Abstract
Paraneoplastic opsoclonus myoclonus ataxia (POMA) is a neurologic disorder thought to be mediated by an autoimmune attack against onconeural disease antigens that are expressed by gynecologic or lung tumors and by neurons. One POMA disease antigen, termed Nova-1, has been identified as a neuron-specific KH-type RNA-binding protein. Nova-1 expression is restricted to specific regions of the central nervous system, primarily the hindbrain and ventral spinal cord, which correlate with the predominantly motor symptoms in POMA. However, POMA antisera recognize antigens that are widely expressed in both caudal and rostral regions of the central nervous system, and some patients develop cognitive symptoms. We have used POMA antisera to clone a cDNA encoding a second POMA disease antigen termed Nova-2. Nova-2 is closely related to Nova-1, and is expressed at high levels in neurons during development and in adulthood, and at lower levels in the adult lung. In the postnatal mouse brain, Nova-2 is expressed in a pattern that is largely reciprocal with Nova-1, including high levels of Nova-2 expression in the neocortex and hippocampus. Functional characterization of Nova-2 in RNA selection and nitrocellulose filter-binding assays reveals that Nova-2 binds RNA with high affinity and with sequence specificity that differs from Nova-1. Our results demonstrate that the immune response in POMA targets a family of highly related sequence-specific neuronal RNA-binding proteins. The expression pattern of the Nova-2 protein is likely to underlie the development of cognitive deficits in some POMA patients.
The paraneoplastic neurologic diseases (PNDs) are an unusual group of diseases at the intersection of neurobiology, immunology, and oncology. Patients with PNDs harbor systemic tumors and develop immune responses against onconeural antigens that are expressed both by their tumors and by neurons (for review, see refs. 1 and 2). The presence of high-titer antibodies in patient sera has allowed the identification and characterization of PND antigens and has provided a unique approach toward understanding the expression and function of these proteins in discrete regions of the brain. For example, the CAR antibody, associated with paraneoplastic blindness and small cell lung cancer (SCLCa), was used to identify recoverin, a protein involved in receptor signaling in the photoreceptor (3, 4), the Yo antibody, associated with paraneoplastic cerebellar degeneration, was used to identify cdr2, a novel leucine-zipper protein expressed in cerebellar Purkinje neurons (5, 6), and the Nb antibody, found in a patient with cerebellar degeneration, was used to identify a neuron-specific, adaptin-like protein called β-NAP (7, 8).
Two distinct families of neuronal RNA-binding proteins, Nova and Hu, have also been identified as PND target antigens (for review, see ref. 2). The Hu proteins were identified in a PND associated with SCLCa (9, 10). This family contains at least four highly related RNA recognition motif (RRM)-type RNA-binding protein members and shares strong homologies with the Drosophila neurogenic gene elav and the splicing and translational control factor sxl (11–14). Within the Hu family, there exists a high degree of complexity, in part because of distinct developmental expression patterns of the multiple family members (15). The Nova-1 protein was identified in paraneoplastic opsoclonus myoclonus ataxia (POMA), which is associated with breast cancer, fallopian cancer, and SCLCa, and is characterized primarily by dysfunction of the motor nervous system (16, 17). The Nova-1 cDNA encodes a sequence-specific K homology (KH)-type RNA-binding protein whose RNA-binding ability can be abrogated in vitro by POMA disease antiserum (17–19). Nova-1 expression is restricted to subcortical structures in the central nervous system (CNS), both during mouse development and in the adult brain (17, 18). However, POMA antisera are reactive against all neurons in the mouse and human CNS by immunohistochemistry (16, 17, 20, 21), and POMA antibodies, affinity-purified with Nova-1 fusion protein (NFP), recognize multiple immunoreactive bands in mouse brain extracts at 50–55 kDa and 70–80 kDa (17), suggesting that additional Nova-1-related POMA antigens may exist. Clinical reports have documented progressive neurological deficits in some POMA patients. In up to 58% of patients, multifocal neurological deficits such as encephalopathy and dementia with cerebral atrophy are seen, suggesting involvement of rostral brain regions that do not express Nova-1 (refs. 16 and 22; for review, see ref. 23).
In the present study, we used POMA antisera to expression clone a second Nova family member, termed Nova-2. Nova-2 is a neuronal KH-type RNA-binding protein expressed in a broader CNS distribution than Nova-1. We have compared the expression patterns and RNA-binding properties of Nova-1 and Nova-2 and conclude that the Nova-2 protein is a PND antigen that is likely to function in neuronal RNA metabolism. Furthermore, the expression pattern of Nova-2 may underlie the neurologic dysfunction in POMA patients who develop encephalopathy and dementia.
MATERIALS AND METHODS
Library Screening and Cloning of Nova-2.
A human SCLCa cDNA expression library (Stratagene) was screened as described (17), by using POMA antiserum. Full-length Nova-2 clones were identified by screening human hippocampal, mouse adult brain, and mouse E12.5 cDNA libraries (Stratagene).
Production of Fusion Proteins.
Full-length T7 epitope-tagged and histidine-tagged NFP was produced as described (18). Full-length T7 epitope-tagged and histidine-tagged Nova-2 fusion protein (N2FP) was produced in the pET21a vector (Novagen).
Western Blot Analysis.
Proteins were separated by SDS/PAGE, transferred to nitrocellulose, and probed with patient and control human antisera. Blots were developed with horseradish peroxidase-linked secondary antibodies and enhanced chemiluminescence (Amersham).
Northern Blot Analysis.
Total mouse RNA was obtained by using a modified guanidine-acid phenol protocol (24), separated on a formaldehyde/agarose gel, transferred to nylon (Biodyne Electronics, Santa Monica, CA; Pall), and probed with a 310-bp radiolabeled fragment from the Nova-2 coding region in an aqueous solution [6× standard saline citrate (SSC) (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7); 2× Denhardt’s solution [1 × Denhardt’s 0.02% polyvinylpyrrolidone/0.02% Ficoll (Pharmacia)/0.02% BSA], 0.1% SDS, 100 μg/ml denatured salmon sperm DNA]. Autoradiograms were exposed with Kodak MS film at −70°C.
In Situ Hybridization.
In situ hybridization was performed as described (15). Frozen sections (12 μm) were fixed with 4% paraformaldehyde. Sense and antisense RNA probes (310 nt) from the Nova-2 coding region were transcribed in vitro with T7 RNA polymerase and 33P-UTP. Slides were hybridized at 55°C for 2 days with 1 × 106 cpm-labeled probe per section in 50% formamide hybridization solution. Slides were dipped in Kodak NTB2 emulsion, exposed for 10–21 days, developed, and counterstained with cresyl violet.
Nova-2-Specific Antipeptide Antibody.
A peptide was synthesized corresponding to the predicted Nova-2 amino acids 392–405 (GGFLTAEKLAAESA). Rabbits were immunized with peptide and methylated BSA, with adjuvant. Nova-2 specific antibody (N2Ab) was affinity-purified with covalently bound N2FP beads; specific antibody was eluted with 0.2 M glycine (pH 3.0), neutralized to pH 7.4, and dialyzed against PBS before use.
Immunoprecipitation.
Mouse embryo extracts were prepared by fresh tissue homogenization in ice-cold NET-2 buffer (150 mM NaCl/50 mM Tris⋅HCl, pH 7.5/0.1% Nonidet P-40), sonication, and centrifugation at 18,000 × g for 15 minutes. Supernatants were precleared with protein A Sepharose; antibody binding was carried out for 4 hours, and immunoprecipitation pellets were washed in RIPA buffer (150 mM NaCl/50 mM Tris⋅Cl, pH 7.5/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS) and analyzed by Western blot analysis.
Immunohistochemical Analysis.
Tissues from mice perfused with Bouins’ solution (Sigma) were postfixed in fresh Bouins’ solution and 70% ethanol and were paraffin embedded. Sections (14 μm) were blocked with normal goat serum and H2O2, were incubated with primary antibody for 2 days at 4°C, then incubated with biotinylated secondary antibody. Signal was intensified with Vectastain (Vector Laboratories) and developed in 0.5 mg/ml diaminobenzidine (Sigma).
Ribohomopolymer Assays.
N2FP (500 ng) and 500 ng control protein were incubated with ribohomopolymer beads (Sigma) in RHPA buffer (10 mM Tris, pH 7.4/2.5 mM MgCl2/0.5% Triton X-100) with the indicated NaCl concentration and 1 mg/ml heparin in a volume of 500 μl. Binding occurred at 4°C for 10 minutes, reactions were washed five times in RHPA buffer with the indicated NaCl concentration, boiled in SDS buffer, and run on Western blots.
RNA Selection Assay.
RNA selection experiments were performed essentially as described (19). For each selection round, radiolabeled RNA was transcribed from an in vitro synthesized oligonucleotide template library with an internal 52-bp random sequence and estimated complexity of 2.1 × 1014. Gel-purified RNA was applied to a precolumn to adsorb nonspecifically bound RNAs, then applied to a N2FP nickel affinity column in selection buffer [20 mM Tris⋅HCl, pH 7.6/1 mM MgCl2/50 mM imidazole, and either 0.5 M LiCl (experiment #1) or 0.3 M LiCl (experiment #2)]. After being washed in 15–30 column volumes of selection buffer, protein and RNA were coeluted by the addition of 1.0 M imidazole in selection buffer. RNA was extracted in phenol/0.5% SDS at 50°C, ethanol precipitated, reverse transcribed, PCR amplified, and RNA was transcribed for RNA selection.
Filter-Binding Assays.
Filter binding by using purified NFP and N2FP was performed as described (19). Fusion proteins were combined with radiolabeled RNA in 50 μl of binding buffer [200 mM KOAc/50 mM Tris-OAc, pH 7.7/5 mM Mg(OAc)2]. After room-temperature incubation, samples were filtered through nitrocellulose, were washed with binding buffer, and retained counts were determined in a scintillation counter.
RESULTS
Cloning of a Nova Family Member.
In situ hybridization has demonstrated that Nova-1 expression is restricted to the hypothalamus, ventral midbrain, hindbrain, and spinal cord (17, 18). To explore the possibility of additional Nova-like proteins in cortical brain regions, we performed Western blot analysis of mouse P0 forebrain vs. hindbrain with POMA disease antiserum (Fig. 1A). Several immunoreactive antigens of 70–75 kDa and a single antigen of 50 kDa are selectively expressed in forebrain, whereas these and additional antigens of 50–55 kDa are present in the hindbrain. The size and distribution of the 50- to 55-kDa hindbrain bands is consistent with the previous characterization of Nova-1. However, because Nova-1 is not expressed in forebrain, and POMA antibodies affinity purified with NFP also recognize these forebrain antigens (ref. 17 and data not shown), the data in Fig. 1A suggest the expression of a Nova-related antigen in forebrain.
Figure 1.
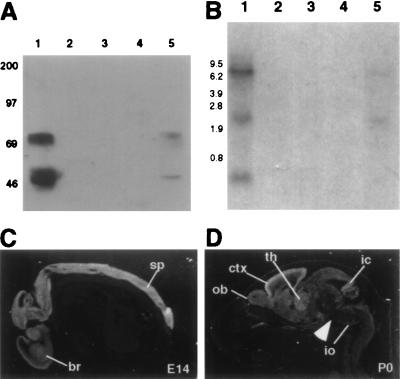
Identification of Nova-2, a PND antigen. (A) Western blot analysis of POMA antigens in P0 mouse brain. Total protein extracts (50 μg per lane) from P0 mouse forebrain (FB) or hindbrain (HB) were analyzed by Western blot analysis with POMA antiserum. Molecular mass markers are indicated on the left (kDa). (B) The full-length human Nova-2 amino acid sequence is shown compared with Nova-1. The Nova-1 sequence includes mini-exon LSK (aa 88–90) and alternatively spliced Exon H (aa 153–176). KH domains are boxed in gray. A potential NLS is boxed in white. The peptide sequence used to generate the Nova-2-specific antibody N2Ab (aa 392–405) is underlined. Dashes indicate gaps inserted to align the sequences, dots indicate identity, and asterisks indicate predicted stop codons. (C) The N2FP is recognized by 5 of 5 POMA patient antisera. Western blots with recombinant N2FP (500 ng per strip) were immunoblotted with POMA antisera (lanes 5–9, serum from five different POMA patients at 1:200, 1:250, 1:250, 1:1000, 1:2000), non-POMA paraneoplastic antisera (lane 3, Yo patient serum at 1:200; lane 4, Nb patient serum at 1:200), or normal human control sera (lane 1, 1:50; lane 2, 1:200). Molecular mass markers are indicated on the left (kDa). (D) The antipeptide antibody N2Ab immunoprecipitates a subset of Nova proteins from mouse brain. Immunoprecipitations from E17 mouse brain extracts were performed with affinity-purified N2Ab (lane 3) or a control affinity-purified rabbit antibody (lane 2). Immunoprecipitates were analyzed for Nova proteins by immunoblotting with POMA antiserum. Extract (50 μg) was loaded (lane 1) to demonstrate the Nova proteins in the starting material. Molecular mass markers are indicated on the left (kDa); the Nova-2 50-kDa band (arrowhead) and IgG bands (asterisk) are indicated on the right.
Because the full complement of Nova immunoreactive bands were expressed in a SCLCa cell line (data not shown), we used POMA antiserum to screen a SCLCa expression library. This screen identified a cDNA encoding an immunoreactive protein related to Nova-1, which we term Nova-2 (Fig. 1B). To determine whether Nova-2 fusion protein (N2FP) can be recognized by antisera from different POMA patients, a full-length recombinant N2FP was produced, and N2FP strips were immunoblotted with POMA or control antisera (Fig. 1C). N2FP was recognized at high titers by 5 of 5 different POMA sera, while all control sera were negative, demonstrating that the Nova-2 cDNA encodes a POMA disease antigen.
The human Nova-2 cDNA contains 1,476 nts of ORF (data not shown), encoding 492 amino acids and predicting a protein of 48.9 kDa. Nova-2 is very closely related to Nova-1, with overall 75% amino acid identity and 85% amino acid homology (Fig. 1B). In the regions of the three KH domains, the amino acid sequence is 98% identical to Nova-1, suggesting that the proteins may share similar RNA-binding characteristics. Nova-1 and Nova-2 share significant homology in the regions of the KH domains to other KH-type RNA-binding proteins (data not shown). In addition, both Nova proteins contain a near-match to the consensus bipartite nuclear localization signal (NLS) (25) positioned in the N terminus before the first KH domain (Fig. 1B); this putative NLS is also an 8/17 (Nova-2) or 7/17 (Nova-1) identical amino acid match to a functional N-terminal NLS identified in heterogeneous nuclear RNP-K (26). Nova-2 is also highly conserved across species, as the mouse- and human-predicted proteins are 99% identical (data not shown).
The spacer region between the second and third KH domains contains the least homology between Nova-1 and Nova-2, with 59% amino acid identity and 74% homology (Fig. 1B). The Nova-2 amino acid sequence in this region contains several long stretches of alanine and/or glycine residues and a short stretch of proline residues, while the Nova-1 amino acid sequence contains shorter stretches of alanine and/or glycine and does not contain a proline-rich region. We used a unique 14 amino acid–peptide sequence from the Nova-2 spacer region (Fig. 1B) to develop an antipeptide antibody termed N2Ab that completely distinguished between purified N2FP and NFP (data not shown). Immunoprecipitation with N2Ab from mouse brain extracts demonstrated specific immunoreactivity against the subset of Nova proteins that are expressed in mouse forebrain (75 kDa and a single band of 50 kDa; Fig. 1 A, D).
Expression Analysis of Nova-2.
We examined the expression of Nova antigens in adult mouse tissues by Western blot analysis using POMA antiserum. Immunoreactive Nova proteins were expressed in brain, at significantly lower levels in lung, and were absent in other tissues (Fig. 2A). The set of proteins recognized at 75 kDa and a single band at 50 kDa correspond to the set of Nova-2 antigens defined with the antipeptide antibody N2Ab (Fig. 1C).
Figure 2.
Expression analysis of Nova-2 in mouse. (A) Nova tissue Western blot. Total protein (50 μg per lane) from adult mouse tissues was immunoblotted with POMA antiserum. Lanes: 1, brain; 2, kidney; 3, heart; 4, spleen; 5, lung. Molecular mass markers are indicated on the left (kDa). (B) Nova-2 tissue Northern blot. Total RNA (20 μg per lane) was isolated from adult mouse tissues and hybridized on a Northern blot with a Nova-2 specific probe. Lanes: 1, brain; 2, kidney; 3, heart; 4, spleen; 5, lung. Molecular weight markers are indicated on the left (kb). The blot was stripped and reprobed for actin to demonstrate RNA integrity (data not shown). (C and D) Nova-2 transcripts in the mouse embryo and perinatal mouse. Sagittal sections (12 μm) of E14 embryo (C) or P0 (D) mouse were hybridized with a 33P-radiolabeled Nova-2 riboprobe and imaged with darkfield microscopy (positive signal is white). At E14, Nova-2 mRNA is detected throughout the CNS, including brain (br) and spinal cord (sp). At P0, Nova-2 transcripts are most abundant in the cortex (ctx), with strong signal also in olfactory bulb (ob), thalamus (th), inferior colliculus (ic), and inferior olive (io). Signal is relatively weak in the brainstem (arrowhead). No signal was seen with control sense riboprobes (data not shown).
To confirm Nova-2 expression in brain and lung, a Nova-2 probe was used on a tissue of Northern blot of mouse total RNA (Fig. 2B). Three Nova-2 transcripts were identified in adult mouse brain at 0.6, 3.0, and ≥9 kb; the two larger transcripts were also detectable in lung at significantly lower levels. Cross-hybridization to the 4.7-kb Nova-1 transcript (17) was not seen. The Nova-2 transcripts were not detected in kidney, spleen, or heart. The 3-kb band is consistent with the sequence in Fig. 1B, and the 0.6-kb band is consistent with a potential alternatively spliced Nova-2 transcript identified by screening a mouse adult brain cDNA library (data not shown). The 9-kb band has not been identified but was present in both total and poly(A)+ RNA (data not shown), suggesting that it is not pre-mRNA and is likely instead to represent either an uncharacterized Nova-2 alternatively spliced RNA or a transcript from a highly related gene (see discussion).
To further examine Nova-2 mRNA expression, a Nova-2 riboprobe was used for in situ hybridization analysis (Fig. 2 C–D). In the E14 mouse embryo, Nova-2 transcripts were detected throughout the entire CNS, with no detectable expression in the peripheral nervous system or in nonneuronal tissues, including lung. At P0, Nova-2 transcripts were detected specifically in the CNS in a widespread pattern, with regional differences in intensity. Highest levels of expression were in the cortex, olfactory bulb, thalamus, inferior colliculus, inferior olive, and the internal and external granule cell layers of the cerebellum, and lowest levels of expression were in the brainstem. A similar pattern of expression was seen in the adult brain at lower levels (data not shown).
Using the Nova-2-specific antibody N2Ab, we carried out immunohistochemical analyses of the Nova-2 protein expression pattern (Fig. 3). N2Ab immunoreactivity at E14 and P0 corresponded precisely with the expression of Nova-2 mRNA defined by in situ hybridization (Fig. 2 C–D), and immunoreactivity in the CNS was completely blocked by preincubation of N2Ab with the peptide against which it was generated (data not shown).
Figure 3.
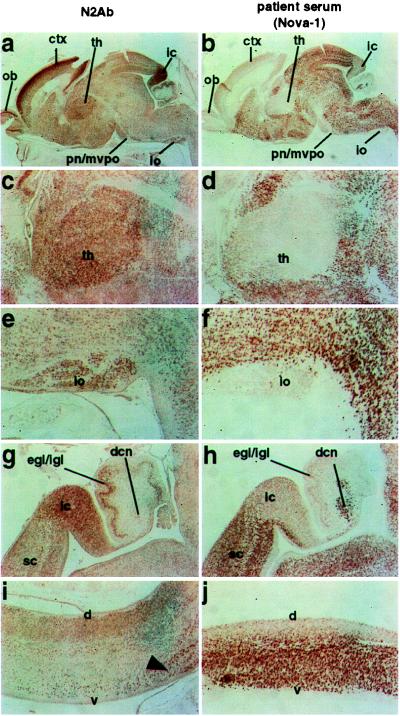
Comparison of Nova-2 expression with Nova-1 at P0. Reciprocal patterns of Nova-2 and Nova-1 protein expression at P0. Nova-2 immunoreactivity in sagittal sections of a P0 mouse brain was assessed with affinity-purified N2Ab (a, c, e, g, i), and compared in serial sections with POMA antiserum under fixation conditions previously demonstrated to preferentially detect the Nova-1 protein (ref. 17; b, d, f, h, j). Whole brain (a, b), and higher magnification views comparing Nova-1 and Nova-2 expression showing the thalamus [th; (c, d)], inferior olive [io; (e, f)], superior colliculus (sc), inferior colliculus (ic), external and internal granule cell layers of the cerebellum (egl/igl), and deep cerebellar nuclei [dcn; (g, h)], and dorsal (d) and ventral (v) spinal cord (i, j). Arrowhead indicates the large motor neurons in the ventral spinal cord (i, j), where moderate Nova-2 and strong Nova-1 expression is overlapping. Olfactory bulb (ob), cortex (ctx), and pontine nuclei/medioventral periolivary nucleus (pn/mvpo) are also shown.
To more directly compare Nova-1 and Nova-2 expression, we performed serial-section immunohistochemistry using either N2Ab or POMA antiserum (using fixation conditions that detect primarily Nova-1 protein; ref. 17). The patterns of Nova-1 and Nova-2 immunoreactivity in the P0 CNS were largely reciprocal (Fig. 3). The highest levels of Nova-2 immunoreactivity were detected in CNS regions that express little or no Nova-1, such as neocortex, thalamus, inferior colliculus, inferior olivary nuclei, and the external granule cell layers of the cerebellum. Conversely, lower levels of Nova-2 immunoreactivity were detected in regions of the CNS overlapping with high levels of Nova-1 expression, including the ventral midbrain, hindbrain, and spinal cord, although some neurons did express significant levels of both Nova-1 and Nova-2, including the large motor neurons of the ventral spinal cord (Fig. 3).
Nova-2 Is a Sequence-Specific RNA-Binding Protein.
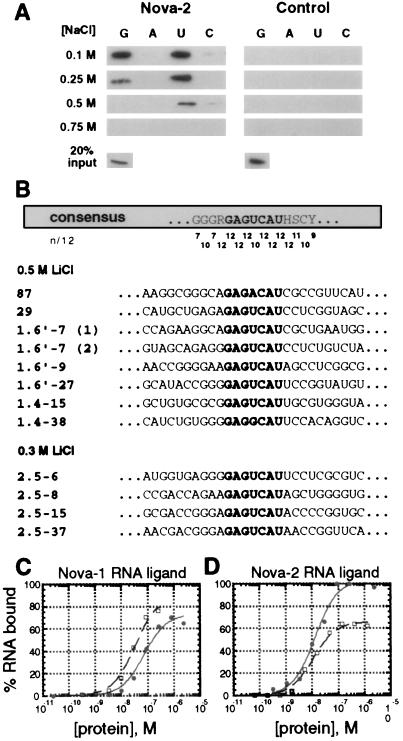
Nova-1 has been characterized as a sequence-specific RNA-binding protein by ribohomopolymer binding assays and RNA selection (18, 19). As an initial comparison of the RNA-binding characteristics of Nova-1 and Nova-2, we performed in vitro ribohomopolymer binding assays and RNA selection with Nova-2. Recombinant T7 epitope-tagged N2FP bound avidly to riboG and riboU in up to 0.5 M NaCl, while a control protein showed no detectable binding at any salt concentration (Fig. 4A). These results demonstrate that Nova-2 is an RNA-binding protein.
Figure 4.
Nova-2 is an RNA-binding protein. (A) Analysis of Nova-2 RNA-binding ability to ribohomopolymer RNA in varying salt concentrations. N2FP (5 μg) or an irrelevant T7 epitope-tagged and histidine-tagged control protein was incubated with poly(G), poly(A), poly(U), and poly(C)-Sepharose ribohomopolymer beads in either 0.1 M, 0.25 M, 0.5 M, or 0.75 M NaCl. After being washed, protein bound to the ribohomopolymers was analyzed by Western blot analysis with an anti-T7 antibody (Novagen). Twenty percent of the input protein is shown (Lower). (B) Affinity elution-based RNA selection identifies a Nova-2 consensus binding sequence. Alignment of 12 Nova-2 RNA ligands selected in two independent Nova-2 selection experiments (in 0.5 and 0.3 M LiCl) is shown. A consensus sequence is shown at the top, with the GAGUCAU motif highlighted. The match to the consensus, scored as the number of exact matches of 12 sequences (n/12), is shown for each position. Bases in RNA ligands with identity to the GAGUCAU motif are also shown highlighted. (C and D). Direct comparisons of Nova-1 and Nova-2 binding to selected RNA ligands. Nitrocellulose filter binding assays were performed to examine Nova-1 (open squares) and Nova-2 (solid circles) binding to RNA ligands mSB2 (C), a 21-nt RNA containing the Nova-1 consensus binding motif [UCAU(N)0–2]3 (19); and 87 (D), a 96-nt RNA ligand isolated by Nova-2 RNA selection (Fig. 4B). RNA ligand mSB2 bound to Nova-1 and Nova-2 with calculated Kd values of 33 ± 5 nM and 73 ± 13 nM, respectively (calculated by least squares fit; ref. 33). Similar results were found with the 96 nt SB2 Nova-1 RNA ligand (data not shown). RNA ligand 87 bound to Nova-1 and Nova-2 with calculated Kd values of 13 ± 2.5 nM and 11 ± 1.5 nM, respectively. Maximal Nova-1 binding to RNA ligand 87 was observed at ≈65% of the total RNA, while maximal Nova-2 binding was 100%. Similar results were found with the Nova-2 RNA ligand 29 (data not shown). Neither Nova protein demonstrated significant binding to a random RNA ligand isolated from the original RNA selection pool, and an irrelevant T7 epitope-tagged recombinant control protein did not demonstrate significant binding to any of the RNA ligands (calculated Kd values > 1 μM, data not shown).
To identify specific RNA sequences that bind Nova-2, we performed affinity elution-based RNA selection with N2FP in two independent selection experiments. We used an in vitro-synthesized 52-randomer template pool with an estimated complexity of 2.1 × 1014 (19). In both experiments, a Nova-2 consensus binding sequence was identified that contained a core GAGUCAU motif (Fig. 4B), demonstrating that Nova-2 is a sequence-specific RNA-binding protein. This sequence is similar to, but distinct from, the RNA consensus-binding sequence identified by Nova-1 RNA selection, which consists of the sequence [UCAU(N)0–2]3 (19). In addition, we note that the Nova-2-selected RNA ligands are predicted to form a stem–loop structure around the GAGUCAU motif, with a sequence preference for purines in the 5′ and pyrimidines in the 3′ half of the predicted stem (data not shown). We assessed the binding of Nova-1 and Nova-2 to selected Nova-1 or Nova-2 RNA ligands in filter-binding assays (Fig. 4 C and D). Both Nova-1 and Nova-2 demonstrated high affinity-binding to RNA ligands containing either Nova-1 or Nova-2 selected binding sites. Both proteins bound the Nova-2 selected sequence with approximately equal affinity, but Nova-1 bound its RNA ligand (mSB2) with a slight (2-fold), but consistently higher, affinity than did Nova-2 (Fig. 4 C and D).
DISCUSSION
Nova Proteins and Paraneoplastic Disease.
Patients with POMA exhibit clinical symptoms of opsoclonus, myoclonus, and ataxia, suggesting that the initial neurologic disorder involves a lack of motor inhibition in the brainstem and spinal cord pathways (for reviews, see refs. 1, 23, and 27). Expression of the POMA antigen Nova-1 is restricted to the subcortical CNS in a pattern that roughly correlates with these motor symptoms (17, 18). However, a subset of POMA patients also develop encephalopathy and cortical deficits, which cannot easily be explained by dysfunction of Nova-1-expressing neurons (refs. 16 and 22; for review, see ref. 23). Although less well documented than the classic POMA symptoms, the cognitive and emotional features reported in POMA patients range from mild emotional lability to dementia, coma, and death. A review of nine case reports reveals that in 19 adults with POMA, mental status changes were noted in 58%, with progression to stupor or coma in 26% (23).
Analysis of Nova-2 transcripts and protein reveals a widespread expression pattern that includes overlapping expression with Nova-1 in the midbrain, hindbrain, and ventral spinal cord (Fig. 3 and data not shown), as well as strong expression in rostral brain regions, including the cortex and thalamus. The different patterns of Nova expression suggest a model in which progression of subcortical motor system dysfunction to widespread neurologic dysfunction in POMA may correspond to initial immunologic targeting of Nova-1 with progression to targeting of common epitopes shared by Nova-2. We have proposed a similar mechanism for the Hu syndrome, a paraneoplastic syndrome in which progression of focal deficits to multifocal neuronal degeneration may correspond to progressive immunologic targeting of epitopes shared by products of the four Hu genes (10, 15). Such progression of immunologic targeting may be a common feature of autoimmune syndromes involving antigenically related families of disease antigens.
The Nova Family of Paraneoplastic Disease Antigens.
The Nova-2 cDNA encodes a protein with striking homology to the previously described paraneoplastic antigen Nova-1. In addition, the third KH domain of the predicted Nova-2 protein is identical to the third KH domain of Nova-1, with additional homology in the surrounding sequences; this region has been identified as the antigenic epitope for POMA antisera and also as a functional domain for sequence-specific RNA binding (17, 19). Identification of Nova-2 thus demonstrates the presence of a family of highly related paraneoplastic disease antigens, a finding that is confirmed by recognition of N2FP by 5 of 5 different POMA antisera at the same high titer as Nova-1. Characterization of Nova-2 by immunoprecipitation and Northern blot analysis revealed a 50-kDa, N2Ab-immunoreactive protein and a 3-kb RNA transcript consistent with the Nova-2 sequence reported in Fig. 1B. Moreover, additional protein bands at 75 kDa and RNA transcript(s) at 9 kb were identified. We have shown that the 75-kDa proteins share two distinct epitopes with Nova-2, including the third KH domain (reactivity with POMA antisera) and a 14 amino acid sequence between the second and third KH domains (reactivity with the antipeptide antibody N2Ab). Similarly, the 9-kb RNA transcript hybridizes with a Nova-2 probe in conditions under which crosshybridization to Nova-1 does not occur. Therefore, while the identification of these larger species remains unknown, it is likely that the 9-kb transcript encodes proteins highly related to Nova-2. A Nova-2 probe used in chromosomal mapping studies hybridized with three different loci, none of which were Nova-1 (28), suggesting the possibility of additional Nova genes.
Sequence-Specific RNA-Binding Proteins in Neuronal RNA Metabolism.
Consistent with the high degree of homology between Nova-1 and Nova-2, the Nova proteins select similar high-affinity RNA ligands in RNA selection experiments and bind individual RNA ligands in vitro with similar affinities (Fig. 4; ref. 19). However, in two independent RNA selection experiments, Nova-2 recognized RNA targets with a core consensus sequence (Fig. 4; GAGUCAU) different from a previously identified Nova-1 RNA target [(UCAU(N)0–2)3; ref. 19]. Moreover, comparison of Nova-1 and Nova-2 binding to [(UCAU(N)0–2)3] RNA revealed slight but consistent differences in RNA binding (Fig. 4), suggesting that the two Nova proteins may bind overlapping but subtly different sets of RNA ligands. If this is the case, neurons expressing Nova-2 alone may differ subtly in their metabolism of RNA from neurons that express a combination of both proteins (Fig. 3).
Several RNA-binding protein families have been described whose members bind like sequences in vivo. HnRNP K and hnRNP E1, two closely related KH-type RNA-binding proteins with homology to the Nova proteins, bind a pyrimidine-rich element in the 15-lipoxygenase mRNA 3′ untranslated region and thereby repress mRNA translation (29). Similarly, the KH-type RNA-binding proteins αCP-1 and αCP-2 bind α-globin 3′-untranslated region elements to stabilize the mRNA (30). Finally, the iron-response proteins IRP1 and IRP2 bind to conserved iron response elements in the 5′ and 3′ untranslated regions of mRNAs involved in regulation of cellular iron levels and function to regulate mRNA stability or translational control (for reviews, see refs. 31 and 32). We have previously shown that Nova-1 protein recognizes the glycine receptor α2 pre-mRNA as an in vivo RNA target (19). Although the Nova-2 in vitro RNA binding data presented here does not necessarily correlate with in vivo function, it is possible that the Nova-1 and Nova-2 proteins may share common in vivo target RNAs. In such cases, differential regulation of metabolism of RNA targets may be mediated by differential protein–protein interactions. Indeed, preliminary data suggest that Nova-1 and Nova-2 interact to form homo- and hetero-oligomers in solution (Yang and Darnell, unpublished observations), suggesting additional complexity in the regulation of neuronal RNA metabolism by these proteins.
Acknowledgments
We thank Svetlana Mojsev for help in designing the peptide used in generating N2Ab, Drs. Stephen Burley and Joan Steitz and members of our laboratory for critical discussions, H. James Okano for assistance with in situ hybridization and immunohistochemistry, and Kirk Jensen for assistance with filter-binding assays. This work was supported by grants to R.B.D. from the National Institute of Neurological Disorders and Stroke (RO1 NS34389) and the Department of Defense (#DAMD017–94-J-4277). Y.Y.L.Y was supported by National Research Service Award training grant #CA 09673-18 and Medical Scientist Training Program (National Institutes of Health) grant 2T32 GM07739.
ABBREVIATIONS
- PND
paraneoplastic neurologic disease
- SCLCa
small cell lung cancer
- RRM
RNA recognition motif
- POMA
paraneoplastic opsoclonus myoclonus ataxia
- KH
K homology
- CNS
central nervous system
- NFP
Nova-1 fusion protein
- N2FP
Nova-2 fusion protein
- N2Ab
Nova-2-specific antibody
- NLS
nuclear localization signal
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. AF083898).
References
- 1. Posner J B, Furneaux H M. In: Immunologic Mechanisms in Neurologic and Psychiatric Disease. Waksman B H, editor. New York: Raven; 1990. pp. 187–217. [Google Scholar]
- 2.Darnell R B. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polans A S, Buczylko J, Crabb J, Palczewski K. J Cell Biol. 1991;112:981–989. doi: 10.1083/jcb.112.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray-Keller M P, Polans A S, Palczewski K, Detwiler P B. Neuron. 1993;10:523–531. doi: 10.1016/0896-6273(93)90339-s. [DOI] [PubMed] [Google Scholar]
- 5.Fathallah-Shaykh H, Wolf S, Wong E, Posner J B, Furneaux H M. Proc Natl Acad Sci USA. 1991;88:3451–3454. doi: 10.1073/pnas.88.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corradi J P, Yang C, Darnell J C, Dalmau J, Darnell R B. J Neurosci. 1997;17:1406–1415. doi: 10.1523/JNEUROSCI.17-04-01406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnell R B, Furneaux H M, Posner J B. J Neurosci. 1991;11:1224–1230. doi: 10.1523/JNEUROSCI.11-05-01224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman L S, McKeever M O, Okano H J, Darnell R B. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 9.Dalmau J, Furneaux H M, Gralla R J, Kris M G, Posner J B. Ann Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Graus F, Rosenblum M K, Posner J B. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner J B, Furneaux H M. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 12.Levine T D, Gao F, King P H, Andrews L G, Keene J D. Mol Cell Biol. 1993;13:3494–3504. doi: 10.1128/mcb.13.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good P J. Proc Natl Acad Sci USA. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma W-J, Cheng S, Campbell C, Wright A, Furneaux H. J Biol Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 15.Okano H J, Darnell R B. J Neurosci. 1997;9:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luque F A, Furneaux H M, Ferziger R, Rosenblum M K, Wray S H, Schold S C, Jr, Glantz M J, Jaeckle K A, Biran H, Lesser M, et al. Ann Neurol. 1991;29:241–251. doi: 10.1002/ana.410290303. [DOI] [PubMed] [Google Scholar]
- 17.Buckanovich R J, Posner J B, Darnell R B. Neuron. 1993;11:657–672. doi: 10.1016/0896-6273(93)90077-5. [DOI] [PubMed] [Google Scholar]
- 18.Buckanovich R J, Yang Y Y L, Darnell R B. J Neurosci. 1996;16:1114–1122. doi: 10.1523/JNEUROSCI.16-03-01114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckanovich R J, Darnell R B. Mol Cell Biol. 1997;17:3194–3201. doi: 10.1128/mcb.17.6.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dropcho E J, Kline L B, Riser J. Neurology. 1993;43:207–211. doi: 10.1212/wnl.43.1_part_1.207. [DOI] [PubMed] [Google Scholar]
- 21.Graus F, Rowe G, Fueyo J, Darnell R B, Dalmau J. Neurosci Lett. 1993;150:212–214. doi: 10.1016/0304-3940(93)90538-v. [DOI] [PubMed] [Google Scholar]
- 22.Hormigo A, Dalmau J, Rosenblum M K, River M E, Posner J B. Ann Neurol. 1994;36:896–902. doi: 10.1002/ana.410360615. [DOI] [PubMed] [Google Scholar]
- 23.Pranzatelli M R. Clin Neuropharmacol. 1992;15:186–228. doi: 10.1097/00002826-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Dingwall C, Laskey R A. Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 26.Michael W M, Eder P S, Dreyfuss G. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darnell R B. In: Current Diagnosis in Neurology. Feldmann E, editor. St. Louis: Mosby; 1993. pp. 137–141. [Google Scholar]
- 28.Fletcher C F, Okano H J, Gilbert D J, Yang Y Y L, Yang C, Copeland N G, Jenkins N A, Darnell R B. Genomics. 1997;45:313–319. doi: 10.1006/geno.1997.4925. [DOI] [PubMed] [Google Scholar]
- 29.Ostareck D H, Ostareck-Lederer A, Wilm M, Thiele B J, Mann M, Hentze M W. Cell. 1997;89:597–606. doi: 10.1016/s0092-8674(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 30.Kiledjian M, Wang X, Liebhaber S A. EMBO J. 1995;14:4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson B R. BioEssays. 1996;18:739–746. doi: 10.1002/bies.950180909. [DOI] [PubMed] [Google Scholar]
- 32.Hentze M W, Kühn L C. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jellinek D, Lynott C K, Rifkin D B, Janjic N. Proc Natl Acad Sci USA. 1993;90:11227–11231. doi: 10.1073/pnas.90.23.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]