Abstract
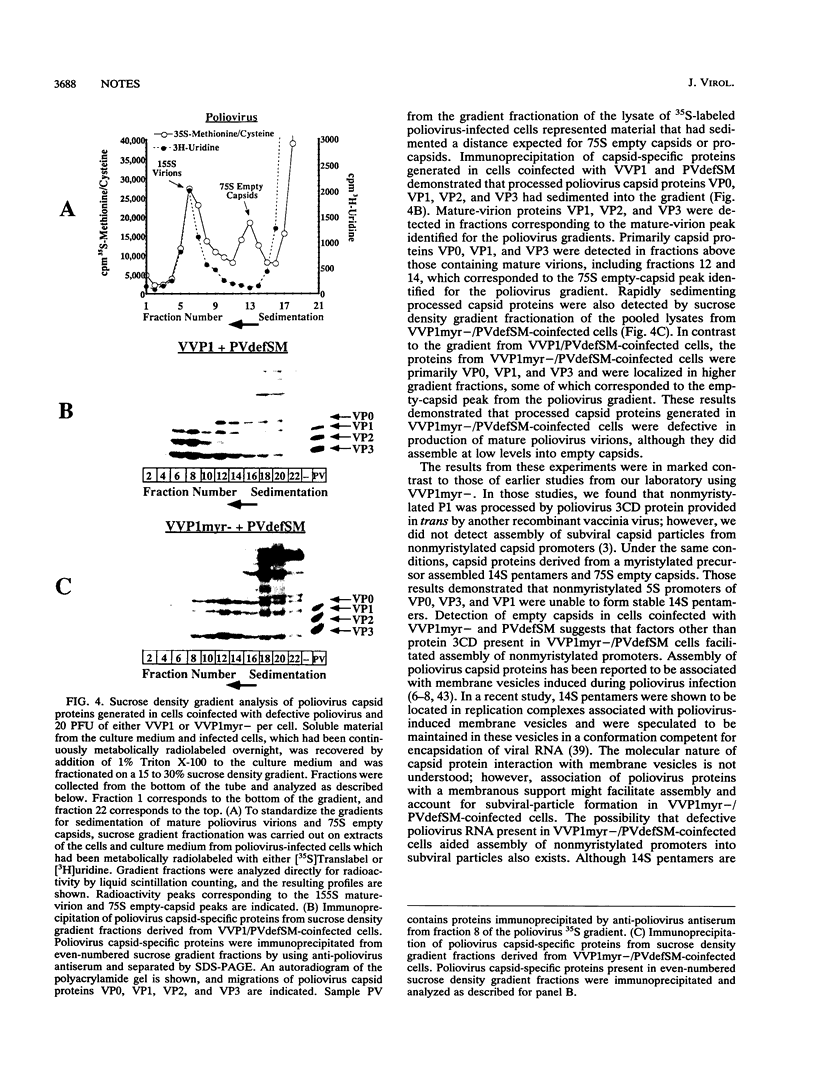
Defective interfering (DI) RNA genomes of poliovirus which contain in-frame deletions in the P1 capsid protein-encoding region have been described. DI genomes are capable of replication and can be encapsidated by capsid proteins provided in trans from wild-type poliovirus. In this report, we demonstrate that a previously described poliovirus DI genome (K. Hagino-Yamagishi and A. Nomoto, J. Virol. 63:5386-5392, 1989) can be complemented by a recombinant vaccinia virus, VVP1 (D. C. Ansardi, D. C. Porter, and C. D. Morrow, J. Virol. 65:2088-2092, 1991), which expresses the poliovirus capsid precursor polyprotein, P1. Stocks of defective polioviruses were generated by transfecting in vitro-transcribed defective genome RNA derived from plasmid pSM1(T7)1 into HeLa cells infected with VVP1 and were maintained by serial passage in the presence of VVP1. Encapsidation of the defective poliovirus genome was demonstrated by characterizing poliovirus-specific protein expression in cells infected with preparations of defective poliovirus and by Northern (RNA) blot analysis of poliovirus-specific RNA incorporated into defective poliovirus particles. Cells infected with preparations of defective poliovirus expressed poliovirus protein 3CD but did not express capsid proteins derived from a full-length P1 precursor. Poliovirus-specific RNA encapsidated in viral particles generated in cells coinfected with VVP1 and defective poliovirus migrated slightly faster on formaldehyde-agarose gels than wild-type poliovirus RNA, demonstrating maintenance of the genomic deletion. By metabolic radiolabeling with [35S]methionine-cysteine, the defective poliovirus particles were shown to contain appropriate mature-virion proteins. This is the first report of the generation of a pure population of defective polioviruses free of contaminating wild-type poliovirus. We demonstrate the use of this recombinant vaccinia virus-defective poliovirus genome complementation system for studying the effects of a defined mutation in the P1 capsid precursor on virus assembly. Following removal of residual VVP1 from defective poliovirus preparations, processing and assembly of poliovirus capsid proteins derived from a nonmyristylated P1 precursor expressed by a recombinant vaccinia virus, VVP1 myr- (D. C. Ansardi, D. C. Porter, and C. D. Morrow, J. Virol. 66:4556-4563, 1992), in cells coinfected with defective poliovirus were analyzed. Capsid proteins generated from nonmyristylated P1 did not assemble detectable levels of mature virions but did assemble, at low levels, into empty capsids.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansardi D. C., Porter D. C., Morrow C. D. Coinfection with recombinant vaccinia viruses expressing poliovirus P1 and P3 proteins results in polyprotein processing and formation of empty capsid structures. J Virol. 1991 Apr;65(4):2088–2092. doi: 10.1128/jvi.65.4.2088-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansardi D. C., Porter D. C., Morrow C. D. Myristylation of poliovirus capsid precursor P1 is required for assembly of subviral particles. J Virol. 1992 Jul;66(7):4556–4563. doi: 10.1128/jvi.66.7.4556-4563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E., Luo M., Vriend G., Rossmann M. G., Palmenberg A. C., Parks G. D., Nicklin M. J., Wimmer E. Implications of the picornavirus capsid structure for polyprotein processing. Proc Natl Acad Sci U S A. 1987 Jan;84(1):21–25. doi: 10.1073/pnas.84.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Compans R. W. The formation of poliovirus particles in association with the RNA replication complexes. J Gen Virol. 1973 Oct;21:99–108. doi: 10.1099/0022-1317-21-1-99. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Mosser A. G. Proteins associated with the poliovirus RNA replication complex. Virology. 1971 Nov;46(2):375–386. doi: 10.1016/0042-6822(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Choi W. S., Pal-Ghosh R., Morrow C. D. Expression of human immunodeficiency virus type 1 (HIV-1) gag, pol, and env proteins from chimeric HIV-1-poliovirus minireplicons. J Virol. 1991 Jun;65(6):2875–2883. doi: 10.1128/jvi.65.6.2875-2883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. 3. Interference and enrichment. J Mol Biol. 1973 May 25;76(3):345–361. doi: 10.1016/0022-2836(73)90509-3. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. IV. Mechanisms of enrichment. J Virol. 1973 Dec;12(6):1414–1426. doi: 10.1128/jvi.12.6.1414-1426.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N. Defective interfering (di) particles of poliovirus. Prog Med Virol. 1975;20:180–207. [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Hagino-Yamagishi K., Nomoto A. In vitro construction of poliovirus defective interfering particles. J Virol. 1989 Dec;63(12):5386–5392. doi: 10.1128/jvi.63.12.5386-5392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S. A., Luo M., Morrow C. D. Enzymatic activity of poliovirus RNA polymerase mutants with single amino acid changes in the conserved YGDD amino acid motif. J Virol. 1991 Sep;65(9):4565–4572. doi: 10.1128/jvi.65.9.4565-4572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Cason J., Burke K. L., Lunney D., Gillen A., Patel D., McCance D. J., Almond J. W. An antigen chimera of poliovirus induces antibodies against human papillomavirus type 16. J Virol. 1990 Mar;64(3):1201–1206. doi: 10.1128/jvi.64.3.1201-1206.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajigaya S., Arakawa H., Kuge S., Koi T., Imura N., Nomoto A. Isolation and characterization of defective-interfering particles of poliovirus Sabin 1 strain. Virology. 1985 Apr 30;142(2):307–316. doi: 10.1016/0042-6822(85)90339-3. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Racaniello V. R. Construction and characterization of poliovirus subgenomic replicons. J Virol. 1988 May;62(5):1687–1696. doi: 10.1128/jvi.62.5.1687-1696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kitson J. D., Burke K. L., Pullen L. A., Belsham G. J., Almond J. W. Chimeric polioviruses that include sequences derived from two independent antigenic sites of foot-and-mouth disease virus (FMDV) induce neutralizing antibodies against FMDV in guinea pigs. J Virol. 1991 Jun;65(6):3068–3075. doi: 10.1128/jvi.65.6.3068-3075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Hölscher C., Reuer Q., Harber J., Wimmer E. Myristoylation of the poliovirus polyprotein is required for proteolytic processing of the capsid and for viral infectivity. J Virol. 1990 May;64(5):2433–2436. doi: 10.1128/jvi.64.5.2433-2436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Saito I., Nomoto A. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J Mol Biol. 1986 Dec 5;192(3):473–487. doi: 10.1016/0022-2836(86)90270-6. [DOI] [PubMed] [Google Scholar]
- Li G. P., Prágai B. M., Rice C. M. Rescue of Sindbis virus-specific RNA replication and transcription by using a vaccinia virus recombinant. J Virol. 1991 Dec;65(12):6714–6723. doi: 10.1128/jvi.65.12.6714-6723.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. E., Sullivan M., Maizel J. V., Jr Characterization of a new isolate of poliovirus defective interfering particles. Cell. 1979 Nov;18(3):759–769. doi: 10.1016/0092-8674(79)90129-6. [DOI] [PubMed] [Google Scholar]
- Marc D., Drugeon G., Haenni A. L., Girard M., van der Werf S. Role of myristoylation of poliovirus capsid protein VP4 as determined by site-directed mutagenesis of its N-terminal sequence. EMBO J. 1989 Sep;8(9):2661–2668. doi: 10.1002/j.1460-2075.1989.tb08406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc D., Girard M., van der Werf S. A Gly1 to Ala substitution in poliovirus capsid protein VP0 blocks its myristoylation and prevents viral assembly. J Gen Virol. 1991 May;72(Pt 5):1151–1157. doi: 10.1099/0022-1317-72-5-1151. [DOI] [PubMed] [Google Scholar]
- Marc D., Masson G., Girard M., van der Werf S. Lack of myristoylation of poliovirus capsid polypeptide VP0 prevents the formation of virions or results in the assembly of noninfectious virus particles. J Virol. 1990 Sep;64(9):4099–4107. doi: 10.1128/jvi.64.9.4099-4107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscufo N., Simons J., Chow M. Myristoylation is important at multiple stages in poliovirus assembly. J Virol. 1991 May;65(5):2372–2380. doi: 10.1128/jvi.65.5.2372-2380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. V., Schultz A., Pincus S. E., Oroszlan S., Wimmer E. Capsid protein VP4 of poliovirus is N-myristoylated. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7827–7831. doi: 10.1073/pnas.84.22.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy N., Barclay W. S., Sullivan M., Almond J. W. A poliovirus replicon containing the chloramphenicol acetyltransferase gene can be used to study the replication and encapsidation of poliovirus RNA. J Virol. 1992 Aug;66(8):5040–5046. doi: 10.1128/jvi.66.8.5040-5046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister T., Pasamontes L., Troxler M., Egger D., Bienz K. Immunocytochemical localization of capsid-related particles in subcellular fractions of poliovirus-infected cells. Virology. 1992 Jun;188(2):676–684. doi: 10.1016/0042-6822(92)90522-q. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R., Pallansch M. A., Nottay B. K., Kew O. M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987 Oct;160(2):311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Rombaut B., Vrijsen R., Boeyé A. New evidence for the precursor role of 14 S subunits in poliovirus morphogenesis. Virology. 1990 Jul;177(1):411–414. doi: 10.1016/0042-6822(90)90502-i. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]