Abstract
Context
Respiratory infections are prevalent in the elderly, resulting in increased morbidity, mortality, and utilization of health care services. Vitamin E supplementation has been shown to improve immune response in the elderly. However, the clinical importance of these findings has not been determined.
Objective
To investigate the effect of 1-year vitamin E supplementation on respiratory infections in elderly nursing home residents
Design
A randomized, double-blind, placebo-controlled trial conducted from April 1998 to August 2001
Setting
33 long-term care facilities in the Boston, Massachusetts area
Participants
617 subjects ≥65 years old, who met the study’s eligibility criteria were enrolled, 73% of whom completed the study. The follow-up time (mean ± SD) was 317±104 and 321±97 days, E and placebo respectively, for all subjects enrolled in the study.
Intervention
A daily vitamin E (200 IU) or placebo capsule; all subjects received a capsule containing 1/2 the Recommended Daily Allowance of essential vitamins and minerals.
Main Outcome Measures
Incidence, number of subjects and number of days with respiratory infections (upper and lower), and number of new antibiotic prescriptions.
Results
There was no statistically significant effect of vitamin E on incidence or number of days with infection for all, upper, or lower respiratory infections. However, fewer vitamin E-supplemented subjects acquired one or more respiratory infections (65% vs 74%, risk ratio=0.88, 95% CI=0.75–0.99, p=0.036 for completed subjects; 60% vs 68%, risk ratio=0.88, 95% CI=0.76–1.00, p=0.048 for all subjects), or upper respiratory infections (50% vs 62%, risk ratio = 0.81, 95% CI=0.66–0.96, p=0.013 for completed subjects; 44% vs 52%, risk ratio=0.84, 95% CI=0.69–1.00, p=0.051 for all subjects). Post hoc sub-group analysis on common colds indicated that the vitamin E group had a lower incidence of common cold (0.66 vs 0.83 per subject-year, rate ratio=0.80, 95% CI=0.64–0.98, p=0.035 for completed subjects; 0.67 vs 0.81 per subject-year, rate ratio=0.83, 95% CI=0.68–1.01, p=0.057 for all subjects) and fewer subjects in the vitamin E group acquired one or more colds (46% vs 57%, risk ratio=0.80, 95% CI=0.64–0.96, p=0.016 for completed subjects; 40% vs 48%, risk ratio=0.83, 95% CI=0.67–1.00, p=0.052 for all subjects). There was no statistically significant vitamin E effect on antibiotic use.
Conclusions
Supplementation with 200 IU per day vitamin E did not have a statistically significant effect on lower respiratory infections in elderly nursing home residents. However, we observed a protective effect of vitamin E supplementation on upper respiratory infections, particularly the common cold, that merits further investigation.
Keywords: Respiratory infections, Upper respiratory infections, Common cold, Vitamin E, Tocopherol, Elderly, Nursing home, Nutritional status
INTRODUCTION
Infections, particularly respiratory infections (RI), are common in the elderly, resulting in decreased daily activity, prolonged recovery times, increased health care service utilization, and more frequent complications, including death 1–11.
It is predicted that 43% of elderly persons will be admitted to a nursing home, with >85% of them admitted to long-term (>1 year) care facilities 12. Infections occur more frequently in nursing home residents than among independent-living elderly 2–10, 13, and RI are a major cause of morbidity and mortality 9, 14, 15.
Contributing to the increased incidence of infection with age is the well-described decline in immune response 16. For example, there is higher morbidity and mortality from cancer, pneumonia, and post-operative complications in those who have diminished delayed-type hypersensitivity skin test responses 17–19.
Nutritional status is an important determinant of immune function 20, 21. Nutritional supplementation has been shown to enhance older subjects’ immune response 22, 23. In our earlier placebo-controlled, double-blind trials in elderly persons, vitamin E (E) supplementation improved immune response, including delayed-type hypersensitivity and response to vaccines 24, 25. Furthermore, subjects receiving E in the 6-month trial 25 had a 30% lower incidence of infectious diseases (primarily RI) compared to those on placebo. That study, however, was not powered to demonstrate statistical significance, and infections were self-reported. To overcome these limitations, the current study determined the effect of one-year supplementation with E on objectively recorded RI in elderly nursing home residents.
METHODS
Study design and intervention
This randomized, double-blind, placebo-controlled trial to investigate the effect of one-year E supplementation on RI in a nursing home population was conducted from April, 1998 to August, 2001. The Tufts-New England Medical Center Institutional Review Board approved the study protocol and informed consent form. A total of 617 subjects entered the study (about 1/3 enrolled in each of the 3 successive years) and were randomly assigned to E or placebo groups.
Nursing home residents have a heterogeneous intake of micronutrients 26, 27, some of which are necessary for proper immune function. To reduce variability, all subjects received a capsule containing 50% of the recommended daily allowance (RDA) 28 for essential micronutrients. Fifty percent RDA was selected since few subjects meeting our eligibility criteria would have intakes <50% of the RDA for micronutrients 29.
The E group received a daily capsule containing 200 IU E (dl-α-tocopherol), and the control group received a placebo capsule containing 4 IU E, both in soybean oil. The E dose was based on earlier studies in the elderly in which 200 IU per day induced the most robust improvement in immune function 25.
Capsules were manufactured by Tishcon Corporation (Westbury, NY) in 2 equal batches with all ingredients from the same sources. The E and placebo capsules were soft gel and identical in color and taste. The manufacturer’s certified ingredient concentrations were confirmed in house. The capsules were packed by Pharmasource Healthcare Inc. (Marlboro, MA) in 30-dose blister packs, and administered by the clinical nursing home staff during routine medication rounds. Nurses and subjects were blinded to treatment group. Adherence to study protocol was verified by nursing home medication records, returned pill count, and quarterly measurement of plasma E levels.
Recruitment and enrollment
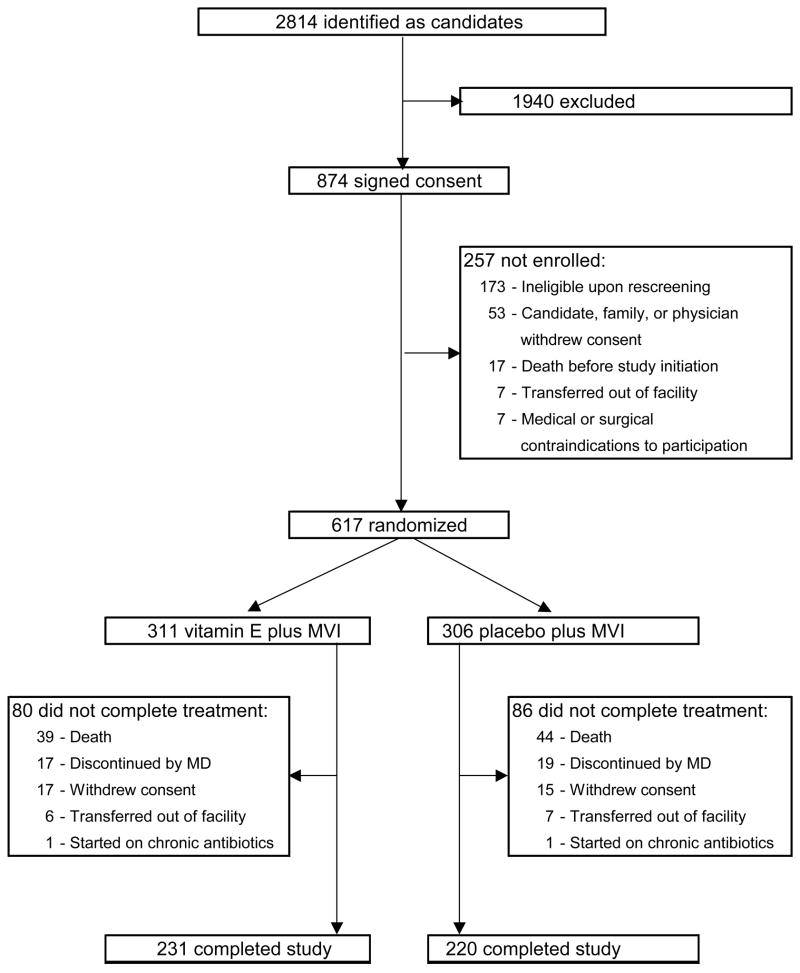
Participants were recruited from 33 long-term care facilities in the Boston area. 2814 residents were initially identified as potential candidates (Figure 1). According to the nursing home staffs, 874 met the following eligibility criteria: ≥65 years old; life expectancy >6 months; no anticipated discharge within 3 months; not room-bound for the past 3 months; absence of active neoplastic disease; no tube feeding, no kidney dialysis; no IV or urethral catheters for the last 30 days; no tracheostomy or chronic ventilator; antibiotics free for >2 weeks; no chronic steroid treatment >10 mg/d, use of immunosuppressive drugs, or >RDA level supplements of vitamins E, C, B6, selenium, zinc, β-carotene, or fish oil; BMI ≥18 kg/m2; serum albumin ≥3.0 g/dL; able to swallow pills; willing to receive influenza vaccine; willing to provide informed consent.
Figure 1. Study profile.
MVI = capsule containing 50% of the recommended daily allowance for essential micronutrients
Subsequent re-screening by our study nurses led to exclusion of 173 of those who had given informed consent. An additional 84 subjects were not enrolled for various reasons detailed in Figure 1. Thus, 617 subjects were randomized to the E (311 subjects) or placebo (306 subjects) groups.
Sample size calculation and randomization
Sample size was based on an expectation that the mean number of RI per subject-year would be 1.00 in the control group 5, 7 and 0.70 in the treatment group. The within-group standard deviation was estimated at 1.27 based on data from a local nursing home. With an expected attrition rate of 25%, the sample size needed to give an 80% chance of detection of the difference in infection rates at the 0.05 level of significance was 320 per treatment group, for a total of 640 subjects.
Subjects were assigned to E or placebo with equal probability in blocks of 4 according to lists generated by the study’s statistician using a computer program. Six randomization lists were constructed for each nursing home according to age (65–79, 80–89, 90+ years) and smoking or chronic obstructive pulmonary disease (COPD) status (Yes or No). Identification codes of newly-enrolled subjects were entered in order by the study statistician into the next available slots in the appropriate list. Those enrolling the subjects had no access to the randomization lists. Subjects were unknown to the statistician.
Outcomes
Primary outcomes of the study included rates of, number of subjects with, and number of days with RI (upper and lower), and number of new antibiotic prescriptions for RI. Because common colds constituted the majority of RI, a post hoc sub-group analysis was performed to determine the effect of E on common colds. Secondary outcomes included emergency room visits, hospitalization, and death.
Data collection
Information regarding subject characteristics, baseline diseases and medications, and vaccination history was obtained from medical records. Fasting blood was collected at baseline and at study completion for clinical chemistries, complete blood count with differential, plasma E, and selected nutrient analyses as previously described 30, 31. In addition, blood was collected after 3, 6, and 9 months of supplementation to measure E levels.
The study nurses collected information weekly relating to infection, including respiratory and heart rates, and temperature. Symptom and physical examination checklists, focused on the respiratory system, were used to record clinical findings. The nurses reviewed each participant’s chart for documentation of laboratory analyses, radiography, medication, nutrient supplementation, weight, and nursing or physician descriptions of symptoms and signs relating to RI.
Study nurses were trained by a study physician to elicit relevant respiratory symptoms and to perform a focused physical examination of the respiratory system. Supervised practice evaluations were repeated throughout the study to reinforce the nurses’ clinical skills and to ensure consistency of the RI data collection.
At the end of the study, data collected from the subjects in each treatment group, by nursing home, were randomly assigned to two of the study physicians for diagnosis of infections. Infection data from any one subject was evaluated by only one physician, except for 18 subjects whose records were used to determine concurrence between physicians.
Diagnosis of respiratory infection
The study physicians evaluated data collected by the nurses from the subject examinations, interviews, and record reviews, to determine incidence and duration of RI. Clinical definitions of RI were developed based on accepted definitions 13, 32–37. In order to increase the specificity of the definitions, a diagnosis of RI had to include at least one physical sign and not be made on symptoms alone. An infection was considered resolved when all symptoms ceased. A new infection was defined as one occurring after ≥7 symptom-free days.
To assess the ability of the study physicians to apply the diagnostic criteria concordantly, the records of 18 subjects were selected at random for each physician to evaluate independently. After each record was reviewed in its entirety, a total of 45 RI infections were identified. The probability that a physician would diagnose an infection if the other physician had diagnosed an infection was estimated to be 0.93 38, 39.
Clinical diagnostic criteria
Common cold
At least 1 of the following signs or symptoms had to be present: rhinorrhea or stuffy nose (nasal obstruction) or sneezing, plus 1 or more of the following: sore or scratchy throat, dry cough, hoarseness, or low-grade fever (temperature no greater than 1°C above normal range). Symptoms had to be new and not due to allergies. Seasonal allergic rhinitis was defined as: clear rhinorrhea or nasal congestion, plus itchiness of the nose or eyes or watery eyes. Fever, sore throat, and cough must be absent. Symptoms had to manifest between April 1 and September 30 and include at least 1 objective sign of rhinitis.
Influenza-like illness
Fever of ≥38°C (100.4°F) plus new or increased dry cough and 1 or more of the following signs or symptoms: chills, new headache or eye pain, myalgias, malaise or loss of appetite, or sore throat.
Pharyngitis
Symptoms of a sore or scratchy throat and at least 1 of the following abnormalities on pharyngeal examination: erythema, exudate, ulceration, vesicles, or edema.
Otitis media
Ear pain plus either erythema or bulging of the tympanic membrane.
Sinusitis
Symptoms could include facial pain, purulent nasal discharge, and nasal congestion. If x-rays were available, the finding of mucosal thickening, opacities or air fluid levels would confirm the diagnosis.
Acute bronchitis
At least 2 of the following signs or symptoms had to be present: increased frequency and severity of cough, new or increased sputum production, burning substernal chest discomfort with coughing or deep inspiration, and fever (T≥38°C). Radiological evidence of pneumonia excluded this diagnosis.
Pneumonia
Symptoms could include cough with or without sputum production, chest pain, dyspnea, and fever. Signs of infection included elevated temperature (≥38°C), tachycardia, tachypnea, abnormal breath sounds, and dullness to percussion of the chest. The diagnosis required radiological findings of 1 or more new pulmonary infiltrates.
Statistical analysis
All randomized subjects were compared at baseline, as were subjects who had final measurements taken, by using Student’s t-test for independent samples (continuous measures) and Pearson’s chi-square test of homogeneity of proportions (categorical measures). Among those completing the study, mean differences between the treatment groups with respect to changes in nutritional status were compared by using Student’s t test for independent samples. Differences in changes in the fraction of completers who were judged nutritionally deficient were assessed using a weighted least-squares linear model with time, treatment, and their interaction as predictors.
Rate ratios and their confidence intervals were obtained by using Poisson regression with the natural logarithm of time as an offset 40. Adjusted rate ratios were adjusted for obstructive lung disease, current smoking, diabetes mellitus, dementia, year of enrollment, baseline albumin, and baseline hemoglobin. The adjusted risk ratio for having one or more infections was obtained by using logistic regression, following the method of Zhang and Yu 41. Two-sided observed significance levels (p values) less than 0.05 were considered to be statistically significant. All calculations were performed using SAS for Windows, version 8.2 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Subject characteristics
Of the randomized subjects, 74% and 72% in the E and placebo group, respectively, completed the one-year study period (Figure 1). There was no statistically significant difference between the two groups in the proportion or causes of discontinuation (Figure 1), or in mortality rates (12.5% and 14.4% for E and placebo, respectively).
Table 1 shows subject characteristics for all who were enrolled in the study (All), and for those who completed 1 year (Completers). The groups were well balanced with regard to baseline characteristics. One exception was a lower percentage of Completers with diabetes mellitus in the E group compared to placebo (p<0.05) (Table 1).
Table 1.
Baseline Characteristics of Elderly Subjects by Completion Category and Treatment Groups
| All
|
Completers
|
|||
|---|---|---|---|---|
| Vitamin E n=311 | Placebo n=306 | Vitamin E n=231 | Placebo n=220 | |
| Age (y), mean (range) | 84.9(65–102) | 84.5(66–103) | 84.7(65–102) | 84.3(66–99) |
| Females, n (%) | 228(73) | 220(72) | 176(76) | 162(74) |
| Caucasians, n (%) | 293(94) | 290(95) | 219(95) | 208(95) |
| COPD*, n (%) | 86(28) | 74(24) | 60(26) | 48(22) |
| Current smoker, n (%) | 17(6)# | 28(9) | 12(5)# | 22(10) |
| Coronary artery disease, n (%) | 116(37) | 97(32) | 80(35) | 63(29) |
| Congestive heart failure, n (%) | 66(21) | 64(21) | 42(18) | 40(18) |
| Hypertension, n (%) | 151(49) | 166(54) | 122(53) | 117(53) |
| Diabetes mellitus, n (%) | 54(17)# | 71(23) | 39(17)## | 54(25) |
| History of malignancy, n (%) | 26(8) | 32(11) | 22(10) | 24(11) |
| Dementia, n (%) | 164(53) | 142(46) | 127(55)# | 101(46) |
| Alzheimer’s, (% of dementia) | 33(20) | 39(27) | 29(23) | 26(26) |
| Subjects taking NSAID, n (%) | 120(39) | 106(35) | 93(40) | 72(33) |
| C-reactive protein, mg/L, mean (SD) | 6.8(10) | 8.4(17) | 6.2(8) | 7.2(12) |
| Total # of medications, mean (SD) | 7.4(4.0) | 7.4(4.0) | 7.2(3.8) | 7.3(4.0) |
includes COPD, chronic bronchitis, and asthma
p<0.10 compared to placebo
p<0.05 compared to placebo
All subjects received influenza vaccine and there was no statistically significant difference between the two groups in the fraction of subjects receiving pneumococcal vaccine (13% vs 9%, E and placebo group, respectively). There was no statistically significant difference in the mean number of days that completing subjects took immune-related medications during the study period, such as NSAIDS (131 vs 110), anti-histamines (4.5 vs 7.9), steroids (16.3 vs 9.2), or nutrient supplements (84 vs 92) for E and placebo groups, respectively.
Biochemical and hematological measurements before and after E supplementation indicated no difference between the two groups (data not shown).
Adherence
Ninety-seven percent of those completing the study consumed the capsules for at least 330 days (>90% of the 1-year supplementation period). There was no statistically significant difference in the number of missed supplements between the E and placebo group (data not shown). Adherence was confirmed by plasma E measurement every 3 months.
Nutritional Status
There were no statistically significant differences between E and placebo groups in BMI or serum levels of vitamins and minerals before or after supplementation (data available upon request). The E group had small, but statistically significant higher hemoglobin levels than the placebo group, both before and after supplementation (12.4±1.4 vs 12.2±1.3 g/dL before, and 12.4±1.3 vs 12.1±1.5 g/dL after, in the E and placebo groups, respectively). There were significantly fewer subjects with low serum albumin levels in the E group compared to placebo at baseline and after supplementation (Table 2). The percentage of subjects with low albumin levels increased significantly during the one-year period for both groups, but there was no statistically significant difference in change over time in serum albumin between the two groups.
Table 2.
Nutritional Status (% deficient) of Elderly Subjects Before and After Supplementation by Treatment Group
| Vitamin E
|
Placebo
|
Measure of deficiency | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Albumin | 19# | 28#& | 27 | 39& | <3.6g/dL female
<3.7g/dL male |
| Hemoglobin | 32# | 31# | 37 | 43 | <11.5g/dL female
<13g/dL male |
| Carotenoids | 11 | 11 | 6 | 12 | <40μg/dL |
| Vitamin A | 1 | 2& | 2 | 4& | <30μg/dL |
| Vitamin D | 2 | 0& | 2 | 0& | <15ng/mL |
| Vitamin E | 3 | 0 | 1 | 0 | <500μg/dL |
| Vitamin B1 | 0 | 2 | 4 | 0 | <0.9A.C.* |
| Vitamin B2 | 0 | 2 | 2 | 2 | <0.9A.C.* |
| Vitamin B6 (pyridoxal phosphate) | 10 | 6& | 9 | 5& | <30nmol/L |
| Vitamin B12 | 0 | 0 | 0 | 0 | <200pg/mL |
| Folate | 0 | 0 | 0 | 0 | <3ng/mL |
| Ferritin | 0 | 0 | 0 | 0 | <10ng/mL female
<20ng/mL male |
| Zinc | 48 | 42 | 50 | 53 | <70μg/dL |
| Copper | 6 | 3 | 7 | 3 | <55μg/dL |
activity coefficient
significantly different (p<0.05) from placebo group at the same time point
significantly different (p<0.05) from before for the same group
Except for E, there was no change over time in the level of micronutrients during the study period in either group. There was a statistically significant increase in plasma E levels in the E group, which doubled after 3 months of supplementation with no further change (1141±391 vs 2119±689 ug/dL before and after supplementation, respectively, p<0.0001). No significant change in serum E levels was observed in the placebo group (1148±429 vs 1209±408 ug/dL before and after supplementation, respectively). There was a small, but statistically significant increase in the fraction of subjects with low serum vitamin A levels, while the fraction of subjects with low vitamin D and B6 levels decreased in both groups (Table 2), with no statistically significant difference between treatments in change over time.
There were significantly fewer subjects with low hemoglobin levels in the E group before and after supplementation (Table 2). There was no statistically significant difference between the two treatment groups in change over time in the fraction of subjects with low hemoglobin levels. Low serum zinc levels were equally prevalent in both groups (Table 2).
Respiratory infections
The highest incidence of RI occurred in the winter and the lowest in the summer (0.41 and 0.24 episodes per placebo subject, respectively).
Conclusions regarding the incidence, percent of subjects acquiring RI, number of sick days, and E-induced effects were minimally affected, whether the data from Completed subjects (Table 3) or All subjects (Table 4) were considered.
Table 3.
Respiratory infection in elderly nursing home residents - subjects completing the study&
| Vitamin E | Placebo | 95% CI | p-value | |||
|---|---|---|---|---|---|---|
| Incidence of infection: | Rate ratio | |||||
| All RI | ||||||
| number of infections | 304 | 320 | ||||
| infections per subject per year | 1.30 | 1.44 | 0.91 | (0.77, 1.06) | 0.220 | |
| Lower RI * | ||||||
| number of infections | 115 | 105 | ||||
| infections per subject per year | 0.49 | 0.47 | 1.05 | (0.80, 1.36) | 0.744 | |
| Upper RI ** | ||||||
| number of infections | 189 | 215 | ||||
| infections per subject per year | 0.81 | 0.96 | 0.84 | (0.69, 1.02) | 0.078 | |
| Colds | ||||||
| number of infections | 155 | 186 | ||||
| infections per subject per year | 0.66 | 0.83 | 0.80 | (0.64, 0.98) | 0.035 | |
| Number of subjects with 1 or more infections: | Risk ratio | |||||
| All RI | 150 | 163 | 0.88 | (0.75, 0.99) | 0.036 | |
| Lower RI | 76 | 71 | 1.02 | (0.77, 1.31) | 0.887 | |
| Colds | 106 | 126 | 0.80 | (0.64, 0.96) | 0.016 | |
| Number of days with infection per subject per year: | Difference(E–P) | |||||
| All RI | 15.36 | 17.32 | −1.97 | (−5.55, +1.62) | 0.281 | |
| Lower RI | 6.69 | 6.45 | +0.24 | (−2.11, +2.59) | 0.843 | |
| Upper RI | 8.66 | 10.87 | −2.21 | (−4.95, +0.54) | 0.115 | |
| Colds | 7.37 | 9.42 | −2.05 | (−4.57, +0.47) | 0.111 | |
| Antibiotic Rx’s for all RI: | Rate ratio | |||||
| number of Rx’s | 153 | 125 | ||||
| Rx’s per subject per year | 0.655 | 0.561 | 1.17 | (0.92, 1.47) | 0.198 | |
number of subjects = 231 Vitamin E, 220 Placebo; total days on study = 85342 Vitamin E, 81436 Placebo CI = confidence interval; Rx's = prescriptions
bronchitis, pneumonia
common cold, influenza-like infection, pharyngitis, otitis media, sinusitis
Table 4.
Respiratory infection in elderly nursing home residents - all subjects enrolled in the study&
| Vitamin E | Placebo | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Incidence of infection: | Rate ratio | ||||
| All RI | |||||
| number of infections | 365 | 394 | |||
| infections per subject per year | 1.35 | 1.47 | 0.92 | (0.80, 1.06) | 0.262 |
| Lower RI * | |||||
| number of infections | 145 | 144 | |||
| infections per subject per year | 0.54 | 0.54 | 1.00 | (0.80, 1.26) | 0.988 |
| Upper RI ** | |||||
| number of infections | 220 | 250 | |||
| infections per subject per year | 0.82 | 0.93 | 0.88 | (0.73, 1.05) | 0.150 |
| Colds | |||||
| number of infections | 180 | 217 | |||
| infections per subject per year | 0.67 | 0.81 | 0.83 | (0.68, 1.01) | 0.057 |
| Number of subjects with 1 or more infections: | Risk ratio | ||||
| All RI | 186 | 207 | 0.88 | (0.76, 1.00) | 0.048 |
| Lower RI | 100 | 100 | 0.99 | (0.78, 1.23) | 0.626 |
| Upper RI | 137 | 159 | 0.84 | (0.69, 1.00) | 0.051 |
| Colds | 125 | 147 | 0.83 | (0.67, 1.00) | 0.052 |
| Number of days with infection per subject per year: | Difference (E–P) | ||||
| All RI | 13.74 | 15.37 | −1.64 | (−4.56, +1.29) | 0.272 |
| Lower RI | 6.28 | 6.38 | −0.10 | (−2.09, +1.88) | 0.919 |
| Upper RI | 7.46 | 8.99 | −1.53 | (−3.70, +0.63) | 0.164 |
| Colds | 6.23 | 7.82 | −1.59 | (−3.56, +0.38) | 0.113 |
| Antibiotic Rx’s for all RI: | Rate ratio | ||||
| number of Rx’s | 185 | 168 | |||
| Rx’s per subject per year | .685 | .626 | 1.10 | (0.89, 1.35) | 0.392 |
number of subjects = 311 Vitamin E, 306 Placebo; total days on study = 98594 Vitamin E, 98091 Placebo CI = confidence interval; Rx’s = prescriptions
bronchitis, pneumonia
common cold, influenza-like infection, pharyngitis, otitis media, sinusitis
Adjustment for obstructive lung disease, current smoking status, diabetes mellitus, dementia, year of enrollment, and baseline albumin and hemoglobin did not have an effect on the outcomes. Further adjustment for nursing home gave essentially the same results. Thus only the unadjusted data are shown (Tables 3, 4), except as noted in the text.
In those subjects completing the study, 1.30 and 1.44 RI per person per year were diagnosed in the E and placebo groups, respectively (Table 3). The rate for all study subjects was 1.35 and 1.47 RI per person-year, E and placebo group, respectively (Table 4). While lower in the E-treated group, this incidence for all RI was not significantly different from the placebo group, nor was the number of days with RI per subject-year (Tables 3, 4). There were, however, fewer subjects in the E-treated group who contracted one or more RI (65% vs 74% for completing subjects, 60% vs 68% for all subjects, in E and placebo groups, respectively).
There was no statistically significant difference in the incidence, proportion, or number of sick days, of lower RI (LRI) between the two treatment groups (Tables 3, 4).
The E-treated subjects had a lower number of upper respiratory infections (URI) per subject-year, and the total number of days with URI per subject-year was lower in the E group (Tables 3, 4). The proportion of subjects contracting one or more URIs in the E-treated group was significantly lower than in the placebo group (50% vs 62%, respectively in completing subjects [Table 3]; 44% v 52%, respectively, in all subjects [Table 4], adjusted risk ratio for all subjects=0.82, 95% CI=0.66–0.98, p=0.030), such that the risk of having an URI was 19% (Completers) and 18% (All) less in the E-treated group.
Among the URIs, 84% were common colds. Post hoc sub-group analysis indicated that E-supplemented subjects had a significantly lower incidence of common colds (Table 3). In addition, significantly fewer subjects in the E group acquired a cold during the study period (for completing subjects 46% vs 57% in the placebo group [Table 3]; for all subjects 40% vs 48% in the placebo group [Table 4], adjusted risk ratio for all subjects=0.81, 95%CI=0.64–0.98, p=0.031). Although statistically not significant, the total number of days with common colds per subject-year was 22% (Completers) and 20% (All) lower in the E-treated group (Tables 3, 4).
Post hoc analysis suggested that there was no statistically significant effect of E on other URI. However, the incidence of these infections was low (0.035 vs 0.050, 0.082 vs 0.073, 0.013 vs 0.009, and 0.030 vs 0.009 per subject-year in the E and placebo groups, respectively, for influenza-like infections, pharyngitis, otitis media, and sinusitis) and the study was not powered to detect differences.
There was no statistically significant effect of E on antibiotic use for all RI (Tables 3, 4), number of emergency room visits (0.086 vs 0.058 per person-year, E and placebo, respectively, rate ratio=1.66, CI=0.80–3.43, p=0.174) or hospitalizations (0.060 vs 0.067 per person-year, E and placebo, respectively, rate ratio=0.91, CI=0.43–1.95, p=0.814) for RI.
DISCUSSION
We found that vitamin E had no statistically demonstrable effect on the incidence or duration of all RI as well as URI and LRI. However, fewer subjects in the E group acquired one or more RI or URI. Post hoc sub-group analysis revealed that the effect of E was mainly due to a reduction of common colds. Supplementation with 200 IU per day E significantly reduced the incidence of common colds in elderly nursing home residents by 20%, while decreasing the risk of acquiring a cold by 21%. A statistically non-significant reduction (22%) in the total number of days with common colds was also observed. Thus, further clinical trials of E supplementation in elderly persons, with common cold as the primary outcome, are warranted.
While our data suggest a protective effect of E against common cold, the most frequently encountered form of URI in this study, there was no apparent protective effect of E on the incidence or duration of other URI or of LRI. This may have been due to the small number of episodes of the other URI and LRI, or differences in the types of pathogens responsible for various forms of URIs and LRI. Most URIs, especially the common cold, are caused by viruses. Animal studies suggest that E protects against viral but not bacterial infection in aged mice 42. We have found that although E supplementation did not protect old mice against primary pulmonary Staphylococcus aureus infection, it was protective against secondary S. aureus infection following influenza infection 43.
The RI definitions applied in our study were derived using commonly accepted criteria from the medical literature 13, 32–37. These criteria do not allow the differentiation of viral from bacterial etiology. Our diagnostic criteria did not include microbiological evaluation due to difficulty in interpreting results, and cost. Furthermore, it was not the aim of this study to determine the etiology of RI. Future studies should include detailed microbiological methods in order to determine whether the beneficial impact of E is primarily on RI of viral etiology.
There are two possible explanations for why supplementation with E did not have an impact on antibiotic use. First, as described above, the protective effect of E may be against viral rather than bacterial RI. Although E might have helped to reduce the number of episodes of viral RI that progressed to secondary bacterial RI, our study was not designed to detect this kind of disease progression. Second, overuse of antimicrobial agents in nursing homes may have impaired our ability to demonstrate an effect of E on antibiotic use. Previous studies have shown that 22% to 89% of antibiotic prescriptions in nursing home patients are inappropriate 44.
Previous studies on E and infection in the elderly have demonstrated mixed results. A retrospective study showed that subjects with plasma E levels above 16.7 mg/L had a significantly lower mean number of infections compared to those with plasma E levels below 12.2 mg/L (1.0 vs 2.3, 95% CI for difference=0.12–2.48) 45. A recent double-blind trial of Dutch elderly 46 living in the community reported a rate ratio for all RI among those receiving E as 1.12 (95% CI=0.88–1.25), compared to those not receiving vitamin E. Our study differed from the Dutch study in population and the way RI was diagnosed. While our subjects were institutionalized elderly, their incidence of RI was similar to the community-dwelling Dutch elderly 46. Furthermore, we have previously shown that E is effective in improving the immune response in community-dwelling elderly 24, 25, and although it did not have sufficient power to demonstrate statistical significance, one of these studies showed a 30% reduction of infection in independently-living elderly 25.
In the Dutch study 46, subjects self-reported their infections by telephone, and then the infections were confirmed by nurse visits. But absence of infection in those not reporting was not confirmed, thus making the study results susceptible to reporting biases. In our study, the presence and type of RI, or absence, was documented by infectious disease specialists based on review of data gathered by trained research nurses during weekly subject interviews, review of medical records, and physical examination focused on RI using standardized case definitions 13, 32–35. Our results indicate that E may reduce URI, particularly common colds, with no effect on LRI or seasonal allergies. Graat et al. 46 did not differentiate between types of infections or between RI and allergies, and thus might have overlooked any E effect on URI. Furthermore, in our study adherence was checked by nursing home medication records and by periodic plasma E measurements, whereas the Graat et al. 46 study measured plasma E levels only at baseline.
Several potential limitations of our study merit comment. First, 27% of the enrolled subjects did not complete the study due to withdrawal or death. This level of loss to follow-up was anticipated in our original study design. It demonstrates the challenges inherent in a one-year study of a frail nursing home population. Since there were minimal differences in the characteristics of those who did and did not complete the study, this did not have an impact on our overall results. However, since attaining plasma and tissue saturation levels of E requires several months 25, the results from completed subjects may better represent the effectiveness of E.
Second, the use of a 1/2 RDA multivitamin 28 capsule in all subjects might have lessened the impact of E on RI by improving the micronutrient status of the placebo group. However, we found no statistically significant differences between the E and placebo groups with change over time in the status of any nutrients other than E. While our E group had a lower proportion of subjects with low albumin and hemoglobin levels at both baseline and follow-up, statistical adjustment for these potentially confounding factors did not change our conclusion.
Rhinoviruses and coronaviruses are the most common documented causes of colds 36, 47–49. They exacerbate COPD 50, and are known to be associated with LRI in the elderly 33, 49, 51. For example, a prospective cohort study of community-based elderly found that rhinoviruses were associated with lower respiratory symptoms in nearly 2/3 of episodes: about 1/5 of patients were confined to bed, and 26% were unable to perform routine household activities 51. Constitutional and lower respiratory tract symptoms and signs have been reported to be more common in the elderly compared to younger adults infected with cold viruses 33. Nursing home populations may also be at risk for epidemic outbreaks of rhinovirus infections 52. The common cold is generally less severe than influenza. However, its much higher incidence and its recognized morbidity in the elderly 33, 49, 51–53 make it an important public health problem in this age group 54. This is particularly relevant since at present no clinically useful vaccine or antiviral therapy is available to combat colds.
In conclusion, we found no effect of E supplementation on the incidence or duration of RI. However, significantly fewer E subjects acquired one or more RI, which was most evident in URI. Post hoc sub-group analysis revealed a statistically significant lower incidence of common cold and a lower number of subjects acquiring a cold. Because of the high rate and increased morbidity associated with common colds in this age group, these findings suggest important implications for the well-being of the elderly and for the economic burden associated with their care. Future studies in the elderly should address the effect of E supplementation on the common cold and incorporate microbiological methods to allow for assessment of the impact of vitamin E on specific types of respiratory pathogens.
Acknowledgments
We are grateful for the contributions made by Paula Murphy-Gismondi for help with recruitment, by the research nurses Susan Fritz, Susan Horsford, Christopher Beck, Karen Reed, Karen Tollins, and Paula O’Connor, our phlebotomist Mureille Despeignes, the personnel in the JMUSDA-HNRCA Nutrition Evaluation Laboratory, the administration and staff at the participating nursing homes, and all the residents there who volunteered for this study.
Footnotes
This work was supported by NIA, National Institutes of Health Grant 1R01-AG13975, and United States Department of Agriculture agreement 58-1950-9-001, and a grant for preparation of study capsules from Hoffmann-LaRoche Inc. The funding organizations did not have input into the design or conduct of the study, into the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. The NIA review panel provided input into data analysis of the study as part of the peer review process when the grant proposal was submitted for funding.
The research for this worked was performed in the Nutritional Immunology Laboratory, Jean Mayer USDA/HNRC at Tufts University, Boston, MA.
References
- 1.Schneider EL. Infectious diseases in the elderly. Ann Intern Med. 1983;98:395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- 2.Gugliotti R. The incidence of nosocomial infections in a skilled nursing facility. Conn Med. 1987;51:287–290. [PubMed] [Google Scholar]
- 3.Alvarez S, Shell CG, Woolley TW, Berk SL, Smith JK. Nosocomial Infections in long-term facilities. J Gerontol. 1988;43:M9–M17. doi: 10.1093/geronj/43.1.m9. [DOI] [PubMed] [Google Scholar]
- 4.Garibaldi RA, Brodine S, Matsumiya S. Infections among patients in nursing homes. N Engl J Med. 1981;305:731–735. doi: 10.1056/NEJM198109243051304. [DOI] [PubMed] [Google Scholar]
- 5.Jackson MM, Fiere J, Barrett-Connor E, et al. Intensive surveillance for infections in a three-year study of nursing home patients. Am J Epidemiol. 1992;135:685–696. doi: 10.1093/oxfordjournals.aje.a116348. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE, McIntyre M, Zacharias H, MacDonell JA. Twelve-month surveillance of infections in institutionalized elderly men. J Am Geriatr Soc. 1984;32:513–519. doi: 10.1111/j.1532-5415.1984.tb02236.x. [DOI] [PubMed] [Google Scholar]
- 7.Farber BF, Brennen C, Puntereri AJ, Brody JP. A prospective study of nosocomial infections in a chronic care facility. J Am Geriatr Soc. 1984;32:499–502. doi: 10.1111/j.1532-5415.1984.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 8.Plewa MC. Altered host response and special infections in the elderly. Emerg Med Clin North Am. 1990;8:193–206. [PubMed] [Google Scholar]
- 9.Crossley KB, Peterson PK. Infections in the Elderly. Clin Infect Dis. 1996;22:209–215. doi: 10.1093/clinids/22.2.209. [DOI] [PubMed] [Google Scholar]
- 10.Mehr DR, Foxman B, Colombo P. Risk factors for mortality from lower respiratory infections in nursing home patients. J Fam Practice. 1992;34:585–591. [PubMed] [Google Scholar]
- 11.Hasley PB, Brancati FL, Rogers J, Hanusa BH, Kapoor WN. Measuring functional change in community-acquired pneumonia. Med Care. 1993;41:649–657. doi: 10.1097/00005650-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Gabrel CS. Characteristics of elderly nursing home current residents and discharges: data from the 1997 National Nursing Home Survey. Adv Data. 2000:1–15. [PubMed] [Google Scholar]
- 13.Ruben FL, Dearwater SR, Norden CW, et al. Clinical infections in the non-institutionalized geriatric age group: methods utilized and incidence of infections. Am J Epidemiol. 1995;141:145–157. doi: 10.1093/oxfordjournals.aje.a117402. [DOI] [PubMed] [Google Scholar]
- 14.Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105:319–30. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 15.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 16.Siskind GW. Immunological aspects of aging: an overview. In: Schimke RT, editor. Biological Mechanism in Aging. USDA, NIH; 1980. pp. 455–467. [Google Scholar]
- 17.Wayne SJRLR, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in the aged. J Gerontol Med Sci. 1990;45:M45–48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- 18.Christou NV, Tellado-Rodriguez J, Chartrand L, et al. Estimating mortality risk in preoperative patients using immunologic, nutritional, and acute-phase response. Ann Surg. 1989;210:69–77. doi: 10.1097/00000658-198907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohn JR, Hohl CA, Buckley CE. The relationship between cutaneous cellular immune responsiveness and mortality in a nursing home population. J Am Geriatr Soc. 1983;31:808–9. doi: 10.1111/j.1532-5415.1983.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 20.Keusch GT, Wilson CS, Waksal SD. Nutrition, host defenses, and the lymphoid system. Adv Host Def Mech. 1983;2:275–306. [Google Scholar]
- 21.Chandra RK. Nutrition is an important determinant of immunity in old age. In: Prinsley DM, Sandstead HH, editors. Nutrition and Aging. New York: Alan R. Liss, Inc; 1990. pp. 321–334. [PubMed] [Google Scholar]
- 22.Chandra RK. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet. 1992;340:1124–1127. doi: 10.1016/0140-6736(92)93151-c. [DOI] [PubMed] [Google Scholar]
- 23.Meydani SN, Blumberg JB. Nutrition and the immune function in the elderly. In: Munro H, Danforth A, editors. Human Nutrition: A Comprehensive Treatise. VII. New York: Plenum Press; 1989. pp. 61–87. [Google Scholar]
- 24.Meydani SN, Barklund PM, Liu S, et al. Effect of vitamin E supplementation on immune responsiveness of healthy elderly subjects. Am J Clin Nutr. 1990;52:557–563. doi: 10.1093/ajcn/52.3.557. [DOI] [PubMed] [Google Scholar]
- 25.Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 26.Sahyoun NR, Otradovec CL, Hartz S, et al. Dietary intakes and biochemical indicators of nutritional status in an elderly, institutionalized population. Am J Clin Nutr. 1988;47:524–533. doi: 10.1093/ajcn/47.3.524. [DOI] [PubMed] [Google Scholar]
- 27.Drinka PJ, Goodwin JS. Prevalence and consequences of vitamin deficiency in the nursing home: a critical review. JAGS. 1991;39:1008–1017. doi: 10.1111/j.1532-5415.1991.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 28.Board FaN., editor. National Research Council. Recommended Dietary Allowances. Washington D. C: National Academy Press; 1989. p. 284. [Google Scholar]
- 29.Fiatarone M. Nutrition in the geriatric patient. Hosp Pract (Off Ed) 1990;25:38–40. 45, 49–54. [PubMed] [Google Scholar]
- 30.Meydani SN, Meydani M, Rall LC, Morrow F, Blumberg JB. Assessment of the safety of high-dose, short-term supplementation with vitamin E in healthy older adults. Am J Clin Nutr. 1994;60:704–9. doi: 10.1093/ajcn/60.5.704. [DOI] [PubMed] [Google Scholar]
- 31.Meydani SN, Meydani M, Blumberg JB, et al. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am J Clin Nutr. 1998;68:311–318. doi: 10.1093/ajcn/68.2.311. [DOI] [PubMed] [Google Scholar]
- 32.Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiol Infect. 1993;111:143–56. doi: 10.1017/s0950268800056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falsey AR, McCann RM, Hall WJ, et al. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45:706–11. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology Ann Allergy Asthma Immunol. 1998;81:478–518. doi: 10.1016/s1081-1206(10)63155-9. [DOI] [PubMed] [Google Scholar]
- 35.McGeer A, Campbell B, Emori TG, et al. Definition of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19:1–7. doi: 10.1016/0196-6553(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 36.Arruda E, Pitkaranta A, Witek TJ, Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–8. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandell GL, Bennett JE, Dolin R. Principles and practice of infectious diseases. New York: Churchill & Livingstone; 1995. [Google Scholar]
- 38.Markus H, Bland JM, Rose G, Sitzer M, Siebler M. How good is intercenter agreement in the identification of embolic signals in carotid artery disease? Stroke. 1996;27:1249–52. doi: 10.1161/01.str.27.7.1249. [DOI] [PubMed] [Google Scholar]
- 39.Bland JM. How do I measure observer agreement when only positive observations are recorded? 2004 http://www.users.york.ac.uk/~mb55/meas/nonos.htm.
- 40.Ramsey F, Schafer D. The Statistical Sleuth: A course in methods of data analysis. Pacific Grove, CA: Duxbury; 2002. [Google Scholar]
- 41.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes Jama. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 42.Hayek MG, Taylor S, Bender BS, et al. Vitamin E supplementation decreases lung viral titer in mice infected with influenza. FASEB J. 1996;9:A500. doi: 10.1086/517265. [DOI] [PubMed] [Google Scholar]
- 43.Gay R, Han SN, Marko M, Belisle S, Bronson RT, Meydani SN. The effect of vitamin E on secondary bacterial infection following influenza infection in young and old mice. The FASEB Journal, Abstracts. 2004;18:A162–163. doi: 10.1196/annals.1331.061. [DOI] [PubMed] [Google Scholar]
- 44.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–4. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 45.Chavance M, Herbeth B, Fournier C, Janot C, Vernhes G. Vitamin status, immunity and infections in an elderly population. Euro J Clin Nutr. 1989;43:827–835. [PubMed] [Google Scholar]
- 46.Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons. JAMA. 2002;288:715–721. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 47.Treanor J, Falsey A. Respiratory viral infections in the elderly. Antiviral Res. 1999;44:79–102. doi: 10.1016/S0166-3542(99)00062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Bmj. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–23. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 51.Nicholson KG, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–23. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123:588–93. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 53.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–41. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–94. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]