 mg/2 wk * | 318 | 93/318 | 47/318 | |||||||
| DMARD | 318 | 169/318 | 35/318 | 10/318 | |||||||
| Total | 636 | 111/318 | |||||||||
| Keystone et al. (32) Adalimumab+MTX vs. MTX 12 months | 40 mg/2 wk +MTX* | 207 | 131/207 | 80/207 | 43/207 | 122/207 | 86/207 | 48/207 | |||
| 20 mg/wk +MTX | 212 | 129/212 | 87/212 | 36/212 | 116/212 | 80/212 | 44/212 | ||||
| Total Adalimumab | 419 | 260/419 | 167/419 | 79/419 | 238/419 | 166/419 | 92/419 | ||||
| MTX | 200 | 59/200 | 19/200 | 5/200 | 48/200 | 19/200 | 9/200 | ||||
| Total | 619 | ||||||||||
| Breedveld et al. (33) (PREMIER) Adalimumab+MTX vs. adalimumab vs MTX 24 months | 40 mg/2 wk+MTX* | 268 | NA | NA | NA | 196/268 | 166/268 | 123/268 | 185/268 | 158/268 | 126/268 |
| 40 mg/2 wk * | 274 | 148/274 | 112/274 | 71/274 | 134/274 | 101/274 | 77/274 | ||||
| Total Adalimumab | 542 | 344/542 | 278/542 | 194/542 | 319/542 | 259/542 | 203/542 | ||||
| MTX | 257 | 162/257 | 118/257 | 72/257 | 144/257 | 111/257 | 72/257 |
* groups receiving doses currently recommended
NA: not available
Figure 2.
Efficacy of all doses of anti-TNFα drugs on ACR20, ACR50 and ACR70 responses. Effect refers to the risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
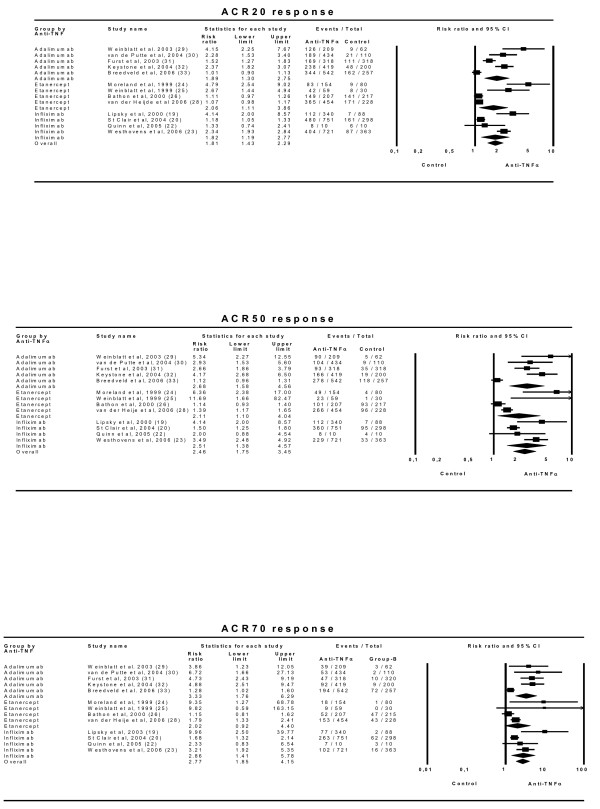
Analysis of this set of 13 trials provided evidence of relevant and statistically significant heterogeneity (Q = 157.7; p < 0.001; I2 92%). Although the limited number of trials reduced the discriminatory power of the funnel plot, it suggested a certain degree of asymmetry (Figure 3), which was statistically confirmed by both the Begg and Mazumdar adjusted rank correlation test (p = 0.033) and Egger's regression asymmetry test (p = 0.001).
Figure 3.
Funnel plot of selected studies. The x-axis shows effect estimates (RR) on a logarithmic scale (any effect estimate greater than zero therefore indicates better results for experimental treatment) while the y-axis measures the precision of each study (as the inverse of the standard error of the effect estimate measured on a logarithmic scale).
Bearing all these aspects in mind, we focused on the analysis of subgroups that appeared more homogeneous on both clinical and trial design grounds: previous exposure and response to DMARDs, mainly MTX, dose of anti-TNFα drug administered and control treatment selected (active or placebo, single or combined). The effects (RR) obtained with different doses of anti-TNFα appear in Table 3. The distinct NNTs and the analysis of heterogeneity appear in Table 4. Evidently the specific subgroups of trials characterised by these features are much less heterogeneous on analysis.
Table 3.
Effects (RR and NNT (95% CI)) obtained with different doses of anti-TNFα drugs
| All doses of anti-TNFα drugs vs. control 4618 vs. 2261* | Recommended doses of anti-TNFα drugs vs. control 2874 vs. 2260** | High-doses drugs vs. control 1169 vs. 921*** of anti-TNFα | Low-doses of anti-TNFα drugs vs. control 251 vs. 252**** | ||||||
| Anti-TNFα | ACR | RR (CI 95%) | NNT | RR (CI 95%) | NNT | RR (CI 95%) | NNT | RR (CI 95%) | NNT |
| Adalimumab | ACR20 | 1.9 (1.3–2.8) | 6 (5–7) | 2.0 (1.3–2.9) | 5 (4–6) | 3.5 (1.6–7.3) | 3 (2–4) | 2.4 (1.4–4.1) | 5 (4–8) |
| ACR50 | 2.7 (1.6–4.4) | 6 (5–7) | 2.8 (1.6–4.7) | 5 (5–6) | 4.7 (1.9–12.0) | 4 (3–5) | 2.9 (1.6–5.1) | 7 (5–13) | |
| ACR70 | 3.3 (1.8–6.3) | 9 (7–11) | 3.5 (1.9–6.7) | 7 (6–8) | 6.1 (1.8–20.8) | 7 (5–11) | 3.0 (1.1–7.9) | 17 (9–77) | |
| Etanercept | ACR20 | 1.7 (1.1–2.6) | 7(5–10) | 1.7 (1.1–2.7) | 6 (5–8) | There are no trials with high doses of Etanercept | 4.3 (1.9–10.1) | 3 (2–5) | |
| ACR50 | 2.1 (1.1–3.9) | 6 (5–9) | 2.2 (1.1–4.3) | 6 (4–7) | 4.7 (1.7–13.4) | 6 (4–13) | |||
| ACR70 | 2.0 (0.9–4.4) | NS | 2.1 (0.9–4.5) | NS | 7.4 (0.9–58.5) | NS | |||
| Infliximab | ACR20 | 1.8 (1.2–2.8) | 5 (4–6) | 1.7 (1.1–2.6) | 5 (4–6) | 2.0 (1.2–3.6) | 5 (4–5) | There are no trials with low doses of Infliximab | |
| ACR50 | 2.6 (1.5–4.7) | 5 (5–6) | 2.2 (1.2–4.1) | 6 (5–7) | 2.8 (1.5–5.5) | 5 (4–6) | |||
| ACR70 | 2.9 (1.4–5.8) | 8 (6–10) | 2.4 (1.2–5.0) | 9 (7–13) | 3.3 (1.5–7.2) | 7 (6–7) | |||
| Overall | ACR20 | 1.8 (1.4–2.3) | 6 (5–7) | 1.8 (1.4–2.3) | 5 (5–6) | 2.5 (1.5–4.2) | 4 (5–4) | 2.9 (1.7–5.1) | 4 (3–6) |
| ACR50 | 2.5 (1.8–3.4) | 6 (5–6) | 2.4 (1.7–3.4) | 5 (5–6) | 3.4 (2.0–5.8) | 5 (4–5) | 3.2 (2.0–5.3) | 6 (5–10) | |
| ACR70 | 2.8 (1.9–4.2) | 8 (7–9) | 2.7 (1.8–4.1) | 7 (7–9) | 3.9 (2.0–7.6) | 7 (6–8) | 3.5 (1.4–8.6) | 15 (10–38) | |
NNT: number of patients needed to be treated
RR (95%CI): relative risk (95% confidence limits)
NS: non-significant results
*4618 patients being treated with anti-TNFα (except 208 patients Bathon's trial being treated with 10 mg of etanercept twice a week) vs 2261 patients of the control groups
**2874 patients with recommended doses of anti-TNFα drugs (Infliximab 3 mg/Kg/8 week; etanercept 25 mg twice a week; adalimumab 40 mg every 2 weeks) vs 2260 patients of the control groups
***1169 patients with high-doses of anti-TNFα drugs (infliximab 3 mg/Kg 4 week, 6 mg/Kg 8 week, 10 mg/Kg 8 week and 10 mg/Kg 4 week; adalimumab 40 mg/week, 80 mg/2 week) vs 921 patients of the control groups
****251 patients with low-doses of anti-TNFα drugs (etanercept 10 mg twice weekly; adalimumab 20 mg/2 week) vs 252 patients of the control groups
Table 4.
Efficacy and heterogeneity
| Comparisons (Anti-TNFα vs. control)*** | ACR response | Anti-TNFα Events/Total | Control Events/Total | RR (CI 95%) | NNT | Q | I2% |
| All doses of anti-TNFα drugs vs. control (4618 vs. 2261) | ACR20 | 2709/4618 | 941/2261 | 1.8 (1.4–2.3) | 6 (5–7) | 157.7* | 92 |
| ACR50 | 1879/4618 | 519/2261 | 2.5 (1.8–3.4) | 6 (5–6) | 109.8* | 89 | |
| ACR70 | 1106/4618 | 2.8 (1.9–4.1) | 8 (7–9) | 52.4* | 77 | ||
| Recommended doses of anti-TNFα drugs vs. control (2874 vs. 2260) | ACR20 | 1808/2874 | 949/2261 | 1.8 (1.4–2.3) | 5 (5–6) | 149.5* | 92 |
| ACR50 | 1247/2874 | 519/2261 | 2.4 (1.7–3.4) | 5 (5–6) | 102.9* | 88 | |
| ACR70 | 733/2874 | 270/2261 | 2.7 (1.8–4.1) | 7 (7–9) | 49.2* | 76 | |
| High-doses of anti-TNFα drugs vs. control (1169 vs. 921) | ACR20 | 697/1169 | 293/921 | 2.5 (1.5–4.2) | 4 (4–5) | 57.2* | 93 |
| ACR50 | 469/1169 | 149/921 | 3.4 (2.0–5.8) | 5 (4–5) | 30.5* | 87 | |
| ACR70 | 295/1169 | 85/921 | 3.9 (2.0–7.6) | 7 (6–8) | 16.6* | 76 | |
| Low-doses of anti-TNFα drugs vs. control (251 vs. 252) | ACR20 | 108/251 | 39/252 | 2.9 (1.7–5.1) | 4 (3–6) | 4.9 | 59 |
| ACR50 | 60/251 | 18/252 | 3.2 (2.0–5.3) | 6 (5–10) | 1.5 | 0 | |
| ACR70 | 23/251 | 6/252 | 3.5 (1.4–8.6) | 15 (10–38) | 1.2 | 0 | |
| Anti-TNFα drugs vs. control in patients with No insufficient response to MTX (1964 vs. 1010) | ACR20 | 1346/1964 | 641/1010 | 1.1 (0.9–1.3) | NS | 2.4 | 9 |
| ACR50 | 1013/1964 | 408/1010 | 1.3 (1.1–1.5) | 9 (7–13) | 8.8 | 55 | |
| ACR70 | 669/1964 | 227/1010 | 1.5 (1.3–1.7) | 12 (9–19) | 7.0 | 43 | |
| Anti-TNFα drugs vs. control in patients with insufficient response to MTX (2654 vs. 1251) | ACR20 | 1427/2654 | 308/1251 | 2.3 (2.0–2.7) | 4 (3–5) | 28.2* | 75 |
| ACR50 | 866/2654 | 113/1251 | 3.6 (2.9–4.4) | 5 (4–5) | 7.2 | 3 | |
| ACR70 | 437/2654 | 43/1251 | 4.4 (3.2–6.0) | 7 (6–8) | 4.2 | 0 | |
| Anti-TNFα drugs at recommended doses plus MTX vs. MTX alone in patients with insufficient response to MTX (779 vs. 743) | ACR20 | 444/779 | 167/743 | 2.6 (2.1–3.3) | 3 (3–4) | 4.3 | 7 |
| ACR50 | 274/779 | 65/743 | 4.1 (2.6–6.6) | 4 (4–5) | 4.6 | 13 | |
| ACR70 | 132/779 | 30/743 | 4.1 (2.4–7.1) | 8 (7–11) | 2.5 | 0 | |
| Anti-TNFα drugs versus placebo at recommended doses (191 vs. 190) | ACR20 | 98/191 | 30/190 | 3.4 (1.6–7.3) | 3 (3–4) | 3.8* | 74 |
| ACR50 | 56/191 | 13/190 | 4.4 (1.5–12.5) | 5 (4–7) | 2.9 | 66 | |
| ACR70 | 25/191 | 3/190 | 8.1 (2.5–26.4) | 9 (7–16) | 0.1 | 0 | |
| Anti-TNFα drugs at recommended doses plus MTX versus MTX alone in patients with no previous resistance to MTX (882 vs. 793) | ACR20 | 631/882 | 500/793 | 1.2 (1.1–1.2) | 10 (7–16) | 0.4 | 0 |
| ACR50 | 504/882 | 315/793 | 1.6 (1.4–1.7) | 6 (5–8) | 1.1 | 0 | |
| ACR70 | 352/882 | 180/793 | 1.8 (1.5–2.1) | 6 (5–8) | 3.9 | 23 | |
| Anti-TNFα drugs versus MTX at recommended doses (704 vs. 702) | ACR20 | 466/704 | 474/702 | 1.0 (0.9–1.1) | NS | 6.9* | 71 |
| ACR50 | 320/704 | 309/702 | 1.0 (0.9–1.2) | NS | 3.6 | 45 | |
| ACR70 | 177/704 | 162/702 | 1.1 (0.9–1.3) | NS | 2.2 | 11 |
RR (95%CI): relative risk (95% confidence limits)
NNT: number of patients needed to be treated
Q: Cochrane's Q
I2: percentage of variability in study results attributable to between-study differences
* statistical heterogeneity
NS: non-significant results
*** number of patients being treated with anti-TNFα versus number of patients in the control groups
Efficacy of anti-TNFα drugs depending on prior exposure and response to MTX
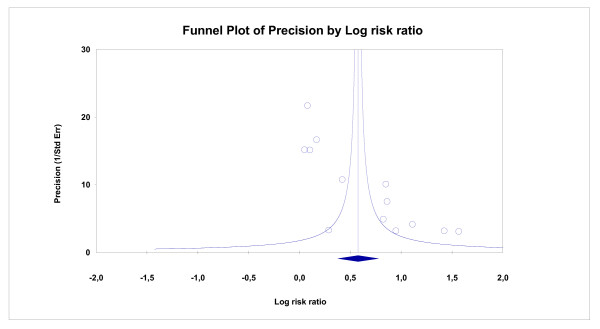
The efficacy results (Figure 4) show that this factor leads to rather different estimates and should be taken into account. When the effect of an anti-TNFα drug is assessed in patients who have received no previous MTX treatment, the relative ACR20 effect is small and only marginally statistically significant: 1.10 (0.96–1.26). On the other hand, when the anti-TNFα drug effect is analysed in patients with previously insufficient responses to MTX, the relative effect is substantially larger (2.32 [1.99–2.72]) and both clinically relevant and statistically significant [NNT of 4 (3–5)]. Similar results are seen with the ACR50 and ACR70 responses, though here the effect in patients naïve to MTX is statistically significant compared to control arms.
Figure 4.
Efficacy of anti-TNFα drugs depending on an insufficient response to MTX prior to trial commencement. Effect refers to risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'Upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
Analysis of the effect of different doses of anti-TNFα drugs
We analysed the efficacy of anti-TNFα drug administration in three separate groups: currently recommended doses (infliximab 3 mg/Kg/8 week; etanercept 25 mg twice a week; adalimumab 40 mg every 2 weeks); high doses (infliximab 3 mg/Kg/4 week, 6 mg/Kg/8 week, 10 mg/Kg/8 week and 10 mg/Kg/4 week; adalimumab 40 mg/week, 80 mg/2 week); and low doses (etanercept 10 mg 10 mg twice weekly; adalimumab 20 mg/2 week). No patient receiving infliximab was prescribed lower than recommended doses, and no patient treated with etanercept received higher than recommended doses. The group given adalimumab 20 mg/week was not included as this dose schedule can be considered neither above nor below the currently recommended regime. The combined and individual effects of the adalimumab, etanercept and infliximab trials at any dose or in subgroups based on the dose level are shown in Table 3. A statistically significantly beneficial effect is apparent with recommended, higher or lower doses in all the comparisons made, except for the ACR70 response to etanercept. Accordingly, the NNTs are very similar for all anti-TNFα drugs.
Analysis of the effect of anti-TNFα drugs at recommended doses
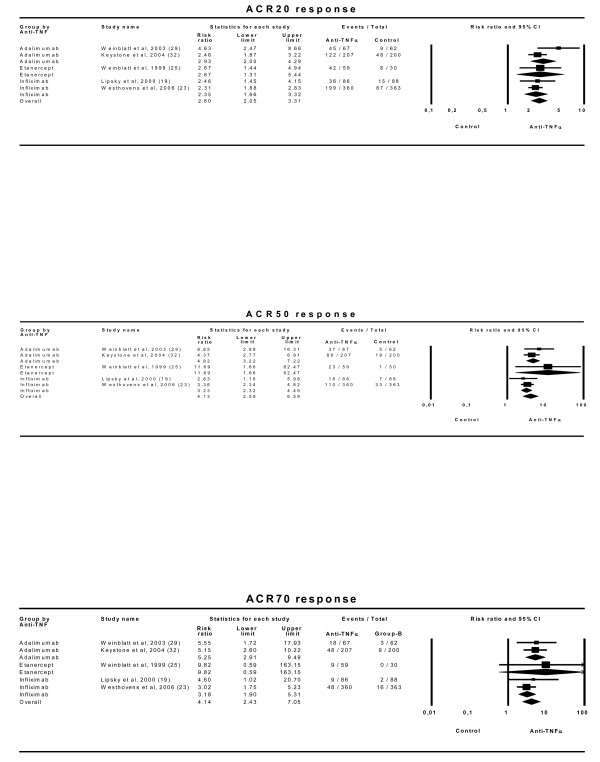
Five trials [19,23,25,29,32] compared the effects of anti-TNFα drugs plus MTX with MTX alone in patients with insufficient responses to MTX. A beneficial combined effect in the ACR20 response is shown: RR 2.60 (2.05–3.31) with an NNT of 4 (3–4). Analyses using the ACR50 and ACR70 responses showed very similar results (Figure 5). There was no evidence of statistical heterogeneity among the different drug classes (Table 4).
Figure 5.
Efficacy of anti-TNFα drugs at recommended doses in combination with MTX compared with MTX alone in patients with insufficient responses to MTX. Effect refers to risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'Upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
Only two trials [24,30] assessed the effect of anti-TNFα drugs versus placebo, showing a combined positive effect on the ACR20 response with an RR of 3.42 (1.60–7.30) and an NNT of 3 (3–4). Although there was a statistically significant heterogeneity of effects according to which drug had been used (Q = 3.8; p = 0.049; I2 = 74) with etanercept apparently more effective than adalimumab (Figure 6), it should be emphasized that there was no direct head to head comparison among them. There was no statistical evidence of heterogeneity in either the ACR50 or the ACR70 response, but the estimates of the effect varied widely between the two drugs, with a pattern similar to that obtained with the ACR20 outcome and based on a rather small number of patients.
Figure 6.
Efficacy of anti-TNFα drugs versus placebo at recommended doses. Effect refers to risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'Upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
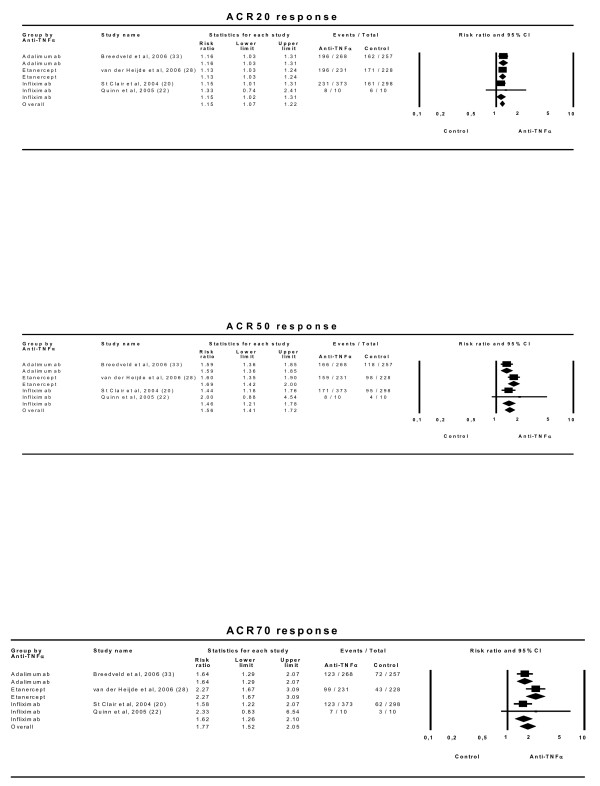
Four trials [20,22,28,33] compared the effect of anti-TNFα drug plus MTX with MTX alone in patients with no previous resistance to MTX. This analysis showed a small but significant combined effect on the ACR20 response of 1.15 (1.07–1.22) with an NNT of 10 (7–16) (Figure 7). The ACR50 showed a combined effect of 1.56 (1.41–1.72) whereas that effect was 1.77 (1.52–2.05) for ACR70. No statistically significant heterogeneity was present for any of these outcomes.
Figure 7.
Efficacy of anti-TNFα drugs plus MTX compared to MTX alone in patients with no previous resistance to MTX (at the recommended doses). Effect refers to risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'Upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
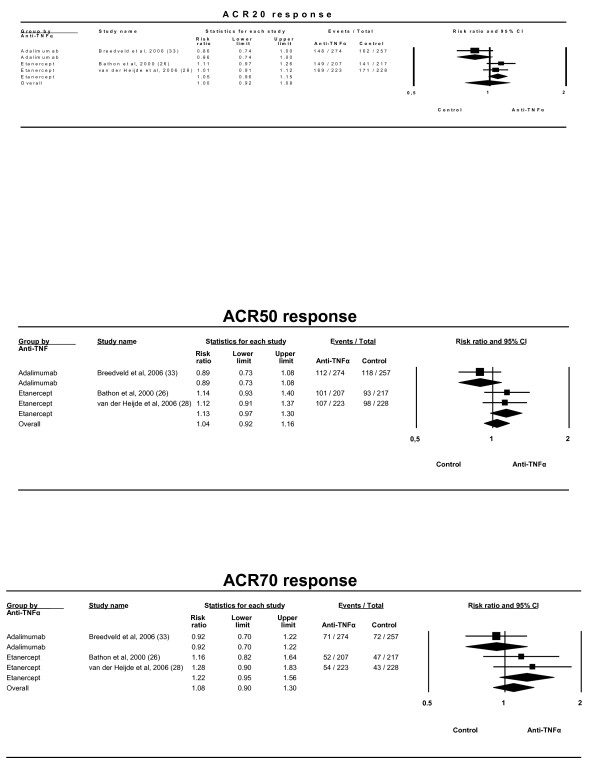
Three trials compared efficacies of anti-TNFα drugs with MTX alone as control [26,28,33]. The ACR20 combined effect showed no significant difference among the arms, with RR = 1.00 (0.92–1.08). Results were similar for the ACR50 and ACR70 responses (Figure 8). The heterogeneity was marginally significant for ACR20 and significant for ACR50 (Table 4).
Figure 8.
Efficacy of anti-TNFα drugs compared to MTX at recommended doses. Effect refers to risk of obtaining the corresponding response with anti-TNFα drug relative to control treatment. 'Lower' and 'Upper' represent the 95% confidence interval limits for the efficacy estimate. Random-effect models.
Safety analysis
An overview of the adverse events reported in the selected trials is displayed in Table 5. The number of withdrawals due to adverse events according to treatment arm was reported in all trials. Information on the incidence of serious infections, malignancies and mortality is also provided, specifying whether patients were in the experimental or control arms, but information about the specific treatment group of the patient was sometimes lacking. Other important safety information (overall number of adverse events, severe adverse events, total number of infections, infusion reactions and injection-site reactions) was provided much less consistently.
Table 5.
Number of patients who presented adverse effects in trials with anti TNFα drugs
| Trial (reference) Anti-TNFα drug | Groups | N of patients | Withdrawn adverse event | Total adverse events | Serious adverse events | Infections | Serious infections | Infusion reactions | Injection- site reactions | Malignancies | Mortality |
| Lipsky et al. (19) Infliximab | 3 mg/Kg 8 | 86 | 5 | _ | _ | _ | _ | _ | _ | ||
| wk +MTX | 86 | 9 | _ | _ | _ | _ | _ | _ | |||
| 3 mg/Kg 4 | 87 | 4 | _ | _ | _ | _ | _ | _ | |||
| wk +MTX | 81 | 8 | _ | _ | _ | _ | _ | _ | |||
| 10 mg/Kg 8 | 340 | 26 | 323 | 58 | 244 | 22 | 5 | 5 | |||
| wk +MTX | 88 | 7 | 83 | 18 | 53 | 7 | NA | 0 | 3 | ||
| 10 mg/Kg 4 | |||||||||||
| wk +MTX | |||||||||||
| Total | |||||||||||
| Infliximab | |||||||||||
| MTX | |||||||||||
| St. Clair et al. (20) Infliximab | 3 mg/Kg 8 | 373 | 34 | _ | _ | 21 | _ | 0 | 1 | ||
| wk +MTX | 378 | 35 | _ | _ | 19 | _ | 4 | 1 | |||
| 6 mg/Kg 8 | 751 | 69 | 103 | 414 | 40 | 135 | 4 | 2 | |||
| wk +MTX | 298 | 9 | NA | 32 | 141 | 6 | 20 | 0 | 2 | ||
| Total | |||||||||||
| Infliximab | |||||||||||
| MTX | |||||||||||
| Quinn et al. (22) Infliximab | 3 mg/Kg 8 | 10 | 1 | 1 | 0 | 1 | 0 | 0 | |||
| wk +MTX | 10 | 0 | NA | 0 | NA | 0 | 0 | 0 | 0 | ||
| MTX | |||||||||||
| Westhovens et al. (23) Infliximab | 3 mg/Kg 8 | 360 | 18 | _ | _ | 6 | 2 | 0 | |||
| wk +MTX | 361 | 20 | _ | _ | 18 | 2 | 2 | ||||
| 10 mg/Kg 8 | 721 | 38 | 512 | 55 | 24 | 4 | 2 | ||||
| wk +MTX | 363 | 8 | 239 | 27 | NA | 6 | NA | 1 | 1 | ||
| Total | |||||||||||
| Infliximab | |||||||||||
| MTX | |||||||||||
| Moreland et al. (24) Etanercept | 25 mg twice | 78 | 2 | 0 | _ | 0 | 0 | ||||
| weekly | 76 | 5 | 0 | _ | 0 | 0 | |||||
| 10 mg twice | 154 | 7 | 0 | 71 | 0 | 0 | |||||
| weekly | 80 | 3 | NA | NA | NA | 0 | 10 | 0 | 0 | ||
| Total | |||||||||||
| Etanercept | |||||||||||
| Placebo | |||||||||||
| Weinblatt et al. (25) Etanercept | 25 mg twice | 59 | 2 | 30 | 0 | 23 | 0 | 0 | |||
| weekly | 30 | 1 | NA | NA | 19 | 0 | 2 | 0 | 0 | ||
| +MTX MTX | |||||||||||
| Bathon et al. (26) Etanercept | 25 mg twice | 207 | 11 | _ | 3 | 1 | |||||
| weekly | 208 | 12 | _ | 2 | 1 | ||||||
| 10 mg twice | 415 | 23 | 140 | 5 | 2 | ||||||
| weekly | 217 | 24 | NA | NA | NA | NA | 16 | 2 | 0 | ||
| Total | |||||||||||
| Etanercept | |||||||||||
| MTX | |||||||||||
| van der Heijde et al. (28) (TEMPO) Etanercept | 25 mg twice | 231 | 37 | _ | _ | _ | 23 | 5 | 1 | ||
| weekly | 223 | 34 | _ | _ | _ | 24 | 5 | 1 | |||
| +MTX | 454 | 71 | 379 | 64 | 285 | 47 | 69 | 10 | 2 | ||
| 25 mg twice | 228 | 47 | 185 | 37 | 147 | 25 | 4 | 2 | 1 | ||
| weekly | |||||||||||
| Total | |||||||||||
| Etanercept | |||||||||||
| MTX | |||||||||||
| Weinblatt et al. (29) (ARMADA) Adalimumab | 40 mg/2 wk | 67 | 0 | _ | _ | _ | _ | ||||
| +MTX | 69 | 4 | _ | _ | _ | _ | |||||
| 20 mg/2 wk | 73 | 1 | _ | _ | _ | _ | |||||
| +MTX | 209 | 5 | 2 | 32 | 1 | 0 | |||||
| 80 mg/2 wk | 62 | 2 | NA | NA | NA | 0 | 2 | 0 | 0 | ||
| +MTX | |||||||||||
| Total | |||||||||||
| Adalimumab | |||||||||||
| MTX | |||||||||||
| van de Putte et al. (30) Adalimumab | 40 mg/2 wk | 113 | 7 | _ | _ | _ | _ | _ | 1 | ||
| 20 mg/2 wk | 106 | 4 | _ | _ | _ | _ | _ | 0 | |||
| 20 mg/wk | 112 | 3 | _ | _ | _ | _ | _ | 0 | |||
| 40 mg/wk | 103 | 3 | 0 | ||||||||
| Total | 434 | 17 | 429 | 53 | 10 | 46 | 4 | 3 | |||
| Adalimumab | 110 | 2 | 105 | 16 | NA | 0 | 1 | 1 | 1 | ||
| Placebo | |||||||||||
| Furst et al. (31) (STAR) Adalimumab | 40 mg/2 wk | 318 | 9 | 275 | 17 | 166 | 4 | 62 | 1 | 1 | |
| DMARD | 318 | 8 | 275 | 22 | 157 | 6 | 37 | 0 | 1 | ||
| Keystone y cols. (32) Adalimumab | 40 mg/2 wk | 207 | 26 | _ | _ | _ | 11 | _ | _ | 2 | |
| +MTX* | 212 | 16 | _ | _ | _ | 5 | _ | _ | 1 | ||
| 20 mg/wk | 419 | 42 | 391 | 97 | 269 | 16 | 101 | 4 | 3 | ||
| +MTX | 200 | 13 | 181 | 37 | 111 | 1 | 48 | 0 | 0 | ||
| Total | |||||||||||
| Adalimumab | |||||||||||
| MTX | |||||||||||
| Breedveld et al. (33) (PREMIER) Adalimumab | 40 mg/2 wk | 268 | 32 | _ | 9 | 2 | 1 | ||||
| +MTX* | 274 | 26 | _ | 3 | 4 | 4 | |||||
| 40 mg/2 wk | 542 | 58 | 524 | 12 | 6 | 5 | |||||
| Total | 257 | 19 | 245 | NA | NA | 7 | NA | 4 | 1 | ||
| Adalimumab | |||||||||||
| MTX |
NA: not available
* Overall data provided although specific data per arm not provided
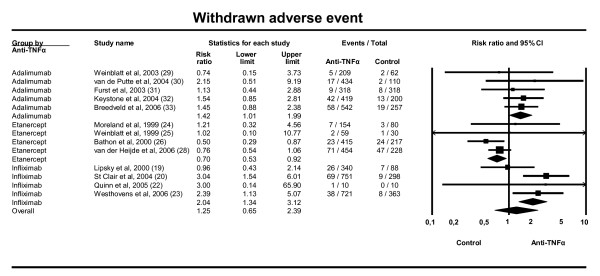
Regarding withdrawals due to adverse events, we found no significant overall difference between the experimental and control groups, with a pooled RR of 1.25 (0.65–2.39) (Figure 9). Results differed depending on the specific anti-TNFα given: patients in the etanercept arms were less likely to withdraw from adverse events than their control counterparts, but the opposite was the case for adalimumab and infliximab, all those comparisons reaching statistical significance. There was statistically significant heterogeneity among the drugs (Q = 29.3; p = 0.003; I2 59) but not within the groups given each specific drug. The results were the same when only groups receiving recommended doses of anti-TNFα drugs were studied. Higher than recommended doses of infliximab led to a higher withdrawal rates. There were no significant differences in withdrawal rates between lower than recommended dose and control arms.
Figure 9.
Adverse event withdrawn in patients with all doses of anti-TNFα drugs.
There were more adverse events in patients allocated to anti-TNFα drugs (RR 1.02 (1.00–1.04)) (p = 0.021) (Table 6). Patients receiving infliximab showed a higher frequency of serious adverse events (p = 0.048) and infections (p = 0.004), but the combined estimates for all three anti-TNFα drugs and safety outcomes were not significant.
Table 6.
Adverse events in patients being treated with anti-TNFα drugs versus control
| ADVERSE EVENTS (anti TNFα vs. control) (references) | Anti-TNFα | Anti-TNFα Adverse events/total | Controls Adverse events/total | RR (95%CI) | NNH(95%CI) | Q | I2 % |
| Withdrawn adverse event | Adalimumab | 131/1922 | 44/947 | 1.4(1.0–2.0) | 47(26–251) | 1.2 | 0 |
| (4826 vs. 2261) | Etanercept | 103/1082 | 75/555 | 0.7(0.5–0.9) | -26(-143 a -14) | 2.4 | 0 |
| ** (19,20,22,23,24,25,26,28,29,30,31,32,33) | Infliximab | 134/1822 | 24/759 | 2.0(1.3–3.1) | 24(17–41) | 4.9 | 0 |
| Total | 368/4826 | 143/2261 | 1.3(0.7–2.4) | NS | 29.3* | 59 | |
| Total adverse events (3228 vs. 1564) (19,23,28,30,31,32,33) | Adalimumab | 1619/1713 | 806/885 | 1.1(0.9–1.1) | NS | 1.9 | 0 |
| (3228 vs. 1564) | Etanercept | 379/454 | 185/228 | 1.0(0.9–1.1) | NS | 0 | 0 |
| (19,23,28,30,31,32,33) | Infliximab | 835/1061 | 322/45 | 1.0(0.9–1.0) | NS | 1.6 | 39 |
| Total | 2833/3228 | 1313/1564 | 1.0(1.0–1.5) | 27(17–59) | 2.9 | 0 | |
| Serious adverse events | Adalimumab | 167/1171 | 75/628 | 1.0(0.7–1.4) | NS | 2.6 | 25 |
| (3235 vs. 1615) | Etanercept | 64/454 | 37/228 | 0.9(0.5–1.6) | NS | 0 | 0 |
| (19,20,22,23,28,30,31,32) | Infliximab | 217/1610 | 77/759 | 1.4(1.0–2.0) | 31(17–167) | 6.2 | 52 |
| Total | 448/3235 | 189/1615 | 1.1(0.8–1.6) | NS | 14.3* | 51 | |
| Infections | Adalimumab | 435/737 | 268/518 | 1.1(0.9–1.2) | NS | 0.7 | 0 |
| (2341 vs. 1162) | Etanercept | 315/513 | 166/258 | 1.0(0.9–1.0) | NS | 0.9 | 0 |
| (19,20,25,28,31,32,33) | Infliximab | 658/1091 | 194/386 | 1.2(1.1–1.3) | 10(7–24) | 0.03 | 0 |
| Total | 1408/2341 | 628/1162 | 1.9(0.9–1.2) | NS | 8.6 | 41 | |
| Serious infections | Adalimumab | 44/1922 | 14/947 | 1.2(0.6–2.8) | NS | 5.8 | 31 |
| (4188 vs.1937) | Etanercept | 47/454 | 25/28 | 0.9(0.4–2.3) | NS | 0 | 0 |
| (19,20,22,23,24,25,28,29,30,31,32,33) | Infliximab | 90/1812 | 19/726 | 1.8(0.9–3.4) | NS | 2.7 | 26 |
| Total | 181/4188 | 58/1937 | 1.4(0.8–2.2) | NS | 11.8 | 32 | |
| Infusión reactions (761 vs. 308) (20,22) |
Infliximab | 136/761 | 20/308 | 2.7(1.7–4.2) | 9(7–14) | 0.005 | 0 |
| Injection-site reactions | Adalimumab | 241/1380 | 88/690 | 1.7(1.0–3.0) | 22(13–67) | 12.6* | 72 |
| (2454 vs. 1245) | Etanercept | 303/1074 | 32/555 | 5.1(2.9–8.8) | 5(4–6) | 2.3 | 0 |
| (24,25,26,28,29,30,31,32) | Total | 544/2454 | 120/1245 | 3.0(1.0–8.6) | 8(7–10) | 51.8* | 86 |
| Malignancies | Adalimumab | 16/1922 | 5/947 | 1.1(0.4–2.7) | NS | 1.6 | 0 |
| (4826 vs. 2261) | Etanercept | 15/1082 | 4/555 | 1.9(0.6–5.7) | NS | 0.3 | 0 |
| (19,20,22,23,24,25,26,28,29,30,31,32,33) | Infliximab | 13/1822 | 1/759 | 2.6(0.6–11.6) | NS | 1.1 | 0 |
| Total | 44/4826 | 10/2261 | 1.5(0.8–3.0) | NS | 3.3 | 0 | |
| Mortality | Adalimumab | 10/1922 | 3/947 | 1.3(0.4–4.7) | NS | 2.0 | 0 |
| (4826 vs. 2261) | Etanercept | 4/1082 | 1/555 | 1.5(0.2–9.5) | NS | 0.2 | 0 |
| (19,20,22,23,24,25,26,28,29,30,31,32,33) | Infliximab | 9/1822 | 5/759 | 0.5(0.2–1.4) | NS | 0.4 | 0 |
| Total | 23/4826 | 9/2261 | 0.8(0.3–2.1) | NS | 4.4 | 0 |
RR (95%CI): relative risk (95% confidence limits)
NNH: number needed to harm
Q: Cochrane's Q
I2: percentage of variability in study results attributable to between-study differences
* statistical heterogeneity
NS: non-significant results
** this figure include 208 patients of the Bathon trial not included in efficacy studies as efficacy date were not reported
Information on severe infections, malignancies and deaths was provided in all trials except for severe infections in the Bathon trial. No significant combined increases in risk were seen for any of these results.
We also inquired whether higher than recommended doses are associated with higher incidences of adverse events. The reported data were incomplete, however, as the Lipsky et al. trial [19] did not assign the 22 severe infections detected to each corresponding infliximab dose arm. The risk of severe infection when receiving high doses of infliximab [20,23] was significantly increased (p = 0.006) with an NNH of 40 (26–91), but the risk of developing malignancies was not increased (p = 0.116). Nor did the two trials [29,30] administering high doses of adalimumab report the dose received by patients experiencing severe infections.
Discussion
This study approached a problem of major clinical and socio-economic importance: the efficacy and safety of anti-TNFα drugs in the treatment of rheumatoid arthritis (RA). We have considered these drugs both individually and as a specific therapeutic group. We have evaluated their efficacy as monotherapy and in combination with MTX. In addition, the efficacies of different doses and the safety of these drugs were explored.
Our search of the literature on the efficacy of anti-TNFα drugs in RA identified thirteen clinical trials fulfilling the required criteria for inclusion in the systematic review and metaanalysis. All thirteen were randomised-controlled trials with a minimal follow-up time of 6 months and used comparable standardised parameters of efficacy. Although the general quality of the trials was high, some difficulties became apparent during the review. The number of trials fulfilling the required criteria was small. Furthermore, there were several sources of clinically relevant heterogeneity: different control treatments were used, populations were not homogeneous, follow-up times differed among trials and the doses administered varied widely (Table 1). Also, the funnel plot asymmetry might indicate publication bias or other types of problems.
In our study, combined analysis of the results from all trials using the recommended doses led us to conclude that anti-TNFα drugs (considered as a therapeutic group) show an effect significantly superior to that of control treatments. However, the heterogeneity was very high, calling for subgroups and more homogeneous comparisons. We only evaluated those trials for which relevant homogeneous comparisons were possible, and a substantial reduction in heterogeneity was apparent when we focused on these groups (Table 4). Comparison of the three anti-TNFα drug plus MTX with MTX alone in patients with insufficient responses to MTX showed no significant heterogeneity of effects, yet despite the absence of head to head comparisons we found no evidence whatsoever that the relative effects of individual drugs are different. Etanercept seemed superior to adalimumab when both drugs were compared to placebo. However, the response observed in the control group of the adalimumab study was substantially higher than that of the etanercept reference group, which casts doubts on the actual comparability of the results and makes it difficult to draw definitive conclusions until the drugs have been compared directly in well designed, head to head randomised trials. Anti-TNFα drug plus MTX had a greater effect than MTX alone in patients with no previous resistance to MTX, but the magnitude of this effect was markedly lower than that obtained in patients with previously inadequate responses to MTX. Trials that assessed this specific efficacy issue recruited patients with short-lasting, less severe disease showing high responses to both experimental and control treatments, thus explaining the lower relative and absolute efficacy estimates (Table 4). In fact, the effects achieved with etanercept and adalimumab in these patients were equivalent to those obtained using MTX for the first time.
When the potential influence on efficacy of doses administered was evaluated, both higher and lower doses than are currently recommended seemed to elicit similar effects, except for the effect of lower doses on ACR70. However, comparisons in this last case are based on a small number of patients.
In the light of these findings it seems sensible to advise that current treatment of moderate and severe RA should be started with MTX. Anti-TNFα drugs should be restricted to patients who do not respond sufficiently to DMARD combinations until experimental evidence demonstrates that the new biological drugs have greater efficacy in earlier stages of RA. It might also be potentially useful to start the indicated treatment with a low dose and then increase it as a function of the magnitude of the response. An alternative option might be to start with the current recommended doses and try to decrease them after a significant stable effect is reached, in order to minimise adverse effects. This issue encompasses important clinical and economic implications probably meriting further research.
For a correct interpretation of our results, the fact that our analyses were based on the ACR response should be taken into account. In recent years, another multidimensional index, the DAS index, has been increasingly used [65]. However, it was not used in any of the trials included in the current review. ACR20, 50 and 70 responses are well-known validated response criteria and they were available in all these anti-TNFα studies, enabling us to conduct a combined analysis and statistical evaluation of the results. Another important subject in the evaluation of the response of RA to anti-TNFα drugs is the quantification of radiological damage (inhibition of progression of structural joint damage). The modified Sharp score was analysed in six trials providing 12-month results and showing the ability of infliximab, etanercept and adalimumab to inhibit the progression of structural joint damage in RA [19,20,26,28,32,33]. Nevertheless, several factors deterred us from using this score as an outcome variable: since it is not normally distributed, the way this index was summarised and displayed in the identified trials did not permit statistical pooling of the results. Moreover the clinical implications of this radiological finding are not yet well understood.
Safety issues are also of central concern. Although we focused solely on published results from well-designed randomised controlled trials, our review shows that patients receiving anti-TNFα drugs are more prone to experience adverse events. Although some of the relative safety estimates are statistically significant, their magnitude is rather small and their clinical relevance should be also addressed. Patients on infliximab and adalimumab withdrew from the trial because of adverse events more frequently than patients on etanercept. Treatment with infliximab is associated with higher frequencies of serious adverse events and infections. If high doses are administered, there is also an increased likelihood of severe infections. All in all, the safety/efficacy relationship as estimated by the NNH/NNT ratio appears to be favourable.
Two metaanalysis have been performed previously [66,67] focusing the problem, although none has been published. Both showed a greater efficacy of etanercept against infliximab. A comprehensive technical report addressing these issues, including an economic evaluation, has recently been published by Chen et al. [68]. Although the deadline for inclusion of studies was February 2005, that article pooled information from 29 studies as its inclusion criteria were much broader: it included studies of shorter duration [46,43,46-48,44], studies using other than the recommended routes of administration [49,51,52], studies in which no arm received recommended doses [62] and a trial in which efficacy was not measured using ACR criteria [58]. It also included unpublished studies. Despite this less restrictive approach, the Chen et al. paper likewise confirms the efficacy of all three marketed anti-TNFα drugs at recommended doses, especially when administered to patients with previous resistance to MTX.
A metaanalysis recently published by Bongartz et al. [7] focused on safety matters regarding infliximab and adalimumab. The risk of malignancies and infections was increased when higher doses were administered. There have been some controversies surrounding its conclusions, involving the accuracy of clinical trials with short follow-up as a means of detecting severe adverse events [69]. With respect to severe infections, our metaanalysis, although it detected a higher frequency in the anti-TNFα arms, showed no significant difference. We pooled safety data from the three available treatments whereas Bongartz et al. [7] only analysed infliximab and adalimumab using a fixed effects pooling method. If we restrict our analysis to infliximab and adalimumab and use a fixed effects model, we also find a significantly higher frequency of severe infections (p = 0.047) with an NNH of 61 (41–126). Therefore, it is likely that the use of both drugs, especially in higher than recommended doses, may increase the risk of severe infections. This risk has not so far been shown for etanercept, but as far as we are aware no study with higher than recommended doses has been published. Our results regarding the incidence of malignancies do not agree with those of Bongartz et al. [7], but they also include malignancies developed at a later stage when the trials are no longer underway.
Recently, two systematic reviews with meta-analysis have been published addressing the role of anti-TNFα drugs as added to MTX vs. MTX alone [70,71]. Both articles select a very limited number of trials, and share an important design limitation, namely, they compare clinical trials that recruit both MTX-naïve patients and MTX-resistant patients, which, from our standpoint leads to their results facing validity problems.
There are limitations to our study that are also shared by other published metaanalyses [7,68] and deserve further comment. The number of published studies is scarce, there is significant heterogeneity in some relevant aspects (patient clinical profiles, comparisons undertaken and lengths of follow-up) and information on safety parameters varies widely among trials. We have attempted to deal with these limitations in the original research by designing and applying rather stringent selection criteria so that our results are based on solid coherent evidence. This reinforcement of internal validity might have been at the expense of some loss of generalizability, yet the quality of information excluded is at least controversial. We have provided these pooled NNTs as a kind of effect estimate for average risk patients. In an attempt to minimise the presence of factors known to influence risks and therefore NNTs, we have selected rather homogeneous studies in terms of minimum follow-up, diagnostic criteria and have further made subgroup analyses accounting for several important clinical characteristics. We have performed an extensive and detailed analysis of available efficacy and safety data.
Conclusion
It may be concluded that the three marketed anti-TNFα drugs are more effective than the corresponding control treatments (MTX or placebo) in RA patients, with an NNT of 5 for ACR20 and ACR50 and of 7 for ACR70 at currently recommended doses (Table 3). High heterogeneity among trials is apparent in key design aspects and is reflected in the results of the combined analysis of all trials, calling for more in-depth assessment of more homogeneous subgroups. When this task is addressed, patients with previously inadequate responses to MTX show similar positive responses when any of the anti-TNFα drugs are added to their treatment regimes. However, when anti-TNFα drug plus MTX is compared with MTX alone in patients with no previous resistance to MTX, the relative efficacy of the combined regime is much lower. Etanercept and adalimumab are superior to the placebo but their effect in monotherapy is similar to that obtained with MTX. Therefore, we advise against starting treatment with anti-TNFα drugs until a lack of adequate response to MTX is clearly documented. Increasing doses lead to no increase in efficacy (Table 3). Analysis of the effect of low anti-TNFα doses suggests that patients treated with etanercept or adalimumab might obtain clinically substantial benefits with doses lower than those currently recommended if indicated on the basis of safety or other grounds.
Overall, patients on anti-TNFα drugs experience adverse events more frequently and those using infliximab and adalimumab have higher withdrawal rates. Infliximab use is associated with a higher likelihood of severe adverse events including severe infections. Interestingly, though, patients using etanercept seem to do so with lower frequency, although this finding might be due to the limitation of the range of doses used to those recommended by the manufacturer. We found no significant difference in the development of malignancies during the follow-up times in the studies. The safety/efficacy relationship is favourable, especially if recommended doses are used. The safety profile of etanercept might be apparently superior because the other drugs were tested over a wider range of doses, including higher than recommended ones.
Although more research is warranted, especially well-powered head to head randomised comparisons of anti-TNFα drugs, our study helps to clarify some frequently encountered questions in the clinical care of RA patients.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
AA-R conceived and designed the study and was involved in data extraction, analysis and preparation of the manuscript. JIP was involved in statistical analysis and preparation of the manuscript. EA developed and conducted the search strategy. MC contributed to data extraction, AU to statistical analysis and AQ to analysis and preparing of the manuscript. All authors critically revised the manuscript and approved it for publication. AA-R is the guarantor.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Alberto Alonso-Ruiz, Email: alonsoru@teleline.es.
Jose Ignacio Pijoan, Email: joseignacio.pijoanzubizarreta@osakidetza.net.
Eukene Ansuategui, Email: eukenean@euskalnet.net.
Arantxa Urkaregi, Email: alonsoru@teleline.es.
Marcelo Calabozo, Email: marcelo.calabozoraluy@osakidetza.net.
Antonio Quintana, Email: kfpquloa@ehu.es.
References
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Maini RN, Feldmann M. TNF α-a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992;31:293–8. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- Furst DE, Breedveld FC, Kalden JR, Smolen JS, Burmester GR, Dougados M, Emery P, Gibofsky A, Kavanaugh AF, Keystone EC, Klareskog L, Russell AS, van de Putte LB, Weisman MH. Updated consensus statement on biological agents for the treatment of rheumatoid arthritis and other immune mediated inflammatory diseases (May 2003) Ann Rheum Dis. 2003;62:ii2–9. doi: 10.1136/ard.62.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg MC, Lebwohl MG, Plevy SE, Hobbs KF, Yocum DE. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin Arthritis Rheum. 2005;34:819–836. doi: 10.1016/j.semarthrit.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–79. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Hochberg MC, Tracy JK, Hawkins-Holt M, Flores RH. Comparison of the efficacy of the tumour necrosis factor α blocking agents adalimumab, etanercept, and infliximab when added to methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2003;62:ii13–ii16. doi: 10.1136/ard.62.suppl_2.ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Boers M, Bonbardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R, Jr, Paulus H, Strand V. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- Jadad Ar, Moore RA, Carroll D, Jenkinson C, Reynols DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trils: is binding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Dees Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiri M, Suarez-Almanzor ME, Wells GA, Robinson V, Tugwell P. Number needed to treat (NNT): implication in rheumatology clinical practice. Ann Rheum Dis. 2003;62:316–21. doi: 10.1136/ard.62.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL, Haynes RB. Summarising the effects of therapy: a new table and some more terms (EBM Notebook) Evidence-Based Medicine. 1997. pp. 103–4.
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequencs of treatment. N Engl J Med. 1988;318:1728–33. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Scheider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg CB, Mazumdar M. Operating characteristic of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St. Clair EW, Furst DE, Breedveld FC, Kolden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1595–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- St. Clair EW, van der Heijde DM, Smolen J, Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang B, Dewoody K, Weiss R, Baker D. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis. A randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- Maini RN, St Clair EW, Breedveld F, Furst D, Kalden JR, Weisman M, Smolen J, Harriman G, Feldmann M, Lipsky P, for the ATTRACT Study Group Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354:1932–9. doi: 10.1016/S0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, Brown C, Fraser A, Jarret S, Emery P. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: Results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:27–35. doi: 10.1002/art.20712. [DOI] [PubMed] [Google Scholar]
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, Rahman MU. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities. A large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54:1075–86. doi: 10.1002/art.21734. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Bulpitt KJ, Martin R, Weinblatt M, Taborn J, Weaver A, Burge DJ, Schiff Mh. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Fink BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malise M, Martin Mola E, Pavelka K, Sany J, Settas L, Wajdula J, Pedersen R, Fateneland S, Sanda M, for the TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- van der Heijde D, Klareskog L, Rodríguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, Tornero-Molina J, Wajdula J, Pedersen R, Fatenejad S, for the TEMPO study investigators Comparison of etaercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis. Two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- van de Putte LBA, Atkins C, Malaise M, Sany J, Russell AS, van Riel PLCM, Settas L, Bijlsma JW, Todesco S, Dougados M, Nash P, Emery P, Walter M, Kaul M, Fischkoff S, Kupper H. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor-α monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J Rheumatol. 2003;30:2563–71. [PubMed] [Google Scholar]
- Keystone EC, Kavanaugh , Sharp JT, Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA, Chartash EK. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid artritis receiving concomitant methotrexate therapy. A randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study. A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- Temekonidis TI, Georgiadis AN, Alamanos Y, Bougias DV, Voulgari PV, Dorsos AA. Infliximab treatment in combination with cyclosporin A in patients with severe refractory rheumatoid arthritis. Ann Rheum Dis. 2002;61:822–5. doi: 10.1136/ard.61.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Cannon GW, Spencer-Green G, Finck BK. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- Barrera P, van der Maas A, van Ede AE, Kiemeney BA, Laan RF, van de Putte , van Riel PL. Drug survival, efficacy and toxicity of monotherapy with a fully human anti-tumour necrosis factor-alpha antibody compared with methotrexate in long-standing rheumatoid arthritis. Rheumatology (Oxford) 2002;41:430–9. doi: 10.1093/rheumatology/41.4.430. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Cohen SB, Baumgartner SW, Tindall EA, Bulpitt K, Martin R, Weinblatt M, Taborn J, Weaver A, Burge DJ, Schiff MH. Longterm safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol. 2001;28:1238–44. [PubMed] [Google Scholar]
- Kavanaugh A, St Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric anti-tumor necrosis factor-alfa monoclonal antibody treatment of patients with rheumatoid arthritis receibing methotrexate therapy. J Rheumatol. 2000;27:841–50. [PubMed] [Google Scholar]
- Elliot MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, Woody JN. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patiens with rheumatoid arthritis. Lancet. 1994;344:1125–27. doi: 10.1016/S0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- O'Dell JR, Petersen K, Leff R, Palmer W, Schned E, Blakely K, Haire C, Fernandez A. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213–18. [PubMed] [Google Scholar]
- Finckh A, Simard JF, Duryea J, Liang MH, Huang J, Daneel S, Forster A, Gabay C, Guerne PA. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54–59. doi: 10.1002/art.21491. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–90. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young M., Jr A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12-week, double-blind, randomized, placebo-controlled study. J Formos Med Assoc. 2004;103:618–23. [PubMed] [Google Scholar]
- Durez P, Nzeusseu Toukap A, Lauwerys BR, Manicourt DH, Verschueren P, Westhovens R, Devogelaer JP, Houssiau FA. A randomised comparative study of the short term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment. Ann Rheum Dis. 2004;63:1069–74. doi: 10.1136/ard.2003.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, Burge DJ. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–63. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- van de Putte LB, Rau R, Breedveld FC, Kalden JR, Malaise MG, van Riel PL, Schattenkirchner M, Emery P, Burmester GR, Zeidler H, Moutsopoulos HM, Beck K, Kupper H. Efficacy and safety of the fully human anti-tumour necrosis factor alpha monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II study. Ann Rheum Dis. 2003;62:1168–77. doi: 10.1136/ard.2003.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, Tindal EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–47. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Elliot MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Breedveld FC, Macfarlane JD, Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alfa(cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–10. doi: 10.1016/S0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Rau R, Simianer S, van Riel PL, van de Putte LB, Kruger K, Schattenkirchner M. Rapid alleviation of signs and symptoms of rheumatoid arthritis with intravenous or subcutaneous administration of adalimumab in combination with methotrexate. Scand J Rheumatol. 2004;33:145–153. doi: 10.1080/03009740410005467. [DOI] [PubMed] [Google Scholar]
- Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, Fischkoff SA, Chartash EK. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25:1700–21. doi: 10.1016/S0149-2918(03)80164-9. [DOI] [PubMed] [Google Scholar]
- Broeder A, van de Putte LBA, Rau R, Schattenkirchner M, van Riedel LCM, Sander O, Binder C, Fenner H, Bankmann Y, Velagapudi R, Kempeni J, Kupper H. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alfa antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29:2288–98. [PubMed] [Google Scholar]
- Moreland LW, Margolies G, Heck LW, Jr, Saway A, Blosch C, Hanna R, Koopman WJ. Recombinant soluble tumor necrosis factor receptor (p80) fusion protein: toxicity and dose finding trial in refractory rheumatoid arthritis. J Rheumatol. 1996;23:1849–55. [PubMed] [Google Scholar]
- Torrance GW, Tugwell P, Amorosi S, Chartash E, Sengupta N. Improvement in health utility among patients with rheumatoid arthritis treated with adalimumab (a human anti-TNF monoclonal antibody) plus methotrexate. Rheumatology (Oxford) 2004;43:712–8. doi: 10.1093/rheumatology/keh153. [DOI] [PubMed] [Google Scholar]
- Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22:128–39. doi: 10.1016/S0149-2918(00)87984-9. [DOI] [PubMed] [Google Scholar]
- van der Heijde D, Klareskog L, Singh A, Tornero J, Melo-Gomes J, Codreanu C, Pedersen R, Freundlich B, Fatenejad S. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis. 2006;65:328–34. doi: 10.1136/ard.2005.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld FC, Emery P, Keystone E, Patel K, Furst DE, Kalden JR, St Clair EW, Weisman M, Smolen J, Lipsky PE, Maini RN. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149–55. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Van Der Heijde DM, St Clair EW, Emery P, Bathon JM, Keystone E, Maini RN, Kalden JR, Schiff M, Baker D, Han C, Han J, Bala M. Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset (ASPIRE) Study Group. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JC, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451–9. doi: 10.1002/art.10302. [DOI] [PubMed] [Google Scholar]
- van de Putte LB, Sander O, Rau R. Therapy of refractory chronic polyarthritis with tumor necrosis factor alpha receptor fusion proteins (TNFR55-IgG1) results of double-blind placebo-controlled studies over 3 months. Z Rheumatol. 1998;57:302–6. doi: 10.1007/s003930050116. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJ, Marsters PA, Wagner CL, McClinton C, Maini RN. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum. 2004;50:1107–16. doi: 10.1002/art.20123. [DOI] [PubMed] [Google Scholar]
- Wiland P, Glowska A, Chlebicki A, Szechinski J. Analysis of efficacy and safety of multiple intravenous infusion of anti-tumor necrosis factor-alpha monoclonal antibody (Remicade) combined with methotrexate compared with sodium aurothiomalate and intramuscular depot methylprednisolone in rheumatoid arthritis. Pol Arch Med Wewn. 2002;108:1055–63. [PubMed] [Google Scholar]
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, Nagaya I. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44. [PubMed] [Google Scholar]
- Van Gestel AM, Haagesma CJ, Van riel PLCM. Validación of rheumatoid arthritis improvement criteria including simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bacon P, Reynols AV. The efficacy of etanercept and infliximab in patients with rheumatoid arthritis who have failed treatment with DMARDs: a meta-analysis (abstract) Arthritis Rheum. 2002;46:s332. [Google Scholar]
- Sing A, Nab H. A meta-analysis of biological response modifiers in the treatment of rheumatoid arthritis for patients failing one or more disease modifying antirheumatic drugs (abstract THU0250) Ann Rheum Dis. 2003;62:185. [Google Scholar]
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:1–248. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- Dixon W, Silman A. Is there an association between anti-TNF monoclonal antibody therapy in rheumatoid arthritis and risk of malignancy and serious infection? Commentary on meta-analysis by Bongartz et al. Arthritis Researh Therapy. 2007;8:111–13. doi: 10.1186/ar2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen LE, Christensen R, Bliddal H, Geborek P, Danneskiold-Samsee B, Saxne T. The number needed to treat for adalimumab, etanercept, and infliximab based on ACR50 response in three randomized controlled trials on established rheumatoid arthritis: a systematic literature review. Scand J Rheumatol. 2007;36:411–417. doi: 10.1080/03009740701607067. [DOI] [PubMed] [Google Scholar]
- Lee YH, Woo JH, Rho YH, Choi SJ, Ji JD, Song GG. Meta-analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatol Int. [DOI] [PubMed]