Abstract
Peroxisome proliferators-activated receptors (PPARs) that are members of the nuclear receptor superfamily have three different isoforms: PPARα, PPARδ, and PPARγ. PPARs are ligand-activated transcription factors, and they are implicated in tumor progression, differentiation, and apoptosis. Activation of PPAR isoforms lead to both anticarcinogenesis and anti-inflammatory effect. It has so far identified many PPAR ligands including chemical composition and natural occurring. PPAR ligands are reported to activate PPAR signaling and exert cancer prevention and treatment in vitro and/or in vivo studies. Although the effects depend on the isoforms and the types of ligands, biological modulatory activities of PPARs in carcinogenesis and disease progression are attracted for control or combat cancer development. This short review summarizes currently available data on the role of PPAR ligands in carcinogenesis.
1. INTRODUCTION
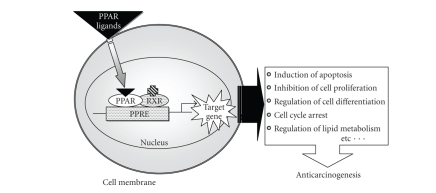
Peroxisome proliferators-activated receptors (PPARs) are member of the nuclear hormone receptor superfamily that were initially characterized as molecules that mediated the proliferation of peroxisomes in rodent liver parenchymal cells in response to the hypolipidemic drug clofibrate [1]. Subsequently, PPARs have been shown to regulate the expression of genes involved in a variety of biological processes, including lipid metabolism and insulin sensitivity [2, 3]. Three isotypes of PPAR exist; PPARα, PPABβ/δ or simply δ, and PPARγ which are known and they are encoded by three separate genes and display distinctly different tissue distributions and functions. PPARα regulates numerous aspects of fatty acid catabolism, where as PPARγ controls adipocyte differentiation, systemic glucose levels, and lipid homeostasis [4, 5]. PPARδ is involved in development, embryo implantation, myelination of the corpus callosum, lipid metabolism, and epidermal cell proliferation [6]. The PPARs are ligand-dependent transcription factors that regulate target genes expression by binding to characteristic DNA sequences termed peroxisome proliferators response element (PPREs) located in the 5′ -flanking region of target genes [7, 8]. Each receptor binds to its PPRE as a heterodimer with the receptor for 9-cis retinoic acid, the retinoid X receptor (RXR) (Figure 1). Upon binding a ligand, the conformation of a PPAR is altered and stabilized such that a binding cleft is created, and recruitment of transcriptional coactivators occurs. The result is an increase in gene transcription, therefore PPARs are able to regulate such divers effects as cell proliferation, differentiation, or apoptosis.
Figure 1.
PPAR activation pathway and its target genes.
2. PPARα LIGANDS AND CARCINOGENESIS
PPARα is the first member of this nuclear receptor subclass to be cloned [9]. PPARα is expressed preferentially in the liver [10] and tissues with high fatty acid catabolism, such as the kidney, heart, skeletal muscle, and brown fat [11–13]. The PPARα isotype is the cellular target for leukotriene B4 (LTB4) fibrates such as bezafibrate and fenofibrate, which are hypolipidemic drugs widely used for reducing triglyceride levels, a risk of cardiovascular diseases. Several studies have established a link between PPARα activation and epidermal differentiation. Fibrates induce differentiation and inhibit proliferation in normal and hyperproliferating mouse epidermis and regulate apoptosis, but are inactive in PPARα-deficient mice [14, 15]. Farnesol also stimulates PPARα-dependent differentiation in epidermal keratinocytes [16]. Topical PPARα ligands have weak preventive effects on tumor promotion in mouse skin, despite upregulation of PPARα in untreated tumors compared with normal epidermis [17]. These observations suggest that the use of PPARα activators may have chemopreventive properties in skin carcinogenesis. PPARα expression is also upregulated in human prostate adenocarcinomas [18]. In addition, PPARα ligands suppress the growth of several cancer lines, including colon [19], endometrial [20], and breast [21] in vivo or in vitro. PPARα ligands are able to suppress the metastatic potential of melanoma cells in vivo and in vitro [22, 23]. More recently, a PPARα ligand WY14643 suppresses both endothelial cell proliferation and tumorigenesis in a PPARα-dependent manner [24]. These data suggest that certain PPARα ligands may act as antitumor agents, although the exact mechanisms remain unclear. PPARα activation has been associated with both anti and proinflammatory actions in rodents. PPARα ligands reduce expression of inflammatory markers [25]. In contrast, the expression of the inflammatory mediator cyclooxygenase (COX)-2 in human breast and colon cancer cells is upregulated by PPARα ligands [26]. The increased COX-2 expression is known to link to the risk of epithelial malignancies [27]. These findings indicate that PPARα ligands may be interesting candidates for the chemoprevention of several types of cancers, but we should consider negative face of influence of PPARα ligands on cancer development.
3. PPARδ LIGANDS AND CARCINOGENESIS
A number of reports have described a variety of biological functions of the PPARα and γ isotypes. These two isotypes also have clinical significance in the treatment of dyslipideamia and type II diabetes mellitus [28]. In contrast, less is known about the physiological role of the PPARδ isoform, although there is some evidence supporting its involvement in embryo implantation and development [6, 29], epidermal maturation and wound healing [30], and regulation of fatty acid metabolism [31]. Recently, the effect of PPARδ function on colon carcinogenesis has been reported. However, the role of PPARδ in colon cancer is still unclear, as there are data suggesting that it either inhibits or promotes colon carcinogenesis. PPARδ expression is increased in colon tumor cells with a mutant Apc (adenomatous polyposis coli) allele (min) [32]. The number of polyps was the same among the multiple intestinal neoplasia (Min) mice that were Ppard −/−, Ppard +/−, or Ppard +/+. These findings suggest that PPARδ is not essential for colon carcinogenesis, but PPARδ may affect size and/or growth of polyps [29]. The most striking results were provided by a study demonstrating that in PPARδ deficient (Ppard−/−) mice, both Min mutants and those with chemically induced cancers, colon polyp formation was significantly greater in those nullizygous for PPARδ [33]. These results suggest that PPARδ attenuates colon carcinogenesis. On the other hand, the following observations strongly suggest that PPARδ enhances colon cancer formation. PPARδ was elevated in colon cancer cells and was repressed by APC gene via the β-catenin/Tcf-4 response elements in its promoter [32]. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells [34]. Nitric oxide donating aspirin is reported to suppress intestinal tumors in Min mice and downregulates the expression of PPARδ and enhance apoptosis and perhaps atypical cell death [35]. This suggests that PPARδ contributes to intestinal carcinogenesis.
GW501516 was shown to be a PPARδ subtype-selective ligand using combinatorial chemistry and structure-based drug design [36]. There are some reports describing the effects of PPARδ ligand on colon carcinogenesis. Exposure of APC min/+ mice to the GW501516 resulted in activation of PPARδ and significant acceleration of intestinal adenoma growth [37]. Furthermore, PPARδ activation by PPARδ ligand promotes tumor growth by inhibiting epithelial tumor cell apoptosis through activation of a VEGF autocrine signaling loop in APC min/+ mice [38]. GW501516 stimulates proliferation of human breast, prostate, and hepatocellular carcinoma cells [39, 40]. In a mouse mammary tumorigenesis model, GW501516 activates 3-phospholinositide-dependent protein kinase-1 that is oncogenic when expressed in mammary ductal cells, and leads to accelerated tumor formation [41]. From these findings, PPARδ selective-ligand tends to exert enhancing effects on carcinogenesis, while its antagonists are expected to prevention and/or treatment of cancer.
4. PPARγ LIGANDS AND CARCINOGENESIS
PPARγ plays an important role in the regulation of proliferation and differentiation of several cell types. PPARγ is known to be expressed in various organs, including adipose tissue [42], mammary glands [43], small intestine [44], lung [45], colon [44], and stomach [46], and is also upregulated in various types of cancer cells.
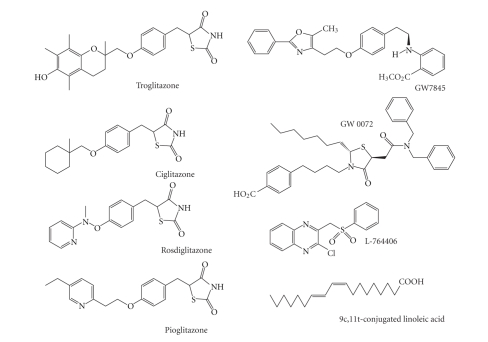
This receptor has the ability to bind a variety of small lipophilic compounds derived from both metabolism and nutrition. These ligands, in turn, direct cofactor recruitment to PPARγ, regulating the transcription of genes in a variety of complex metabolic pathways. Several specific ligands (Figure 2) have been identified, such as the thiazolidinediones (including pioglitazone, rosiglitazone, and troglitazone), naturally occurring lipid, polyunsaturated fatty acids (PUFA) (including arachidonic, oleic, and linoleic acid) and the cyclopentenone prostaglandin (PG) 15-deoxy Delta12,14-PGJ2, a metabolite of PGD2. PPARγ ligands have been reported to induce cell differentiation and apoptosis in several types of cancer [47–51], suggesting potential application as anticancer agents. Furthermore, some reports recently suggested that PPARγ ligands can be used as chemopreventive agents for colon, breast, and tongue carcinogenesis [52–54].
Figure 2.
Synthetic and naturally occurring ligands for PPARγ.
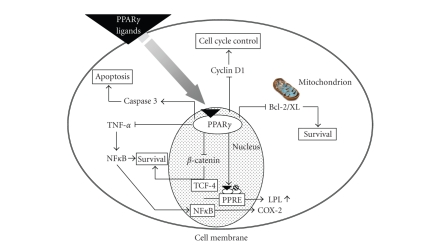
The most widely used synthetic agents belong to the thiazolidinedione class of antidiabetic drugs (also referred to as glitazones). These include ciglitazone, troglitazone, pioglitazone, rosiglitazone, and LY171.833. Pioglitazone, rosiglitazone, and troglitazone have already been used clinically to treat type 2 diabetes, making use of the ability of synthetic PPARγ ligands to sensitize insulin and to lower blood glucose concentration. Recent evidence indicates that certain thiazolidinedione members, especially troglitazone and ciglitazone, exhibit moderate anitproliferative activities against epithelial-derived human cancer cell lines, including those of prostate [55], breast [56], colon [57], thyroid [51], lung [58], and pituitary carcinoma [50]. PPARγ is known to be expressed in a variety of cancer, and the treatment of these cancer cells with PPARγ ligands often induces cell differentiation and apoptosis [47–51], and exerts antiproliferative effects on human colon cancer [59], breast cancer [47], pituitary adenomas [50], gastric cancer [60], and bladder cancer [61]. Furthermore, postulated mechanisms by which PPARγ ligands exert their effects include modulation of the oncogenic Wnt pathway, inhibition of nuclear factor kappaB (NF-κB), and modulation of cell cycle pro and antiapoptotic proteins (Figure 3). Wnt signaling is a complex pathway in which β-catenin binds to transcription factors in the nucleus and plays a role as a central mediator in regulating cell proliferation and differentiation [62]. PPARγ activation causes a decrease in β-catenin expression in adipocytes in vitro and in normal intestinal mucosa in mice [63]. In the cultured human monocytes, PPARγ inhibits NF-κB activation thus influencing the transcription of both survival- and apoptosis-related genes [64]. PPARγ activation also induces the activation of the proapoptotic caspase-3 protein in human liver cancer cell lines and a reduction in antiapoptotic Bcl-2 and Bcl-XL protein level in human colon and gastric cancer cell lines, respectively [65–67]. Furthermore, colon cancer development is related to hyperlipidemia [68], with clear links to high level of serum triglycerides (TGs) [69]. A PPARγ ligand, pioglitazone, suppresses both hyperlipidemia and intestinal polyp formation in the APC-deficient mice in conjunction with elevation of lipoprotein lipase (LPL), which catalyzes TG hydrolysis [70].
Figure 3.
Molecular mechanisms for anticarcinogenic and/or chemopreventive effects of PPARγ ligands.
We previously investigated the modifying effects of PPARγ or α ligands (troglitazone, pioglitazone, or bezafibrate) on early phase of colon carcinogenesis with or without colitis in male F344 rats [19, 71]. The role of PPARγ in AOM-induced colon tumorigenesis was directly demonstrated by the study showing that the incidence of colonic tumors increased in the hemizygous knockout of PPARγ that received AOM [72]. Although thiazolidinediones inhibit AOM-induced colon carcinogenesis in the wild type mice, the observation using APC-deficient mouse models showed conflicting results regarding the effects of PPARγ ligand treatment [73–76]. This may be caused by use of different PPARγ agonists (troglitazone versus pioglitazone), and different doses (100–2000 ppm in diet) examined. Colonic inflammation is associated with a high risk of colorectal cancer (CRC) [77]. CRC is thus one of the most serious complications of inflammatory bowel disease, such as ulcerative colitis and Crohn's disease [77]. In the experiments, dietary administration of PPARα or γ ligands effectively suppressed azoxymethane (AOM)-induced or dextran sodium sulfate (DSS)/AOM-induced aberrant crypt foci, which are precursor lesions for colon carcinoma (Table 1). Our findings suggested that synthetic PPARγ and PPARα ligands are able to inhibit the early stages of colon tumorigenesis with or without colitis, and the findings were confirmed by the study conducted by Osawa et al. [78]. Furthermore, we demonstrated ligands for PPARγ and PPARα inhibit colitis-related colon carcinogenesis [79] using our AOM/DSS mouse model [80]. In the experiment, dietary administration (0.05% in diet for 14 weeks) with troglitazone and bezafibrate significantly inhibited both the incidence and multiplicity of colonic adenocarcinoma induced by the treatment with AOM/DSS, although bezafibrate feeding did not significantly lower the multiplicity (Table 2). Dietary exposure of troglitazone and bezafibrate suppressed cell proliferation and induced apoptosis and lowered immnoreactivity of COX-2, inducible nitric oxide, and nitrotyrosine in the colonic malignancies.
Table 1.
Effects of PPAR ligands on ACF formation in rats.
| Treatment (No. of mice) | ACF/colon (% inhibition) | ACs/colon (% inhibition) |
|---|---|---|
| AOM alone (12) | 83 ± 6(a) | 2.0 ± 0.24 |
| AOM + 0.01% troglitazone(8) | 68 ± 16 (18%) | 1.7 ± 0.21(15%) |
| AOM + 0.05% troglitazone(8) | 55 ± 13(b) (34%) | 1.5 ± 0.13(c) (25%) |
| AOM + 0.01% bezafibrate (8) | 75±8 (10%) | 2.0 ± 0.20 (0%) |
| AOM + 0.05% bezafibrate (8) | 53± 9(d) (36%) | 1.9 ± 0.10(5%) |
| None | 0 | 0 |
|
| ||
| 1% DSS + AOM (10) | 115 ± 22 | 2.4 ± 0.29 |
| 1% DSS + AOM + 0.01% pioglitazone (7) | 71 ± 24(e) (38%) | 1.8 ± 0.17(f) (25%) |
| 1% DSS + AOM + 0.01% troglitazone(7) | 57 ± 14(g) (50%) | 1.6 ± 0.14(g) (33%) |
| 1% DSS + AOM + 0.01% bezafibrate (7) | 59 ± 18(h) (49%) | 1.7 ± 0.16(i) (29%) |
| None | 0 | 0 |
(a)Mean ±SD.
(b–d)Significantly different from the AOM alone group: (b) P < .01; (c) P < .005; and (d) P < .001.
(e–i)Significantly different from the DSS/AOM group: (e) P < .05; (f) P < .01; (g) P < .001; (h) P < .005; and (i) P < .002.
Table 2.
Effects of PPAR ligands on colon carcinogenesis in mice.
| Treatment (no. of mice) | Incidence/Multiplicity (% inhibition) | ||
|---|---|---|---|
| Total | Adenoma | Adenocarcinoma | |
| AOM/DSS | 100%/5.2 ± 3.0(a) | 100%/2.1 ± 1.8 | 100%/3.0 ± 1.8 |
| AOM/DSS/0.05% Troglitazone | 90%/2.5 ± 1.8(b)(52%,) | 90%/1.6 ± 1.1 (24%) | 40%(c)/1.2 ± 2.5(b) (60%) |
| AOM/DSS/0.05% Bezafibrate | 80%/2.6 ± 2.5(b)(50%) | 70%/1.1 ± 1.0(b) (48%) | 60%(b)/1.8 ± 2.6 (40%) |
| None | 0%/0 | 0%/0 | 0%/0 |
(a)Mean ±SD.
(b,c)Significantly different from the AOM/DSS group: (b) P < .05; and (c) P < .01.
PPARγ receptors are activated by certain lipophilic ligands, such as PUFAs and eicosanoid derivatives. They bind to the PPARγ receptor at micromolar concentrations. The essential fatty acids (arachidonic acid, docosahexanoic acid, and eicosapentaenoic acid) as well as modified oxidized lipids (9-hydroxyoctadecanoic acid and 13-hydroxy-octadecanoic acid) bind to and activate PPARγ [5]. Recently, conjugated linoleic acid (CLA) was shown to act as a high affinity ligand and an activator of PPARγ [81]. Anticarcinogenic activity of CLA is mediated by PPARγ activation in susceptible tumors [81]. When treated with CLA, PPARγ expression is increased, and APC and c-myc proteins are downregulated in the human colon cancer cells, and finally proliferation of cancer cells is inhibited by CLA [82–85]. In fact, feeding with seed oils containing 9c, 11t, 13t-, 9c, 11t, 13c-, and 9t, 11t, 13c-conjugated linolenic acid, which are converted to 9c, 11t- and 9t, 11t-CLA within colonic and liver cells, suppresses AOM-induced colon carcinogenesis by increased expression of PPARγ protein in the colon mucosa [86–89].
5. CLINICAL TRIAL FOR PPARγ LIGANDS AGAINST TUMORS
There are several clinical studies on the effects of PPARγ ligands on malignancies (Table 3). The beneficial effects of glitazones on liposarcomas have been demonstrated in a small clinical trial [90]. Three patients with intermediate to high-grade liposarcomas were given troglitazone (800 mg/day orally). In the patients, differentiation of the neoplasms occurred as revealed by histological and biochemical analysis. The clinical outcome of these patients was not reported, but the therapy was well tolarated [90]. However, a phase II study on 12 patients with liposarcoma showed that the PPARγ ligand rosiglitazone did not significantly improve clinical outcome [94]. In prostate, PPARγ immunoreactivity was significantly higher in prostate cancer and prostatic intraepithelial neoplasia than in those with benign prostate hyperplasia and with healthy prostate [98]. A high incidence of prolonged stabilization of serum prostate-specific antigen (PSA) was observed in phase II clinical study, where patients with advances prostate cancer who had no symptoms of metastasis were treated with troglitazone (800 mg/day orally). Moreover, one patient had a striking decrease in PSA concentration to almost undetectable amounts [91]. In a 75-year-old man with occult recurrent prostate cancer showed a decrease in PSA after oral treatment with toroglitazone (600–800 mg/day for 1.5 years) [92]. Thus, PPARγ is expressed in prostate cancer and activation of PPARγ might offer an additional therapeutic option for treatment of prostate cancer in the near future. At present, most of the available data suggest that PPARγ has antineoplastic effect on malignant neoplasms [99], including colonic malignancies. However, in a clinical phase II study on CRC, orally administrated troglitazone did not lengthen median progression-free survival or median survival in 25 patients with chemotherapy-resistant metastatic colon carcinoma [93]. In a phase II study [95] for the use of troglitazone to treat patients with advanced refractory breast cancer, no objective tumor response was observed. However, the study was incomplete because troglitazone was withdrawn from commercial availability after a warning by the US Food and Drug Administration about hepatic toxic effects. On the other hand, it is important to note that neither hormone status of the tumors nor the amount of PPARγ protein is assessed before patients were included in the study. In an open labeled phase II study where ten patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer were enrolled and they were given oral rosiglitazone treatment (4 mg/day for 1 week, then 8 mg per day for 7 weeks), rosiglitazone treatment resulted in a 40% partial response rate, but no complete responses, and the expression level of PPARγ mRNA and protein in the neoplasm appeared unrelated to rosiglitazone treatment response [96]. The findings also suggest that higher doses and longer duration of rosiglitazone therapy may be useful to better define the role of rosiglitazone as a redifferentiation agent in differentiated thyroid cancer. There is a phase I clinical study of a PPARγ ligand (LY293111) that is not thiazolidinedione members [97]. LY293111 is a novel diaryl ether carboxylic acid derivative and is known as PPARγ agonist and LTB4 antagonist. The study suggested the dose (600 mg) of LY293111 in combination with irinotecan (200 mg/m2 IV every 21 days for phase II clinical study against solid tumors.
Table 3.
Clinical trials on the anticancer effects of PPARγ ligands.
| Clinical trials | Drug | Results | Reference no. |
|---|---|---|---|
| Patients with intermediate to high-grade liposarcomas (case reports) | Troglitazone | Histlogical and biochemical differentiation | [90] |
| Phase II study on patients with histologically-confirmed prostate cancer and no symptomatic metastatic disease | Troglitazone | Lengthened stabilization of prostate-specific antigen | [91] |
| 75-year-old patient with an occult recurrent prostate cancer (case reports) | Troglitazone | Reduced prostate-specific antigen | [92] |
| Phase II study on patients with metastatic colon cancer | Troglitazone | No significant effect | [93] |
| Phase II study on patients with liposarcoma | Rosiglitazone | Lengthened mean time of progression | [94] |
| Phase II study on patients with refractory breast cancer | Troglitazone | No significant effect | [95] |
| Phase II study on patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer | Rosiglitazone | Induced radioiodine uptake | [96] |
| Phase I study on patients with solid tumors | LY293111 | The recommended oral dose (600 mg/day) for phase II trial | [97] |
6. CONCLUSIONS
PPARs were originally recognized to be genetic regulators of complex pathways of mammalian metabolism, including fatty acid oxidation and lipogenesis. However, the receptors have been shown to be implicated in carcinogenesis and inflammation. PPARs are involved in cell proliferation and differentiation of a variety of cancer. Numerous reports indicate that PPARs ligands could play an important role in prevention and inhibition of cancer development. Synthetic PPAR ligands used for drugs or those of naturally occurring lipids are promising cancer chemopreventive agents with slight side effects against several types of cancer. We should characterize expression patterns of different isoforms of PPAR in cancerous and precancerous tissues and determine their precise roles in the carcinogenic process for development of PPARs ligands as a novel class of cancer preventive/theraputic drugs. Based on current data from preclinical and clinical studies, we believe that thiazolidinediones, especially PPARγ agonists, have important role in short-term prophylactic therapy designed to reduce the number of putative preneoplasia, ACF, in patients who are at high risk for CRC development.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Cancer Research, for the Third-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan; a Grant-in-Aid (no. 18592076 to T.T., 17015016 to T.T., and 18880030 to Y.Y.) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant (H2007-12 to T.T. and S2006-9 to Y.Y.) for the Project Research from the High-Technology Center of Kanazawa Medical University.
ABBREVIATIONS
- AOM:
Azoxymethane
- APC:
Adenomatous polyposis coli
- CLA:
Conjugated linoleic acid
- COX-2:
Cyclooxygenase-2
- CRC:
Colorectal cancer
- DSS:
Dextran sodium sulfate
- LPL:
Lipoprotein lipase
- LTB4:
Leukotriene B4
- PG:
Prostaglandin
- PPARs:
Peroxisome proliferators-activated receptor
- PPRE:
Peroxisome proliferators response element
- PSA:
Prostate-specific antigen
- PUFA:
Polyunsaturated fatty acid
- RXR:
Retinoid X receptor
- TG:
Triglyceride
References
- 1.Hess R, Stäubli W, Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy- isobutyrate in the rat. Nature. 1965;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- 2.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. The Lancet. 1999;354(9173):141–148. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 3.Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annual Review of Pharmacology and Toxicology. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- 4.Michalik L, Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Current Opinion in Biotechnology. 1999;10(6):564–570. doi: 10.1016/s0958-1669(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 5.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 6.Lim H, Gupta RA, Ma WG, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ . Genes & Development. 1999;13(12):1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer CNA, Hsu M-H, Griffin KJ, Johnson EF. Novel sequence determinants in peroxisome proliferator signaling. Journal of Biological Chemistry. 1995;270(27):16114–16121. doi: 10.1074/jbc.270.27.16114. [DOI] [PubMed] [Google Scholar]
- 8.Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson J-Å. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(4):1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 10.Palmer CNA, Hsu M-H, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-α expression in human liver. Molecular Pharmacology. 1998;53(1):14–22. [PubMed] [Google Scholar]
- 11.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 12.Auboeuf D, Rieusset J, Fajas L, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46(8):1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez FJ. The role of peroxisome proliferator activated receptor alpha in peroxisome proliferation, physiological homeostasis, and chemical carcinogenesis. Advances in Experimental Medicine and Biology. 1997;422:109–125. doi: 10.1007/978-1-4757-2670-1_9. [DOI] [PubMed] [Google Scholar]
- 14.Kömüves LG, Hanley K, Lefebvre AM, et al. Stimulation of PPARα promotes epidermal keratinocyte differentiation in vivo. Journal of Investigative Dermatology. 2000;115(3):353–360. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 15.Komuves LG, Hanley K, Man M-Q, Elias PM, Williams ML, Feingold KR. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPARα activators restores tissue homeostasis. Journal of Investigative Dermatology. 2000;115(3):361–367. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanley K, Kömüves LG, Ng DC, et al. Farnesol stimulates differentiation in epidermal keratinocytes via PPARα . Journal of Biological Chemistry. 2000;275(15):11484–11491. doi: 10.1074/jbc.275.15.11484. [DOI] [PubMed] [Google Scholar]
- 17.Thuillier P, Anchiraico GJ, Nickel KP, et al. Activators of peroxisome proliferator-activated receptor-α partially inhibit mouse skin tumor promotion. Molecular Carcinogenesis. 2000;29(3):134–142. doi: 10.1002/1098-2744(200011)29:3<134::aid-mc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Collett GP, Betts AM, Johnson MI, et al. Peroxisome proliferator-activated receptor α is an androgen-responsive gene in human prostate and is highly expressed in prostatic adenocarcinoma. Clinical Cancer Research. 2000;6(8):3241–3248. [PubMed] [Google Scholar]
- 19.Tanaka T, Kohno H, Yoshitani S, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 20.Saidi SA, Holland CM, Charnock-Jones DS, Smith SK. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Molecular Cancer. 2006;5, article 13 doi: 10.1186/1476-4598-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchanek KM, May FJ, Robinson JA, et al. Peroxisome proliferator-activated receptor α in the human breast cancer cell lines MCF-7 and MDA-MB-231. Molecular Carcinogenesis. 2002;34(4):165–171. doi: 10.1002/mc.10061. [DOI] [PubMed] [Google Scholar]
- 22.Grabacka M, Placha W, Plonka PM, et al. Inhibition of melanoma metastases by fenofibrate. Archives of Dermatological Research. 2004;296(2):54–58. doi: 10.1007/s00403-004-0479-y. [DOI] [PubMed] [Google Scholar]
- 23.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor α activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clinical Cancer Research. 2006;12(10):3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi A, Ibanez MR, Gatica AE, et al. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. Journal of Biological Chemistry. 2007;282(24):17685–17695. doi: 10.1074/jbc.M701429200. [DOI] [PubMed] [Google Scholar]
- 25.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. Journal of Biological Chemistry. 1999;274(45):32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 26.Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. Journal of Biological Chemistry. 1999;274(12):8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 27.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Advances in Cancer Research. 1999;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 28.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends in Pharmacological Sciences. 2004;25(6):331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Barak Y, Liao D, He W, et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di-Poï N, Michalik L, Tan NS, Desvergne B, Wahli W. The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. Journal of Steroid Biochemistry and Molecular Biology. 2003;85(2–5):257–265. doi: 10.1016/s0960-0760(03)00215-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 32.He T-C, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nature Medicine. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 34.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2598–2603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)δ expression in APC min/+ mice proportionally to their tumor inhibitory effect: implications for the role of PPARδ in carcinogenesis. Carcinogenesis. 2006;27(2):232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 36.Oliver WR, Jr, Shenk JL, Snaith MR, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nature Medicine. 2004;10(3):245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Wang H, Guo Y, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephen RL, Gustafsson MCU, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Research. 2004;64(9):3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 40.Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARδ activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochemical and Biophysical Research Communications. 2003;308(2):361–368. doi: 10.1016/s0006-291x(03)01384-6. [DOI] [PubMed] [Google Scholar]
- 41.Yin Y, Russell RG, Dettin LE, et al. Peroxisome proliferator-activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Research. 2005;65(9):3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 42.Keller H, Mahfoudi A, Dreyer C, et al. Peroxisome proliferator-activated receptors and lipid metabolism. Annals of the New York Academy of Sciences. 1993;684(1):157–173. doi: 10.1111/j.1749-6632.1993.tb32279.x. [DOI] [PubMed] [Google Scholar]
- 43.Gimble JM, Pighetti GM, Lerner MR, et al. Expression of peroxisome proliferator activated receptor mRNA in normal and tumorigenic rodent mammary glands. Biochemical and Biophysical Research Communications. 1998;253(3):813–817. doi: 10.1006/bbrc.1998.9858. [DOI] [PubMed] [Google Scholar]
- 44.Mansén A, Guardiola-Diaz H, Rafter J, Branting C, Gustafsson J-Å. Expression of the peroxisome proliferator-activated receptor (PPAR) in the mouse colonic mucosa. Biochemical and Biophysical Research Communications. 1996;222(3):844–851. doi: 10.1006/bbrc.1996.0832. [DOI] [PubMed] [Google Scholar]
- 45.McGowan SE, Jackson SK, Doro MM, Olson PJ. Peroxisome proliferators alter lipid acquisition and elastin gene expression in neonatal rat lung fibroblasts. American Journal of Physiology. 1997;273(6):L1249–L1257. doi: 10.1152/ajplung.1997.273.6.L1249. [DOI] [PubMed] [Google Scholar]
- 46.Huin C, Corriveau L, Bianchi A, et al. Differential expression of peroxisome proliferator-activated receptors (PPARs) in the developing human fetal digestive tract. Journal of Histochemistry & Cytochemistry. 2000;48(5):603–611. doi: 10.1177/002215540004800504. [DOI] [PubMed] [Google Scholar]
- 47.Elstner E, Müller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rumi MAK, Sato H, Ishihara S, et al. Peroxisome proliferator-activated receptor γ ligand-induced growth inhibition of human hepatocellular carcinoma. British Journal of Cancer. 2001;84(12):1640–1647. doi: 10.1054/bjoc.2001.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heaney AP, Fernando M, Melmed S. PPAR-γ receptor ligands: novel therapy for pituitary adenomas. Journal of Clinical Investigation. 2003;111(9):1381–1388. doi: 10.1172/JCI16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. Journal of Clinical Endocrinology & Metabolism. 2001;86(5):2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 52.Badawi AF, Badr MZ. Chemoprevention of breast cancer by targeting cyclooxygenase-2 and peroxisome proliferator-activated receptor-gamma (Review) International Journal of Oncology. 2002;20(6):1109–1122. [PubMed] [Google Scholar]
- 53.Brown PH, Lippman SM. Chemoprevention of breast cancer. Breast Cancer Research and Treatment. 2000;62(1):1–17. doi: 10.1023/a:1006484604454. [DOI] [PubMed] [Google Scholar]
- 54.Kopelovich L, Fay JR, Glazer RI, Crowell JA. Peroxisome proliferator-activated receptor modulators as potential chemopreventive agents. Molecular Cancer Therapeutics. 2002;1(5):357–363. [PubMed] [Google Scholar]
- 55.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Research. 1998;58(15):3344–3352. [PubMed] [Google Scholar]
- 56.Yin F, Wakino S, Liu Z, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochemical and Biophysical Research Communications. 2001;286(5):916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 57.Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H. Induction of differentiation and peroxisome proliferator-activated receptor γ expression in colon cancer cell lines by troglitazone. Journal of Cancer Research and Clinical Oncology. 2004;130(2):73–79. doi: 10.1007/s00432-003-0510-2. [DOI] [PubMed] [Google Scholar]
- 58.Tsubouchi Y, Sano H, Kawahito Y, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-γ agonists through induction of apoptosis. Biochemical and Biophysical Research Communications. 2000;270(2):400–405. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- 59.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi N, Okumura T, Motomura W, Fujimoto Y, Kawabata I, Kohgo Y. Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Letters. 1999;455(1-2):135–139. doi: 10.1016/s0014-5793(99)00871-6. [DOI] [PubMed] [Google Scholar]
- 61.Guan Y-F, Zhang Y-H, Breyer RM, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptor γ (PPARγ) in human transitional bladder cancer and its role in inducing cell death. Neoplasia. 1999;1(4):330–339. doi: 10.1038/sj.neo.7900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103(2):311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 63.Gerhold DL, Liu F, Jiang G, et al. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-γ agonists. Endocrinology. 2002;143(6):2106–2118. doi: 10.1210/endo.143.6.8842. [DOI] [PubMed] [Google Scholar]
- 64.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda M, Takagi H, Horiguchi N, et al. A ligand for peroxisome proliferator activated receptor γ Inhibits cell growth and induces apoptosis in human liver cancer cells. Gut. 2002;50(4):563–567. doi: 10.1136/gut.50.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen GG, Lee JFY, Wang SH, Chan UPF, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and Nf-κB in human colon cancer. Life Sciences. 2002;70(22):2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida K, Tanabe K, Fujii D, Oue N, Yasui W, Toge T. Induction mechanism of apoptosis by troglitazone through peroxisome proliferator-activated receptor-γ in gastric carcinoma cells. Anticancer Research. 2003;23(1A):267–273. [PubMed] [Google Scholar]
- 68.Bruce WR, Wolever TMS, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutrition and Cancer. 2000;37(1):19–26. doi: 10.1207/S15327914NC3701_2. [DOI] [PubMed] [Google Scholar]
- 69.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiology Biomarkers & Prevention. 1994;3(8):687–695. [PubMed] [Google Scholar]
- 70.Mutoh M, Niho N, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation by increasing lipoprotein lipase activity in Apc-deficient mice. Biological Chemistry. 2006;387(4):381–385. doi: 10.1515/BC.2006.051. [DOI] [PubMed] [Google Scholar]
- 71.Kohno H, Yoshitani S, Takashima S, et al. Troglitazone, a ligand for peroxisome proliferator-activated receptor γ, inhibits chemically-induced aberrant crypt foci in rats. Japanese Journal of Cancer Research. 2001;92(4):396–403. doi: 10.1111/j.1349-7006.2001.tb01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APC min/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 74.Niho N, Takahashi M, Kitamura T, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Research. 2003;63(18):6090–6095. [PubMed] [Google Scholar]
- 75.Niho N, Takahashi M, Shoji Y, et al. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPARγ ligand. Cancer Science. 2003;94(11):960–964. doi: 10.1111/j.1349-7006.2003.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 77.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osawa E, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124(2):361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 79.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5, article 46 doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Science. 2003;94(11):965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarty MF. Activation of PPARgamma may mediate a portion of the anticancer activity of conjugated linoleic acid. Medical Hypotheses. 2000;55(3):187–188. doi: 10.1054/mehy.1999.1010. [DOI] [PubMed] [Google Scholar]
- 82.Bozzo F, Bocca C, Colombatto S, Miglietta A. Antiproliferative effect of conjugated linoleic acid in Caco-2 cells: involvement of PPARγ and APC/β-catenin pathways. Chemico-Biological Interactions. 2007;169(2):110–121. doi: 10.1016/j.cbi.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Yasui Y, Hosokawa M, Kohno H, Tanaka T, Miyashita K. Troglitazone and 9cis,11trans,13trans-conjugated linolenic acid: comparison of their antiproliferative and apoptosis-inducing effects on different colon cancer cell lines. Chemotherapy. 2006;52(5):220–225. doi: 10.1159/000094865. [DOI] [PubMed] [Google Scholar]
- 84.Yasui Y, Hosokawa M, Kohno H, Tanaka T, Miyashita K. Growth inhibition and apoptosis induction by all-trans-conjugated linolenic acids on human colon cancer cells. Anticancer Research. 2006;26(3A):1855–1860. [PubMed] [Google Scholar]
- 85.Yasui Y, Hosokawa M, Sahara T, et al. Bitter gourd seed fatty acid rich in 9c, 11t, 13t-conjugated linolenic acid induces apoptosis and up-regulates the GADD45, p53 and PPARγ in human colon cancer Caco-2 cells. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73(2):113–119. doi: 10.1016/j.plefa.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Kohno H, Yasui Y, Suzuki R, Hosokawa M, Miyashita K, Tanaka T. Dietary seed oil rich in conjugated linolenic acid from bitter melon inhibits azoxymethane-induced rat colon carcinogenesis through elevation of colonic PPARγ expression and alteration of lipid composition. International Journal of Cancer. 2004;110(6):896–901. doi: 10.1002/ijc.20179. [DOI] [PubMed] [Google Scholar]
- 87.Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Science. 2004;95(6):481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki R, Yasui Y, Kohno H, et al. Catalpa seed oil rich in 9c, 11t, 13t-conjugated linolenic acid suppresses the development of colonic aberrant crypt foci induced by azoxymethane in rats. Oncology Reports. 2006;16(5):989–996. [PubMed] [Google Scholar]
- 89.Yasui Y, Suzuki R, Kohno H, et al. 9trans,11trans conjugated linoleic acid inhibits the development of azoxymethane-induced colonic aberrant crypt foci in rats. Nutrition and Cancer. 2007;59(1):82–91. doi: 10.1080/01635580701419055. [DOI] [PubMed] [Google Scholar]
- 90.Demetri GD, Fletcher CDM, Mueller E, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hisatake J, Ikezoe T, Carey M, Holden S, Tomoyasu S, Koeffler HP. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer Research. 2000;60(19):5494–5498. [PubMed] [Google Scholar]
- 93.Kulke MH, Demetri GD, Sharpless NE, et al. A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer Journal. 2002;8(5):395–399. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Debrock G, Vanhentenrijk V, Sciot R, Debiec-Rychter M, Oyen R, Van Oosterom A. A phase II trial with rosiglitazone in liposarcoma patients. British Journal of Cancer. 2003;89(8):1409–1412. doi: 10.1038/sj.bjc.6601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Research and Treatment. 2003;79(3):391–397. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 96.Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140(6):960–967. doi: 10.1016/j.surg.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 97.Baetz T, Eisenhauer E, Siu L, et al. A phase I study of oral LY293111 given daily in combination with irinotecan in patients with solid tumours. Investigational New Drugs. 2007;25(3):217–225. doi: 10.1007/s10637-006-9021-8. [DOI] [PubMed] [Google Scholar]
- 98.Segawa Y, Yoshimura R, Hase T, et al. Expression of peroxisome proliferator-activated receptor (PPAR) in human prostate cancer. The Prostate. 2002;51(2):108–116. doi: 10.1002/pros.10058. [DOI] [PubMed] [Google Scholar]
- 99.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. The Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]