Abstract
AA amyloidosis is one of the principal causes of morbidity and mortality in captive cheetahs (Acinonyx jubatus), which are in danger of extinction, but little is known about the underlying mechanisms. Given the transmissible characteristics of AA amyloidosis, transmission between captive cheetahs may be a possible mechanism involved in the high incidence of AA amyloidosis. In this study of animals with AA amyloidosis, we found that cheetah feces contained AA amyloid fibrils that were different from those of the liver with regard to molecular weight and shape and had greater transmissibility. The infectious activity of fecal AA amyloid fibrils was reduced or abolished by the protein denaturants 6 M guanidine·HCl and formic acid or by AA immunodepletion. Thus, we propose that feces are a vehicle of transmission that may accelerate AA amyloidosis in captive cheetah populations. These results provide a pathogenesis for AA amyloidosis and suggest possible measures for rescuing cheetahs from extinction.
Keywords: feces, transmissibility
The amyloidoses are a group of protein misfolding disorders characterized by the accumulation of amyloid fibrils formed from a variety of proteins that, under normal physiological conditions, are harmless and soluble. Currently, >25 amyloid diseases have been identified, such as the prion diseases, Alzheimer's disease, type 2 diabetes, and various systematic amyloidoses (1). Although the various proteins that can polymerize into amyloid fibrils have unrelated sequences, they can all form fibrils with a similar ultrastructural appearance. Among them, prion diseases such as transmissible spongiform encephalopathy (TSE), including scrapie in sheep, bovine spongiform encephalopathy (BSE), and chronic wasting disease (CWD) of deer and elk, are highly infectious (2). In these diseases, prion (PrPSc), an abnormal form of the host cellular prion protein (PrPC), induces the conformational change of PrPC to the PrPSc and causes a detectable phenotype or disease in the affected individual.
AA amyloidosis, known as reactive or secondary amyloidosis, is generally recognized as the predominant form of systemic amyloidosis that occurs in domestic animals and the animal kingdom (3). This disease is characterized by the systemic deposition of extracellular fibrils composed of amyloid A protein, primarily in the spleen; liver; and, to a lesser extent, in other organs. In most species, AA amyloidosis occurs sporadically and is typically secondary to chronic inflammation, infection, or neoplasia. Intriguing recent data suggest that AA amyloidosis could be transmitted by a prion-like infectious process through a seeding-nucleation mechanism (4–7). Thus, the fibrillar nuclei formed by the aggregation of misfolded protein monomers (rich in β-sheet structures) act as seeds to induce and stabilize conversion of the native monomeric protein (8–9). This mechanism provides a plausible explanation for the transmissible nature of AA amyloidosis. AA amyloidosis can be easily induced when mice are given an extract from AA amyloid-laden tissue (10, 11) or synthetic amyloid-like fibrils (12, 13), providing further evidence for transmissibility.
The cheetah species (Acinonyx jubatus) is in danger of extinction and is included on The World Conservation Union list of vulnerable species. Although efforts have been made in wildlife sanctuary parks and zoos worldwide to prevent extinction, a steady increase in the size of the cheetah population is hampered by the high prevalence of certain diseases in captive cheetahs. In particular, systemic AA amyloidosis is regarded as an increasingly important cause of morbidity and mortality in captive cheetahs as prevalence increased from 20% in pre-1990 reported necropsies to an unusual 70% of necropsied cheetahs in 1995 (14). Despite much effort, the pathogenesis for AA amyloidosis in cheetahs is still only partially understood. Inflammatory diseases, especially chronic lymphoplasmacytic gastritis, found in 100% of cheetahs with AA amyloidosis (14), and genetic homogeneity have been considered as causes for the increased incidence of AA amyloidosis (15). However, environmental epidemiological studies indicate that breeding conditions have a prominent effect on the incidence of AA amyloidosis. A high rearing density is always associated with early age of onset, and with the high incidence and severity of AA amyloidosis, findings similar to sheep scrapie and cervid CWD. Thus, sustained epidemics of sheep scrapie and cervid CWD appear to be principally due to horizontal (animal to animal) transmission, although the routes of natural transmission remain to be clarified (16, 17). The propagation of AA amyloidosis among captive cheetah populations may also depend on a horizontal transmission pathway. Identification of the mode of transmission is a prerequisite for disease control.
In this study, we show that the feces from a cheetah with AA amyloidosis can act as a possible transmission origin to accelerate the transmission of AA amyloidosis.
Results
Cheetah AA Amyloid Fibril Proteins and Specific Antiserum Against Them.
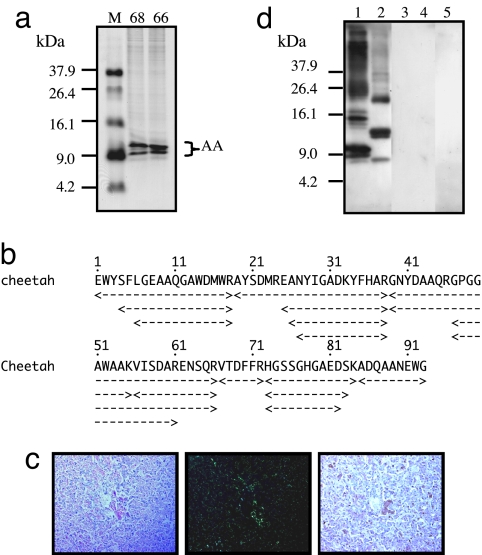
Until now, specific anti-cheetah AA antiserum has not been produced. To produce anti-cheetah AA antiserum, we isolated AA amyloid fibril fraction from the liver of Cheetah 68 (C68) with severe AA amyloidosis. When the isolated fibril fraction was separated with 16.5% SDS/PAGE, two major bands with molecular masses of ≈9.0 kDa and two faint bands were seen by Coomassie Brilliant Blue (CBB) staining (Fig. 1a). Based on their molecular masses, the four bands were determined most likely to be the monomeric forms of AA amyloid fibril protein. The four bands were cut and subjected to amino acid sequencing (Fig. 1b). This revealed that the sequences of the four bands were consistent with cheetah AA sequence determined in ref. 18. It also revealed that the isolated amyloid fibril fraction contained several cheetah AA amyloid fragments with different lengths. The longest fragment was the 93-amino acid-long fragment, and N-terminal and/or C-terminal deleted fragments also existed (Fig. 1b). In addition to C68, we also isolated the AA amyloid fibril fraction from the liver of C66 with severe AA amyloidosis followed by sequencing. The result was identical to the result from C68 (Fig. 1a; sequence data not shown).
Fig. 1.
Cheetah AA amyloid fibril proteins and specific antiserum. (a) The isolated AA amyloid fibril fractions from the livers of C68 and C66 (20 μg) were applied to 16.5% Tris·tricine SDS/PAGE gel followed by CBB staining. M, molecular weight markers. Molecular masses of two main and two faint bands corresponded to the AA monomer. (b) Amino acid sequencing from four bands in C68 sample. AA amyloid fragments with different lengths were obtained. The amyloid fibril fraction from C68 was used as antigen to produce specific anti-cheetah AA antiserum. (c) Amyloid deposition in the liver of C68 was detected in Congo red-stained sections by standard microscopy (Left), by green birefringence under polarized microscopy (Center), and by immunohistochemical staining (Right). The anti-cheetah AA antiserum was used as primary antibody (dilution 1:1,000). (Magnification, ×200.) (d) AA amyloid fibril fractions from the liver of C68 (lane 1), human (lane 2), mouse (lane 3), cow (lane 4), and murine AApoAII(C) amyloid fibril fraction (lane 5) were used (12 μg of protein per well) to perform Western blot analysis with anti-cheetah AA antiserum as primary antibody (dilution 1:3,000).
We used the isolated fibril fraction from C68 to produce antiserum against cheetah AA protein. To evaluate the specificity of anti-cheetah AA antiserum, we performed immunohistochemical staining of liver specimens that had been identified with severe amyloid deposition by Congo red staining. The amyloid deposition was stained positively with anti-cheetah AA antiserum (Fig. 1c). In addition, Western blot analysis was also performed by using the de novo antiserum as primary antibody (Fig. 1d). The anti-cheetah AA antiserum recognized all forms of cheetah AA proteins, including the monomer, dimer, and oligomer. It also reacted with human AA protein, because three dominant bands corresponding to the monomer, dimer, and trimer of human AA protein were recognized. All forms of cheetah AA protein (monomer, dimer, and oligomer) had relatively larger molecular mass compared to human AA protein. This was due to an eight-amino acid insertion in the C-terminal part (from amino acids 69–76) of cheetah AA protein (18). In contrast, the antiserum did not recognize murine or bovine AA proteins [Fig. 1d and supporting information (SI) Fig. S1]. There was also no immunoreaction with AApoAII protein, the amyloid protein of AApoAII amyloidosis that is another systemic amyloidosis occurring in mice.
Existence of Distinctive AA Amyloid Fibrils in the Feces of a Cheetah with Amyloidosis.
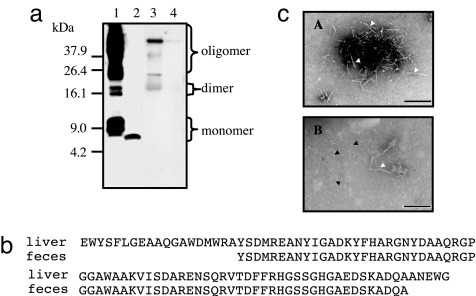
We isolated the amyloid fibril fraction from the feces of C68 and performed Western blot analysis with anti-cheetah AA antiserum. A clear single immunoreactive band (presumptive monomer) at a 7.0 kDa molecular mass was seen in fecal samples (Fig. 2a). As a control, we also isolated the amyloid fibril fraction from the liver of C68, and several bands corresponding to the monomer, dimer, and oligomers were seen by Western blot analysis. Unexpectedly, we found that the molecular mass of the fecal AA amyloid protein monomer was ≈2.0 kDa smaller than that from the liver (Fig. 2a). In addition to C68, we also examined three fecal samples from C67, C78, and C90. The same results were obtained; a single band with a molecular mass of 7.0 kDa was present in all fecal samples (data not shown). The difference in molecular mass was confirmed by amino acid sequencing, indicating the deletion of both N- and C-terminal sequences from the fecal AA amyloid protein compared to the liver AA amyloid protein (Fig. 2b). Transmission electron microscopy of the negatively stained liver amyloid sample revealed amyloid-characteristic straight and unbranched fibril images. However, the fibril image from feces was different from the liver exhibiting much thinner and smaller fibrils, implicating a divergent ultrastructure between the two kinds of fibrils (Fig. 2c). To exclude the possibility that soluble serum precursor SAA was excreted into the feces and then formed amyloid fibrils during the isolation process, we also examined the fecal sample from TT-253, a cheetah that had very minor AA amyloidosis limited to the esophagus. No reactive band was found (data not shown).
Fig. 2.
Analysis of AA amyloid protein in fecal matter from cheetahs with amyloidosis. (a) AA amyloid fibril fractions were analyzed by Western blot. Lane 1, sample (20 μg) from liver of C68. Lane 2, sample (20 μg) from feces of C78. Lane 3, 100 μg of protein from lyophilized whole urine of C68. Lane 4, pellets centrifuged at 100,000 × g from 4.0 ml of urine of C68. (b) Amino acid sequences of AA amyloid proteins from the liver and the feces corresponding to monomer detected in Fig. 2a. The fecal AA amyloid protein lacked several amino acids from both the N and C termini compared with the liver AA amyloid protein. (c) The AA amyloid fibrils from the liver (A) and the feces (B) were observed by transmission electron microscopy. Open triangles indicate characteristic amyloid fibrils; filled triangles represent fecal-specific fibrils. (Scale bar, 100 nm.)
Urine from C68 was dialyzed against PBS, and centrifuged at 100,000 × g for 1 h. Only a few bands with high molecular mass were observed in the whole urine sample, whereas no bands were found to react with anti-cheetah AA antiserum in the urine pellet (Fig. 2a). From these results, we presumed that the fecal AA amyloid proteins might be involved in the propagation of AA amyloidosis and further characterized its transmissibility with mouse experimental AA amyloidosis.
High Transmissibility of Feces from the Cheetah with Amyloidosis.
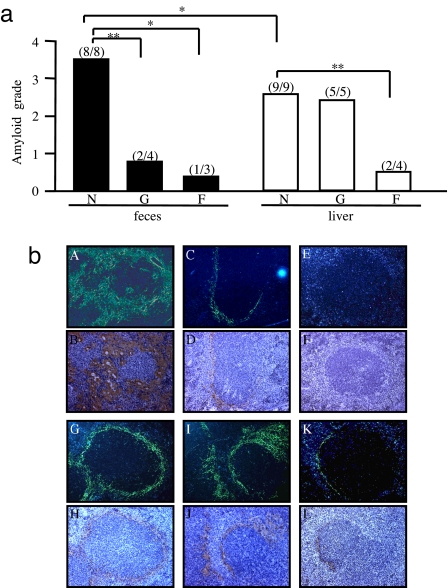
Transmissibility of the disease to mice by feces was examined by i.v. injection of isolated fecal AA amyloid fibril fraction with s.c. injection of 1% AgNO3 (0.5 ml) to induce AA amyloidosis. For comparison, an equal quantity of AA amyloid fibril fraction (100 μg) from the liver of C68 was injected. Ten days after injection, amyloid deposition in the spleen was observed and graded by Congo red staining. All mice induced by either the fecal fraction or the liver fraction suffered from AA amyloidosis. However, mice induced by the fecal fraction had more severe amyloidosis (P < 0.05) (Fig. 3a).
Fig. 3.
High transmissibility of feces from the cheetah with amyloidosis. (a) Fecal (C68 and C67) and liver (C68) AA amyloid fibril fractions were untreated (N), or treated by guanidine-hydrochloride (G) or formic acid (F) and injected into mice to induce AA amyloidosis. Equal quantities of amyloid fractions (100 μg) were used in each experiment. The degree of amyloidosis was determined by the amyloid deposition observed in Congo red-stained sections of the spleen (*, P < 0.05; **, P < 0.01). (b) AA deposition in the spleens of mice exposed to untreated fecal fibrils (A and B) (grade 4) or untreated liver fibrils (G and H) (grade 3). Deposition observed in mice exposed to guanidine-hydrochloride treated fecal fibrils (C and D) (grade 1) or liver fibrils (I and J) (grade 3) and to formic acid treated fecal fibrils (E and F) (grade 0) or liver fibrils (K and L) (grade 1). Deposition was detected by green birefringence in Congo red-stained sections under polarized microscopy (Upper) and immunohistochemical staining with anti-mouse AA antiserum (Lower). (Magnification, × 200.)
The amyloid-inducing activity of the fecal fraction was disrupted by both 6 M guanidine·HCl and formic acid denaturation as most injected mice did not develop amyloidosis, and only a few had very minor degrees of deposition. With regard to the liver fraction, formic acid treatment caused a nearly complete loss of amyloid-inducing activity, whereas the guanidine·HCl treated fraction retained high amyloid-inducing activity (Fig. 3a). The amyloid depositions that stained positively with Congo red were identified as AA amyloid deposition by immunohistochemical staining with anti-mouse AA antiserum (Fig. 3b). These results suggested that the amyloid fraction isolated from the feces might possess greater amyloid-inducing activity.
Quantitation of the Transmissibility of AA Amyloid Fibrils from the Feces.
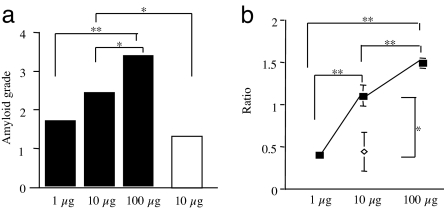
The amyloid fibril fraction from the feces was serially diluted in distilled water (DW) and injected into mice at doses of 1 μg, 10 μg, and 100 μg to induce AA amyloidosis. The degree of amyloid deposition, graded with Congo red staining, increased in a dose-dependent manner (Fig. 4a). We also induced the disease in mice by injection of 10 μg of the liver amyloid fibril fraction. We found that these mice had lower amyloid deposition compared to those receiving 10 μg of fecal fibrils.
Fig. 4.
Quantitation of transmissibility of AA amyloid fibrils from feces. (a) The degree of amyloid deposition in spleens of mice induced by fecal amyloid fibril fractions (filled squares) (1 μg, 10 μg, and 100 μg) or the liver fractions (open squares) (10 μg) was determined in Congo red-stained tissue sections (four mice per group). (b) The degree of AA deposition in induced mice was determined by isolation of AA amyloid fibril fractions from the spleens of mice in each group (filled squares, fecal; open diamonds, liver) followed by Western blot analysis (20 μg of protein per well) and quantification using National Institutes of Health Images. The means and SE were determined by the relative ratios of AA amyloid protein levels in each group versus the group receiving 10 μg of amyloid fibrils fraction from the feces (*, P < 0.05; **, P < 0.01).
In addition to Congo red staining, the degree of amyloid deposition was evaluated by Western blot analysis, using equal quantities of the AA amyloid fibril fractions isolated from spleens of induced mice in each group, followed by AA protein quantification, using National Institutes of Health image software (Fig. 4b). This showed that the intensity of the bands stained positively with anti-mouse AA antiserum increased proportionately to the logarithm of the AA amyloid fibril fraction dosage (1–100 μg). In mice injected with 10 μg of liver amyloid fibril fraction, the intensity of bands was appreciably lower than from mice injected with 10 μg of fecal amyloid fibril fraction and was only similar in degree to mice injected with 1 μg of fecal matter. These results confirmed that AA amyloid fibrils in feces had a higher transmissibility than that in liver. No samples isolated from mouse spleens reacted with anti-cheetah AA antiserum, which demonstrated that the amyloid deposition observed in the mouse tissue was derived from mouse AA fibrils but not the cheetah fibrils in the inocula (Fig. S2).
The Requisite of Fecal AA Amyloid Protein for Transmission.
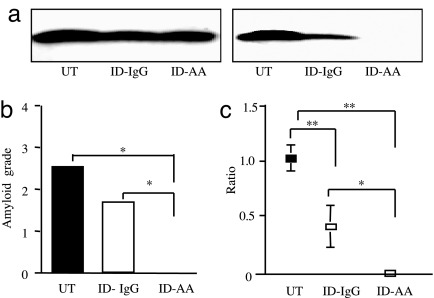
To establish whether AA amyloid protein contained in the fecal amyloid fibril fraction was a requisite for the amyloid-inducing activity, the amyloid fraction was immunodepleted with anti-cheetah-AA antiserum. Control experiments showed that five cycles of immunodepletion were required to remove AA proteins from the fecal fraction (Fig. 5a). Thus, fecal amyloid fractions were depleted five times with either anti-cheetah AA antiserum or normal rabbit IgG and were subsequently used to induce AA amyloidosis in mice. As shown in Fig. 5b, immunodepletion with anti-cheetah-AA antiserum completely prevented the amyloid-inducing activity of the fecal AA amyloid fraction, because no induced mice suffered from amyloidosis. In contrast, five cycles of immunodepletion with normal rabbit IgG antibody only partially decreased the amyloid-inducing activity, which was associated with only a partial loss of AA amyloid protein. The reliability of the amyloid grade was confirmed by Western blot analysis of the amyloid fractions isolated from the spleens of mice (Fig. 5c). These results show that cheetahs suffering from AA amyloidosis pass fecal matter that possesses amyloid-inducing activity because of the presence of AA amyloid fibril protein.
Fig. 5.
AA amyloid protein in the fecal amyloid fibril fraction is required for transmission. (a) The fecal amyloid fibril fraction from C90 was untreated (UT), immunodepleted with normal rabbit IgG (ID-IgG) or anti-cheetah AA antiserum (ID-AA) for one cycle (Left) or five cycles (Right) and then were separated with 16.5% SDS/PAGE followed by immunoblotting with anti-cheetah AA antiserum (10 μl per well). (b) UT and ID-IgG or ID-AA for five cycles of immunodepletion were used to induce AA amyloidosis in C57BL/6 mice (four mice per group). The amount of amyloid deposited was graded in Congo red-stained sections. (c) AA amyloid protein levels in the spleens from mice exposed to UT, ID-IgG, or ID-AA for five cycles of immunodepletion. AA amyloid proteins were isolated from each group mice, and Western blots were analyzed (20 μg of protein per well) followed by quantitative analysis using National Institutes of Health Image. The means and SE were determined by the relative ratios of AA amyloid protein levels for each induced group versus the group induced by the untreated sample (*, P < 0.05; **, P < 0.01).
Discussion
It is currently accepted that systemic AA amyloidosis is an increasingly important cause of morbidity and mortality in captive cheetah populations (14). For conservation of this species, therefore, it is critical to elucidate the etiology of AA amyloidosis. As with sheep scrapie and cervid CWD, the routes of transmission are among the most debated and intriguing issues. Infectious CWD prions in saliva have been identified to be involved in transmission in high-density captive situations (19, 20). Recently, available evidence indicates that an environmental reservoir of infectivity contributes to the continuation of these diseases in affected populations. These infectious agents can be transmitted by flesh flies (21) or hay mites (22) and can directly enter the environment from decomposing carcasses of infected animals (23). Environmental contamination by excreta from infected cervids has also seemed the most plausible explanation for the dissemination of CWD (24). Scrapie-infected hamsters and Creutzfeldt–Jakob disease (CJD) patients were reported to excrete urinary protease-resistant PrP isoform (25), indicating that urinary excretion from infected animals may provide a vector for horizontal transmission. However, there are studies that are not consistent with these findings (26, 27). Perhaps unrecognized nephritic conditions may underlie these discrepant observations, because it has been reported that urinary prion excretion is found only in scrapie-infected mice with lymphocytic nephritis (28). In this study, we observed several bands with high molecular weights that reacted with anti-cheetah AA antiserum in the whole urine sample, but not in the urine pellet in which AA amyloid fibrils should be recovered. We thought that the possibility for a transmission pathway through urine might be low, but it could not be ruled out.
In addition to urine, the alimentary shedding route has been considered as a possible transmission pathway (29). Abnormal prion protein is present in gut-associated lymphoid tissues of mule deer infected with CWD, consistent with an alimentary shedding route (30). In this study, we showed that the fecal fraction from a cheetah with amyloidosis had AA amyloid fibrils and possessed high transmissibility. In mouse AApoAII amyloidosis, regarded recently as another transmissible amyloidosis (5–7), we also demonstrated that the feces could serve as an agent to induce amyloidosis in recipient mice (31). These results shed new light on the etiology involved in the high incidence of AA amyloidosis in cheetahs.
In this study, we unexpectedly found that the amyloid fibril fraction from feces had smaller amyloid fibrils and higher sensitivity to denaturation treatment than the liver amyloid fibril fraction. In mammalian prion, it has been demonstrated that there is a very strong correlation between seeding capability and amyloid fibril conformation (32, 33). Similarly, in yeast prion, it also has been indicated that [PSI+] with stronger infectivity typically have less stable fibrils in vivo than strains with weaker infectivity (34), and the prion strain with relatively smaller prion particles is always associated with greater frangibility and increased sensitivity to denaturants (35). The enhanced frangibility is presumably involved in the increase in seeding efficiency and prion infectivity, while the high sensitivity probably results from structural differences in inter-molecular contacts and a shorter, less stable amyloid core. The divergent ultrastructure between the fecal and the liver fibrils identified by transmission electron microscopy may be responsible for the different characteristics of transmissibility and sensitivity to denaturation treatment, analogous to prion protein.
It has been reported that AA amyloidosis can be experimentally induced by i.v. or i.p. administration of AA amyloid fibrillar extracts in recipient mice (10). A few recent studies have shown that AA-containing extracts also had amyloid-inducing activity when administered orally to mice (36, 37). In AApoAII amyloidosis, we reported that an oral administration of AApoAII amyloid fibrils induced amyloidosis in recipient mice (38). Thus, it is plausible that oral ingestion of AA-containing fecal matter caused amyloid deposition in the cheetah population. At this juncture, the manner in which fecal matter is initially absorbed by the cheetahs is not clear. This may occur during mutual grooming (licking of the fur contaminated by fecal matter). Recently it was shown that a prion agent could bind to whole soil and common soil minerals and retain infectivity for a prolonged period (23, 39). Thus, soil may act as a reservoir capable of contaminating both food and fur. It is also unknown how AA fibril proteins enter the feces. Because AA amyloidosis was also in the small intestines of AA amyloidosis cheetahs, it is possible that AA proteins enter the feces through exfoliated mucosa.
In conclusion, we found that cheetahs with amyloidosis pass fecal matter that had strong seeding efficiency and should be regarded as a transmission medium. To control the incidence of AA amyloidosis and reduce the likelihood of the animal's extinction, prevention of the transmission with excretion from cheetahs with amyloidosis should be considered along with reduction of precursor SAA levels.
Materials and Methods
Animals.
C68 was a domestic male cheetah that died of AA amyloidosis at 4 years of age. AA amyloidosis was systemic and severe. C66 (died at 8 years of age) and C90 (died at 11 years of age) were female cheetahs that had systemic AA amyloidosis similar to C68. C67 was a female cheetah that died at age 13 years with minor AA amyloidosis seen in the kidney. C78 was a male cheetah that died at 1 year of age and had a minor systemic AA amyloidosis. TT253 was a female cheetah that died at 17 years of age with very minor AA amyloidosis localized only in the esophagus. Because the amount of fecal amyloid fibril fraction obtained from a single cheetah was not sufficient, we used fecal fractions from C68 and C67 for induction of experimental AA amyloidosis, fecal fraction from C90 in immunodepletion experiments, and fecal amyloid fibril fraction from C78 for amino acid sequencing.
Eight-week-old male C57BL/6 mice (Japan SLC) were used for the induction of experimental AA amyloidosis. Mice were raised in the Division of Laboratory Animal Research, Research Center for Human and Environmental Sciences, Shinshu University, under clean conventional conditions at 24 ± 2°C with a 12-h/12-h light/dark cycle. A commercial diet (MF; Oriental Yeast) and tap water were provided ad libitum. A female JW/CSK rabbit bought from Japan SLC was used for the production of antiserum against AA amyloid fibril proteins. All experiments were performed with the consent of the Animal Care and Use Committee of Shinshu University School of Medicine.
Production of Antiserum for Cheetah AA Amyloid Fibril Proteins.
Crude amyloid fibrils were isolated in a water suspension fraction from the liver of C68 and further purified by ultracentrifugation (40). The amyloid fibrils were partially degraded in 0.1 N of NaOH at 37°C for 1 h and used for immunization. Antiserum was produced in a rabbit by s.c. injection of the partially degraded AA amyloid fibrils suspended in DW with TiterMax (CytRX Norcross) adjuvant. The rabbit was given booster doses after 2 and 4 weeks with incomplete Freund's adjuvant and bled 2 weeks after the last dose.
Isolation of Amyloid Fibrils and Western Blot Analysis.
AA amyloid fibril fractions were isolated from feces of cheetah and from human (an autopsied thyroid of a 49-year-old female patient with rheumatoid arthritis), mouse (the liver of a C57BL/6 mouse with induced severe AA amyloidosis), and bovine (the liver of an aged cow with severe spontaneous AA amyloidosis), using the same method as in ref. 40. Murine AApoAIIC amyloid fibril fraction was isolated from the liver of an R1.P1-Apoa2c mouse. Urine from C68 was treated as described in ref. 41. Protein contents in the fibril preparations were determined with a protein assay kit (Bio-Rad). Proteins in the amyloid fibril fractions were separated by 16.5% SDS/PAGE with a Tris·tricine buffer system and electro-transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). AA on the blots was detected with either anti-cheetah AA antiserum (1:3,000 dilution) or anti-mouse AA antiserum (1:5,000 dilution).
Histological and Immunohistochemical Studies.
Specimens from the livers of cheetahs or spleens of mice induced by various fibrils were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were stained with Congo red and observed by using polarizing microscopy. For identification of amyloid protein, an immunohistochemical study, using labeled streptavidin-biotin, was performed with rabbit anti-cheetah AA antiserum (1:1,000 dilution) or anti-mouse AA antiserum (1:3,000 dilution) as primary antibodies.
Amino Acid Sequence Analysis of Amyloid Fibril Proteins.
The isolated liver amyloid protein from C68 was separated with 16.5% SDS/PAGE followed by CBB staining, and the AA protein bands were excised for amino acid sequence analysis described in SI Text. The fecal AA amyloid fibril fractions were also isolated followed by CBB staining. Because the fecal amyloid fibril sample did not show a clean detectable band with CBB, the gel region corresponding to the band immunodetected by anti-cheetah AA antiserum was excised for sequence analysis.
Transmission Electron Microscopy.
Aliquots (20 μl, 0.5 μg/μl) of amyloid fibril fractions from the liver and feces of the cheetah were applied to 400-mesh collodion-coated copper grid (Nissin EM) for 1 min and subjected to negative staining with 1% phosphotungstic acid (pH 7.0) for 1 min. The negatively stained samples were observed with a JEOL 1200EX electron microscope (JEOL) at 80 kV. Electron micrographs were taken with a Gatan multiscan camera model 791 with Gatan digital micrograph software version 3.6.4 (Gatan).
Treatment of AA Amyloid Fibrils by Guanidine·HCl and Formic Acid.
Lyophilized AA amyloid fibrils were dissolved in buffer [6 M guanidine·HCl, 0.1 M Tris·HCl (pH 10.0), and 50 mM DTT] at a final concentration of 1.0 mg/ml and were denatured for 24 h with gentle stirring at room temperature followed by dialysis against 10 mM NH4HCO3 and lyophilization. The lyophilized fibrils were dissolved in DW (1.0 mg/ml) and used immediately for injection (42). The other lyophilized AA fibrils were treated with 88% formic acid for 8 h at room temperature followed by dialysis against DW for 6 h before injection (38).
AA Immunodepletion of AA Amyloid Fibril Fraction from the Feces.
Six hundred μl of protein G-Agarose beads were washed three times in DW and incubated overnight at 4°C with 60 μl of anti-cheetah AA antiserum or normal anti-rabbit IgG, then washed three times in DW. The AA amyloid fibril fractions isolated from the feces were diluted to a final concentration of 1 mg/ml in DW. The diluted AA amyloid fractions (120 μl) were allowed to react with antibody-beads complexes overnight at 4°C, then pelleted by centrifugation at 13,400 × g for 1 min at 4°C. Supernatants were harvested and aliquots were used for Western blot with anti-cheetah AA antiserum to assess immunodepletion. Five cycles of immunodepletion were used to remove the AA amyloid proteins in the cheetah feces and the supernatant was used (10 μl for each mouse) in induction experiments.
Induction of Experimental AA Amyloidosis.
Each mouse was injected intravenously with a single dose of sonicated amyloid fibrils and, simultaneously, with a s.c. injection of 1% AgNO3 (0.5 ml). Ten days after treatment, all mice were killed by cardiac puncture under diethyl ether anesthesia. Half of each organ was fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 4-μm serial sections for Congo red staining and immunohistochemical staining, and the other half was stored at −80°C for future use. The scoring of severity of induced AA amyloidosis was performed based on the amyloid deposition in the spleen identified in Congo red stained sections. The amount of amyloid deposited in each spleen was graded as follows: 0, no amyloid deposition; 1, minute amount of amyloid deposition; 2, small amounts of amyloid deposition only in the perifollicular regions of the spleen; 3, moderate amount of amyloid deposition (< 30% of the area of the red pulp of the spleen); 4, extensive amyloid deposition (30–80% of the area of the red pulp of the spleen). All sections were examined by two independent observers who were blinded to the experimental protocol. For quantitative analysis of the degree of AA amyloid deposition, the AA amyloid fibril fraction was isolated from the spleen of each mouse induced, applied to Western blot analysis with anti-mouse AA antiserum and then quantified by using a densitometric image analyzer with National Institutes of Health Image version 1.61.
Statistical Analysis.
The scores for amyloid deposition in the spleens among the various groups of mice were compared by using the Mann–Whitney U test. Student's t test was used for the AA protein quantitative analysis based on the results of Western blot analysis, using National Institutes of Health Image, Version 1.61. Differences were considered significant if P values were <0.05.
Supplementary Material
Acknowledgments.
We gratefully acknowledge Kiyoshi Matsumoto (Research Center for Human and Environmental Science, Shinshu University) for animal care, and Kiyokazu Kametani for assistance with the histological studies and electron microscopic studies. This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grants-in-Aid for Priority Areas 17028018 and Scientific Research (B) 17390111 and by a grant from the Intractable Disease Division, the Ministry of Health, Labor and Welfare, Research Committees for Amyloidosis in Japan and for Epochal Diagnosis and Treatment of Amyloidosis in Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7113.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800367105/DCSupplemental.
References
- 1.Westermark P, et al. Amyloid fibril protein nomenclature-2002. Amyloid. 2002;9:197–200. doi: 10.3109/13506120209114823. [DOI] [PubMed] [Google Scholar]
- 2.Caughey B. Transmissible spongiform encephalopathies, amyloidoses and yeast prion; common threads? Nat Med. 2000;6:751–754. doi: 10.1038/77476. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KH, Westermark P, Sletten K, O'Brien TD. Amyloid proteins and amyloidosis in domestic animals. Amyloid Int J Exp Clin Invest. 1996;3:270–289. [Google Scholar]
- 4.Sigurdsson EM, Wisniewski T, Frangione B. Infectivity of amyloid diseases. Trends Mol Med. 2002;8:411–413. doi: 10.1016/s1471-4914(02)02403-6. [DOI] [PubMed] [Google Scholar]
- 5.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Walker L, LeVine H, Jucker M. Koch's postulates and infectious proteins. Acta Neuropathol. 2006;112:1–4. doi: 10.1007/s00401-006-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker LC, LeVine H, Mattson MP, Jucker M. Inducible proteopathies. Trends Neurosci. 2006;29:438–443. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 9.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 10.Lundmark K, et al. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci USA. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niewold TA, Hol PR, van Andel AC, Lutz ET, Gruys E. Enhancement of amyloid induction by amyloid fibril fragments in hamster. Lab Invest. 1987;56:544–549. [PubMed] [Google Scholar]
- 12.Johan K, et al. Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganowiak K, Hultman P, Engstrom U, Gustavsson A, Westermark P. Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem Biophys Res Commun. 1994;199:306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- 14.Papendick RE, Munson L, O'Brien TD, Johnson KH. Systemic AA amyloidosis in captive cheetahs (Acinonyx jubatus) Vet Pathol. 1997;34:549–556. doi: 10.1177/030098589703400602. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien SJ, et al. Genetic basis for species vulnerability in the cheetah. Science. 1985;227:1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- 16.Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech. 1996;15:827–852. doi: 10.20506/rst.15.3.959. [DOI] [PubMed] [Google Scholar]
- 17.Miller MW, Williams ES. Horizontal prion transmission in mule deer. Nature. 2003;425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KH, et al. Amino acid sequence analysis of amyloid protein A (AA) from cats (captive cheetahs: Acinonyx jubatus) with a high prevalence of AA amyloidosis. Amyloid Int J Exp Clin Invest. 1997;4:171–177. [Google Scholar]
- 19.Mathiason CK, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;6:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 20.DeJoia C, Moreaux B, O'Connell K, Bessen RA. Prion infection of oral and nasal mucosa. J Virol. 2006;80:4546–4556. doi: 10.1128/JVI.80.9.4546-4556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post K, Riesner D, Walldorf V, Mehlhorn H. Fly larvae and pupae as vectors for scrapie. Lancet. 1999;354:1969–1970. doi: 10.1016/S0140-6736(99)00469-9. [DOI] [PubMed] [Google Scholar]
- 22.Carp RI, et al. Characteristics of scrapie isolates derived from hay mites. J Neurovirol. 2000;6:137–144. doi: 10.3109/13550280009013157. [DOI] [PubMed] [Google Scholar]
- 23.Brown P, Gajdusek DC. Survival of scrapie virus after 3 years' interment. Lancet. 1991;337:269–270. doi: 10.1016/0140-6736(91)90873-n. [DOI] [PubMed] [Google Scholar]
- 24.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaked GM, et al. A protease-resistant prion protein isoform is present in urine of animals and humans affected with prion diseases. J Biol Chem. 2001;276:31479–31482. doi: 10.1074/jbc.C100278200. [DOI] [PubMed] [Google Scholar]
- 26.Serban A, Legname G, Hansen K, Kovaleva N, Prusiner SB. Immunoglobulins in urine of hamsters with scrapie. J Biol Chem. 2004;279:48817–48820. doi: 10.1074/jbc.M409107200. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa H, et al. A pitfall in diagnosis of human prion diseases using detection of protease-resistant prion protein in urine. Contamination with bacterial outer membrane proteins. J Biol Chem. 2004;279:23661–23667. doi: 10.1074/jbc.M400187200. [DOI] [PubMed] [Google Scholar]
- 28.Seeger H, et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 29.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 30.Spraker TR, et al. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet Pathol. 2002;39:110–119. doi: 10.1354/vp.39-1-110. [DOI] [PubMed] [Google Scholar]
- 31.Xing Y, et al. Transmission of mouse senile amyloidosis. Lab Invest. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- 32.Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan R, Lindquist SL. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature. 2005;435:765–772. doi: 10.1038/nature03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 36.Cui D, et al. Acceleration of murine AA amyloidosis by oral administration of amyloid fibrils extracted from different species. Pathology International. 2002;52:40–45. doi: 10.1046/j.1440-1827.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- 37.Solomon A, et al. Amyloidogenic potential of foie gras. Proc Natl Acad Sci USA. 2007;104:10998–11001. doi: 10.1073/pnas.0700848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Transmissibility of mouse AApoAII amyloid fibrils: Inactivation by physical and chemical methods. FASEB J. 2006;20:1012–1014. doi: 10.1096/fj.05-4890fje. [DOI] [PubMed] [Google Scholar]
- 39.Johnson CJ, et al. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:296–302. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing Y, et al. Induction of protein conformational change in mouse senile amyloidosis. J Biol Chem. 2002;277:33164–33169. doi: 10.1074/jbc.M111570200. [DOI] [PubMed] [Google Scholar]
- 41.Korenaga T, et al. Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis. Am J Pathol. 2006;168:898–906. doi: 10.2353/ajpath.2006.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuch K, et al. Fibrilization in mouse senile amyloidosis is fibril conformation-dependent. Lab Invest. 1998;78:1535–1542. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.