Abstract
Acute kidney injury (AKI) occurs in about half of patients in septic shock and the mortality of AKI with sepsis is extremely high. An effective therapeutic intervention is urgently required. Statins are HMG-CoA reductase inhibitors that also have pleiotropic actions. They have been reported to increase survival of septic or infectious patients. But the effect of simvastatin, a widely used statin, on sepsis-induced AKI is unknown. The effects of simvastatin and TNF-alpha neutralizing antibody were studied in a clinically relevant model of sepsis-induced AKI using cecal ligation and puncture (CLP) in elderly mice. Simvastatin siginificantly improved CLP-induced mortality and AKI. Simvastatin attenuated CLP-induced tubular damage and reversed CLP-induced reduction of intrarenal microvascular perfusion and renal tubular hypoxia at 24 hours. Simvastatin also restored towards normal CLP-induced renal vascular protein leak and serum TNF-alpha. Neither delayed simvastatin therapy nor TNF-alpha neutralizing antibody improved CLP-induced AKI. Simvastatin improved sepsis-induced AKI by direct effects on the renal vasculature, reversal of tubular hypoxia, and had a systemic anti-inflammatory effect.
Introduction
Acute kidney injury (AKI) is a common life-threatening disease whose mortality has remained at about 45% over three decades, despite advances in supportive care. Sepsis is a contributing factor in about half of patients of severe AKI [1]. Septic shock is the most common contributing factor to AKI in intensive care unit [2]. AKI occurs in half of septic shock patients whose blood cultures are positive [3]. The mortality is higher in AKI patients with sepsis (75%) than in those without sepsis (45%) [4]. AKI independently increases the morbidity and mortality although other organ failures also contribute [5]. Thus, the strategy of treatment for sepsis-induced AKI is urgently required. Activated protein C decreases mortality from severe sepsis [6] and intensive insulin therapy or early goal-directed therapy including early resuscitation is beneficial in patients with severe sepsis or septic shock [7, 8]. However, there are no drugs to prevent or treat sepsis-induced AKI [9, 10].
We have recently developed a clinically relevant sepsis-induced AKI model based on a classic cecal ligation and puncture (CLP) model of polymicrobial sepsis that can be used to screen drugs and investigate the pathogenesis of sepsis. CLP differs from endotoxin injection models because there is bacterial infection that mimics human sepsis [11-13]. Serum creatinine starts to increase at 12 hours (hrs) but not 6 hrs after CLP, although tubular damage can be detected at 6 hrs by MRI techniques [14] and renal cyr61 expression, a tubular damage marker [15]. The renal pathophysiology after CLP is currently unknown.
HMG-CoA reductase inhibitors (statins) such as simvastatin have pleiotropic effects independent of lipid lowering [16-18]. Statin therapy has clinically beneficial effects on cardiovascular, cerebrovascular and acute and chronic kidney diseases via diverse effects [17, 19-21]. The protective effects of statins on both human and animal sepsis have been recently shown. A retrospective study in humans reported that statin therapy reduced both overall and attributable mortality in patients with bacteremia [22]. A controlled study revealed that prior statin therapy was associated with a reduction of severe sepsis and intensive care unit admission [23]. In animals, simvastatin improved survival in a murine CLP model [24, 25]. Despite these observations, the possible role of statins on sepsis-induced AKI remains unknown.
In the present study, we investigated whether simvastatin has an effect on sepsis-induced AKI and studied its mechanism of action. Specifically, we investigated renal vascular permeability, microperfusion, tubular hypoxia and histologic damage. Because simvastatin decreased circulating TNF-alpha during sepsis, treatment with anti TNF-alpha antibody was examined.
Results
Effect of simvastatin on sepsis-induced mortality and acute kidney injury
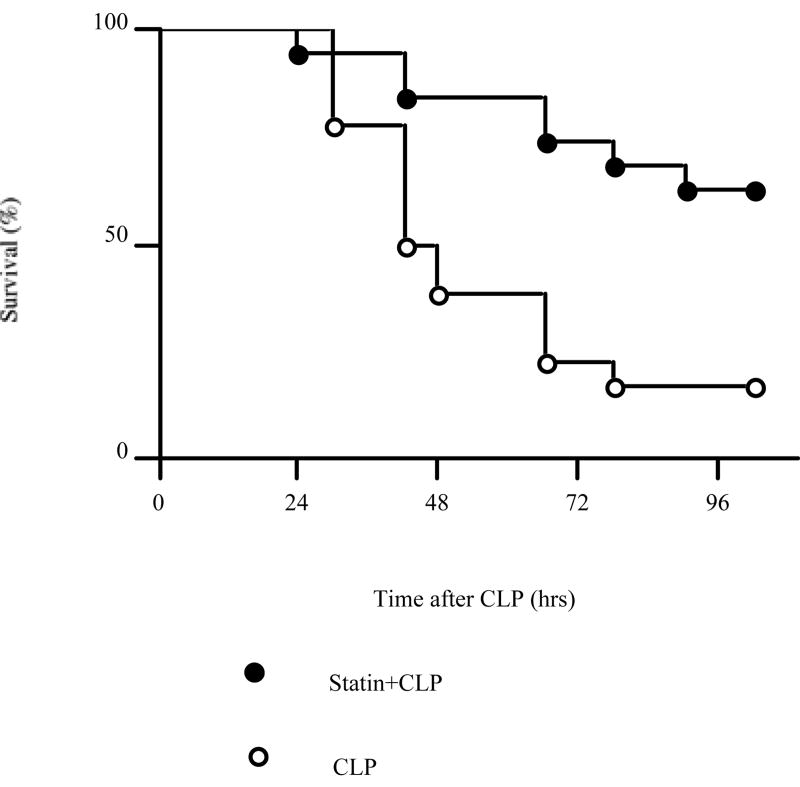
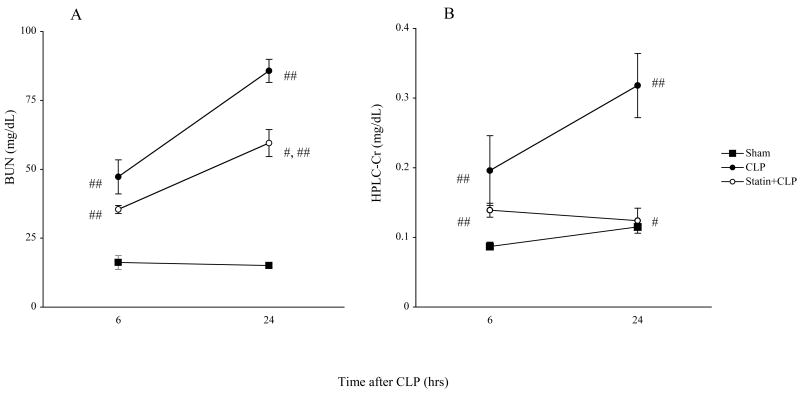
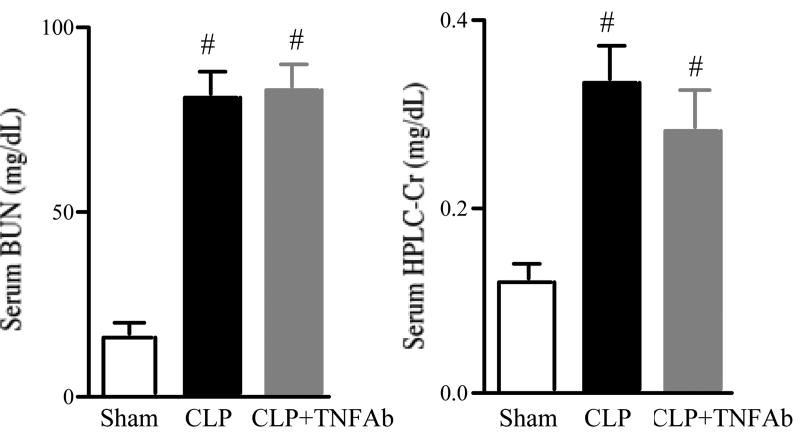
To determine whether simvastatin had an effect on CLP-induced mortality and renal dysfunction in aged mice treated with fluid and antibiotics, we measured survival and renal function. The survival for mice treated with saline was 100% at 24 hrs, 42% at 48 hrs and 26% at 72 hrs after CLP. The survival for aged mice treated with simvastatin was 95% at 24 hrs, 84% at 48 hrs and 73% at 72 hrs (Fig1). Simvastatin significantly improved survival after CLP. This survival advantage is consistent with the previous reports [24, 25] of the effect of simvastatin on sepsis in mice. However, previous studies did not evaluate renal function. Serum creatinine and BUN were significantly increased at 6 hrs after CLP compared to sham and further worsened at 24 hrs. Prior statin treatment significantly prevented the renal dysfunction at 24 hrs but not 6 hrs after CLP as detected by BUN and HPLC creatinine (Fig. 2).
Figure 1. Effect of simvastatin on survival after CLP.
Aged mice were subjected to CLP. Simvastatin (40mg/kg) or vehicle was administered for 3 days before CLP. Open circles indicate CLP group (N=19). Closed circles indicate Statin+CLP group (N=19). (P < 0.05)
Figure 2. Effect of simvastatin on renal function following surgery.
Mice were treated as in Figure 1. Mice were sacrified at indicated times for measurement of serum BUN (A) and creatinine by HPLC method (B). Closed circles indicate CLP group. Open circles indicate Statin+CLP group. Closed squares indicate Sham group. Values are mean ± SE (N= 6∼16 per group). #, P < 0.05 vs. CLP. ##, P < 0.05 vs. Sham.
The effect of simvastatin on sepsis-induced tubular damage
As reported previously, CLP caused very subtle changes in renal histology consisting of patchy tubular vacuolization but no thrombosis, tubular necrosis or cast formation [15]. The renal histology in both of the cortex and the outer stripe of the outer medulla (OSOM) worsened significantly after CLP (Fig.3). Simvastatin significantly prevented the deterioration of tubular damage induced by CLP in both the cortex and the OSOM (Fig.3).
Figure 3. Effect of simvastatin on renal histology.
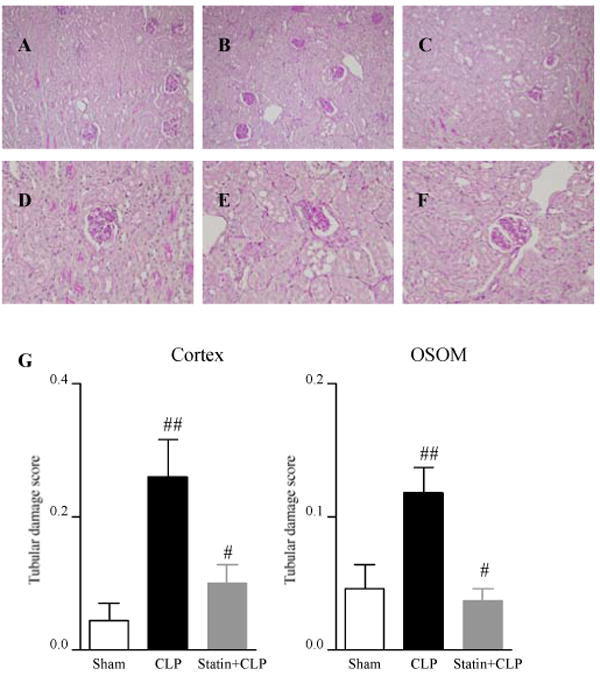
Mice were treated as in Figure 1. Mice were sacrified at 24 hrs after surgery. Histology of cortex in Sham group (A, D), CLP group (B, E), Statin+CLP group (C, F), Original magnification: X200 (A, B, C), X400 (D, E, F). (G) The tubular damage score (see Methods section) was measured in the cortex (left panel) and the outer stripe of the outer medulla (OSOM) (right panel). Values are mean ± SE (N=6∼16 per group). #, P < 0.05 vs. CLP. ##, P < 0.05 vs. Sham.
The effect of simvastatin on sepsis-induced vascular permeability
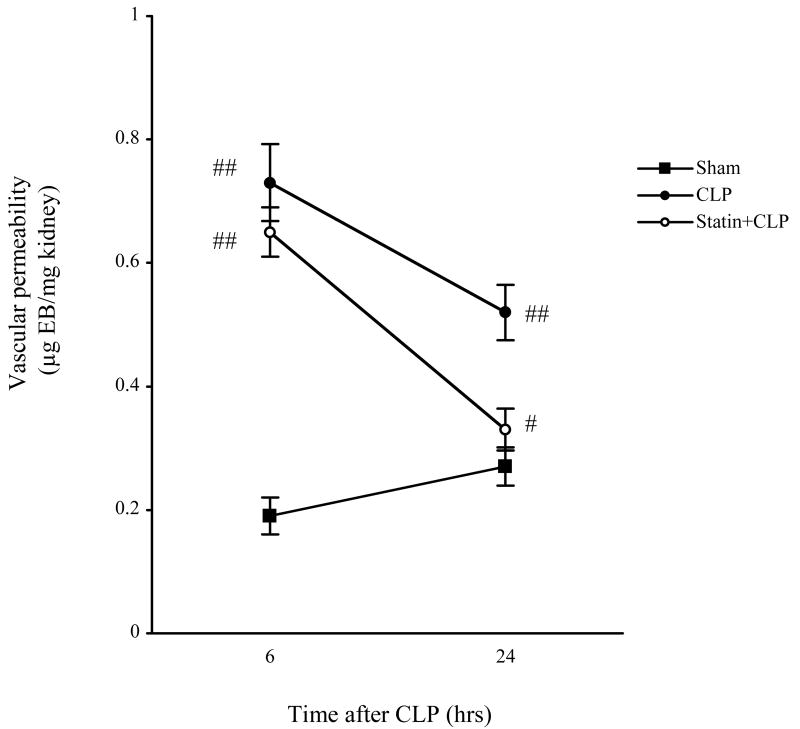
Changes in vascular permeability are thought to be important in the pathogenesis of sepsis-induced organ injury [26]. LPS injection, which is another septic model, increases vascular permeability in various organs including kidney, lung, liver, and heart [24, 27-29]. However, the renal vascular permeability after CLP is unknown. Therefore, vascular permeability was assessed by Evans blue dye leakage. Since CLP did not significantly change vascular permeability at 2 hrs after CLP (data not shown), we evaluated it at 6 hrs and 24 hrs after surgery. Renal vascular permeability was similar at 6 hrs and 24 hrs in sham mice. CLP significantly increased renal vascular permeability at 6 hrs and remained high at 24 hrs (Fig. 4). Simvastatin significantly decreased the CLP-induced increase in renal vascular permeability at 24 hrs but not at 6 hrs after CLP.
Figure 4. Effect of simvastatin on renal vascular permeability.
Mice were treated as in Figure 1. Mice were sacrified at 24 hrs after surgery. Evans blue dye (EBD) leakage in kidney tissue (see Methods) was measured at 6 hrs and 24 hrs following surgery. Values are mean ± SE (N = 4∼6 per group). #, P < 0.05 vs. CLP. ##, P < 0.05 vs. Sham.
The effect of simvastatin on renal tubular hypoxia
Tissue hypoxia is thought to be a dominant factor in organ dysfunction of sepsis [30]. Therefore, we assessed renal tubular hypoxia by pimonidazole incorporation, which is considered to react with cellular proteins at oxygen tensions below 10 mmHg [31]. Pimonidazole incorporation was detected in the tubules of the cortex and the OSOM but not in the inner stripe of outer medulla or inner medulla after CLP (Fig. 5). Pimonidazole incorporation was significantly increased in both the cortex and the OSOM at 24 hrs after CLP (Fig. 5). Simvastatin decreased pimonidazole incorporation in both the cortex and the OSOM at 24 hrs. These findings suggest that CLP caused renal hypoxia at 24 hrs and that simvastatin improved renal hypoxia.
Figure 5. Effect of simvastatin on pimonidazole incorporation following surgery.

Mice were treated as in Figure 1. Mice were sacrified at 24 hrs after surgery. Histology of the cortex and the OSOM in Sham group (A), CLP group (B), and Statin+CLP group (C). Original magnification, X200. (D) The hypoxic score (see Methods) was measured in the cortex and the OSOM. Values are mean ± SE (N=3∼7 per group). #, P < 0.05 vs. CLP. ##, P < 0.05 vs. Sham.
The effect of simvastatin on intra-renal microcirculation
Since simvastatin improved CLP-induced renal hypoxia at 24 hrs, we hypothesized that simvastatin might have a vascular effect during the late phase of CLP. Therefore, we evaluated perfusion of the renal microcirculation by physiologic infusion of FITC-labeled lectin, which binds to the capillary endothelium. In sham-surgery animals, peritubular capillaries were clearly detected in the cortex and in the OSOM. Perfused capillaries were decreased in the cortex and the OSOM at 24 hrs after CLP (Fig. 6). Simvastatin partially reversed the decrease in peritubular capillary flow in the cortex and in the OSOM at 24 hrs after CLP (Fig. 6). As a control for lectin binding, we evaluated FITC-labeled lectin binding to unperfused kidney sections to confirm the ability of endothelium to bind lectin after CLP. There was no difference in direct, topically applied lectin staining between CLP and sham sections (data not shown). Therefore, the changes shown here can be attributed to altered perfusion, and not to changes in lectin binding.
Figure 6. Effect of simvastatin on renal microvascular perfusion following surgery.
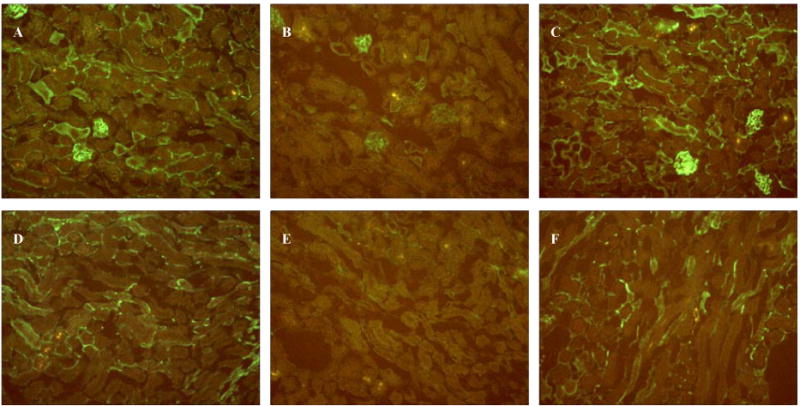
Mice were treated as in Figure 1. Mice were sacrified at 24 hrs after surgery. Histology of cortex (A, B, C) and OSOM (D, E, F) in Sham group (A, D), CLP group (B, E), Statin+CLP group (C, F). Original magnification, X400.
The effect of short-term administration of simvastatin on serum lipids
CLP did not significantly alter serum cholesterol or triglyceride at 24 hrs when compared to sham (serum cholesterol; 62±6.2 vs 72±4.9 mg/mL, serum triglyceride; 44±6.0 vs 59.2±8.3 mg/mL). There was no significant difference in serum cholesterol and triglyceride at 24 hrs between Statin+CLP and CLP groups (serum cholesterol; 62±6.2 vs 55.4±3.9 mg/dL, serum triglyceride; 44±6.0 vs 40±2.0 mg/dL). CLP significantly increased serum CK at 24 hrs as previously reported [15] (data not shown); however, simvastatin did not alter serum CK at 24 hrs after CLP surgery (data not shown), which suggests that rhabdomyolysis, a potentially serious side effect of statin therapy [32], did not occur.
Effect of TNF-alpha neutralizing antibody on sepsis-induced acute kidney injury
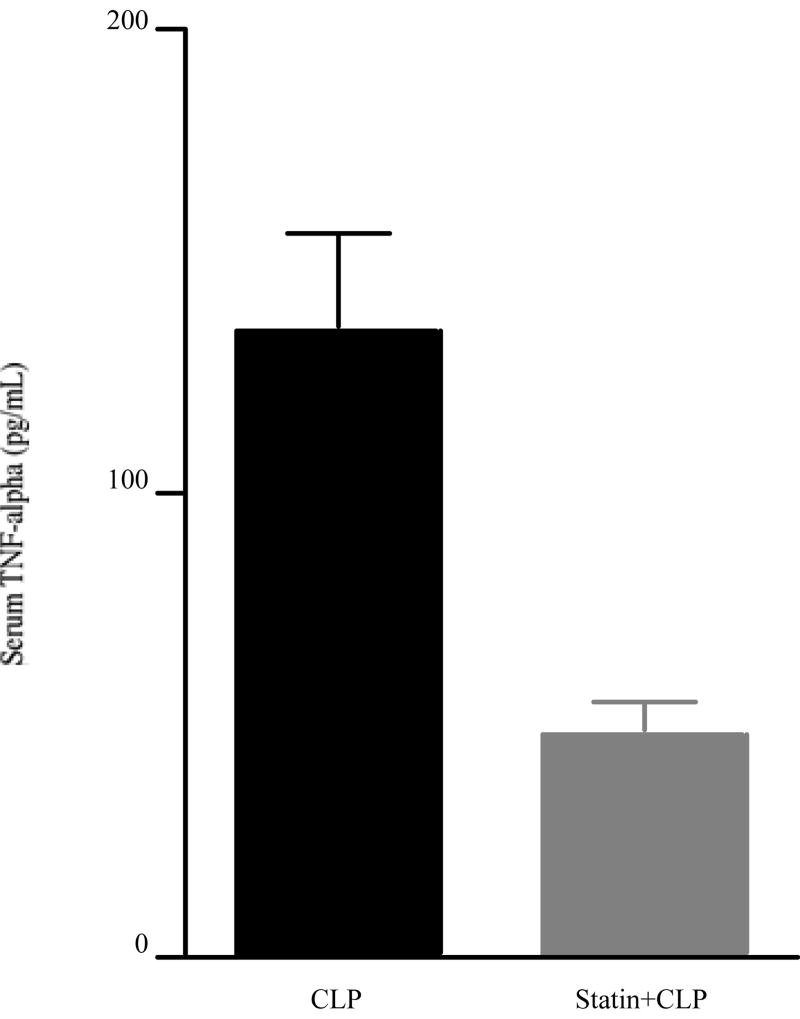
TNF-alpha is a prototypical pro-inflammatory cytokine that is elevated in some sepsis models, and is considered to be one of key mediators in developing AKI in several other renal injury models [33]. We found that simvastatin inhibited serum TNF-alpha elevation at 24 hrs after CLP (Fig. 7). We evaluated if simvastatin might improve renal injury via reduction of circulating TNF-alpha. However, neutralization of TNF-alpha did not significantly reduce serum BUN and creatinine at 24 hrs after CLP (Fig. 8).
Figure 7. Effect of simvastatin on serum TNF-alpha.
Mice were treated as in Figure 1. Mice were sacrified at 24 hrs after surgery. Values are mean ± SE (N= 9 per group). #P < 0.05 vs. CLP.
Figure 8. Effect of TNF-alpha neutralizing antibody on renal function following CLP surgery.
TNF-alpha neutralizing antibody or normal mouse IgG was administered at a dose of 800 ug/kg BW immediately after CLP. Mice were sacrified at 24 hrs after CLP for measurement of serum BUN (A) and creatinine by HPLC method (B). Values are mean ± SE (N=5∼16 per group). #, P < 0.05 vs. sham.
Effect of delayed simvastatin treatment on sepsis-induced acute kidney injury
We also examined delayed administration of simvastatin using two different protocols. 1) simvastatin administered subcutaneously 40 mg/kg at 6 hrs after CLP did not improve serum BUN or creatinine (serum BUN; vehicle : 93.0±6.5 vs simvastatin : 102.3±2.7 mg/dL, serum creatinine; 0.49 ± 0.07 vs 0.69 ± 0.17 mg/dL, N=6 mice per group). 2) 2 doses of simvastatin administered subcutaneously (40 mg/kg) at 6 and 18 hrs after CLP did not improve serum BUN or creatinine (serum BUN; 87.2±16.3 vs 89.7±4.7 mg/dL, serum creatinine; 0.39 ± 0.08 vs 0.45 ± 0.16 mg/dL, N=6 mice per group).
Discussion
We demonstrated that simvastatin, a widely used lipid-lowering drug, improved sepsis-induced mortality and AKI. Simvastatin reversed the late renal microvascular injury and microvascular perfusion defect with corresponding improvement in tissue oxygenation, and reduced a serum pro-inflammatory cytokine (serum TNF-alpha). These results suggest that simvastatin has both vascular and anti-inflammatory effects in this model.
Effects of simvastatin in sepsis-induced acute kidney injury
We found that simvastatin significantly improved survival after CLP in mice, which is consistent with Merx's previous animal studies [24, 25]. Two clinical natural history studies showed that statin usage was associated with reduced mortality from sepsis and/or bacterial infection [22, 23]. We also demonstrated for the first time that simvastatin had a beneficial effect on sepsis-induced AKI. Pretreatment with Simvastatin improved renal function, as measured by serum BUN and creatinine (Fig. 2). Miyaji et al. showed that CLP caused subtle injury to the renal tubules of the cortex and the OSOM [15]. Simvastatin also inhibited the patchy tubular vacuolar degeneration found after CLP in both the cortex and the OSOM (Fig. 3). Statins have been shown to have beneficial effects in other kidney diseases including ischemia-reperfusion injury, transplantation and chronic kidney disease [16-18, 34, 35]. Interestingly, these effects, including an effect on cardiovascular morbidity and mortality, were independent of lowering serum cholesterol. Although the site of action of simvastatin is unclear, simvastatin could have direct tubular effects. The mevalonate pathway or cholesterol synthesis pathways are activated in cortical tubules after sepsis induced by LPS or E. Coli injection, however, cholesterol accumulation was not reversed by statin therapy [36-38].
Vascular effects
Because of the rather subtle tubular changes in this model, and the known effects of statins on the vasculature, we focused our remaining studies on the renal vasculature. Renal vascular permeability measured by Evans blue dye extravasation was increased after CLP, and the late increase at 24 hrs was reversed by simvastatin (Fig. 4). Changes in vascular permeability are thought to be important in the pathogenesis of sepsis-induced organ injury [26]. Increased vascular permeability can cause compression of peritubular capillaries [39], hemoconcentration [40] and reduced microvascular flow. Indeed, alteration of vascular permeability is thought to modulate renal function [41] and play an important functional role in the pathophysiology of ischemic AKI [42, 43]. Vascular permeability may be altered by several multiple factors, including the interaction between activated leukocytes and the endothelium, endothelial NO synthase (eNOS), vascular endothelial growth factor (VEGF), angiopoietins, shear stress and matrix metalloproteinases [42, 44, 45], which might in turn be therapeutic targets in sepsis. Statins have been shown to protect the endothelium with preservation of eNOS function and to regulate several mediators associated with vascular permeability such as VEGF and metalloproteinases [17, 34]. Simvastatin enhanced eNOS expression and improved vascular permeability in lungs subjected to ischemia-reperfusion injury [46].
Interestingly, in our studies, simvastatin did not improve vascular permeability at 6 hrs, although vascular permeability was improved at 24 hrs (Fig. 4). The late reversal was quite unexpected, and suggests that prolonged increases in vascular permeability may contribute to renal dysfunction as recently proposed in ischemic injury [47].
We next focused on renal microperfusion and hypoxia, two consequences of altered vascular permeability. We detected decreased renal microvascular perfusion measured by FITC-lectin infusion (Fig. 6) and renal tubular hypoxia measured by pimonidazole staining (Fig. 5) at 24 hrs after CLP. Interestingly, both microvascular hypoperfusion and tubular hypoxia were found in both the cortex and the OSOM at 24 hrs. A similar loss of global tubular function was detected by dendrimer contrast MRI after CLP [14]. Taken together, our data suggests that increased microvascular permeability and global worsening of renal microperfusion and tubular hypoxia might be responsible for developing AKI after CLP since tissue hypoperfusion and hypoxia are considered to be dominant factors in organ dysfunction of sepsis [30, 48]. Also, the global pattern of tubular and vascular injury differs from ischemia-reperfusion injury, which is limited to the OSOM [43, 49, 50]. On the other hand, this reduced renal microvascular perfusion might contribute to reduced tubular damage in our model because reduced cellular metabolism might increase cell survival [51].
Another late effect of simvastatin was that it reversed the microperfusion defect and tubular hypoxia at 24 hrs after CLP (Fig. 5, 6). This reversal might be caused by beneficial hemodynamic effects and/or local effects on renal tubular or microvasculature. For example, simvastatin preserved cardiac function and improved hemodynamic stability [24, 25] in a CLP model, although renal end points were not measured. Simvastatin could decrease tubular oxygen consumption since it has been shown to reduce cardiac oxygen consumption [52, 53]. However, that would not easily account for the reversal effect. Statins may also have direct vascular protective effects via preservation of endothelial function, in part, mediated by eNOS [54]. It is likely that simvastatin has a direct effect on the vasculature since renal vascular permeability was restored towards normal after CLP by simvastatin (Fig. 4).
Inflammatory effects
Statins also have anti-inflammatory effects [17, 20, 21, 34]. We found that simvastatin inhibited serum TNF-alpha elevation at 24 hrs after CLP (Fig. 7). Inhibition of the TNF alpha pathway reduced kidney injury in LPS model [55, 56]. However, Anti-TNF alpha treatment does not decrease mortality in CLP mice [57, 58], and did not improve sepsis-induced AKI in our studies (Fig. 8). Although anti-TNF-alpha did improve sepsis-induced AKI in a small human study [59], the role of TNF-alpha in sepsis is still controversial.
Effect of delayed administration of simvastatin on sepsis-induced acute kidney injury
A recent study found that delayed administration of simvastatin starting at 6 hrs improved mortality in a slightly different CLP model [25]. However, we found that delayed administration of simvastatin starting at 6 hrs after CLP did not improve renal function. How to reconcile these observations is not clear. Conceivably, delayed administration of simvastatin could have a beneficial effect of mortality but not kidney injury. Alternatively, subtle differences between animal models might be responsible.
Conclusion
We showed that simvastasin improved sepsis-induced mortality and acute kidney injury. The mechanism of its renal protection may include effects on systemic circulation, direct effects on the renal vasculature and subsequent reversal of tubular hypoxia and a systemic anti-inflammatory action. The advantage of simvastatin in clinical settings is that it is clinically well established and already widely used, has an adequate safety profile in septic patients [22, 23] and is relatively inexpensive. There are clinical situations where preventive therapy with simvastatin might be useful. For example, one could prophylactically administer simvastatin to septic patients who have been identified to be at high risk for sepsis-induced AKI by either clinical characteristics or biomarker findings. Thus, simvastatin may be a potential preventive intervention for sepsis-induced AKI.
Methods
Animals
Animal care followed by National Institutes of Health (NIH) criteria for the care and use of laboratory animals in research (K100-MDB-02). Male (38 to 44 week old) C57BL/6 mice (NIH, Frederick, MD, USA) had free access to water and chow before and after surgery and housed individually.
Polymicrobial CLP sepsis-induced acute kidney injury
CLP was performed as previously described with small modifications [15]. Briefly, a 4-0 silk ligature was placed 15 mm from the cecal tip after laparotomy under isoflurane anesthesia. The cecum was punctured twice with a 21-gauge needle and gently squeezed to express a small amount of fecal material, then returned to the central abdominal cavity. In sham-operated animals, the cecum was located, but neither ligated nor punctured. The abdominal incision was closed in two layers with 6-0 nylon sutures. After surgery, 1 mL per 25 g body weight of pre-warmed normal saline was given subcutaneously [13]. All animals received antibiotic and fluid therapy subcutaneously (imipenem/cilastatin; 14 mg/kg in 1.5 mL of 2/3 saline at 6 hours and 7 mg/kg in 1.5 mL of 2/3 saline at 18 hours after surgery). Twenty-four hours after surgery, blood was collected from the abdominal aorta for measurement of serum creatinine, BUN and TNF-alpha. Serum creatinine was measured by HPLC [60]. Serum BUN and TNF-alpha were measured as previously described [15]. Kidneys were fixed in 10% formalin or snap-frozen in liquid nitrogen before storage at −80°C until further study.
Treatment of simvastatin and anti-TNF-alpha antibody
Simvastatin (EMD Biosciences, Inc. CA, USA) was prepared by dissolving 10 mg of simvastatin in 500 μl of ethanol and 0.407 μl of 1N NaOH, incubating at 50°C for 2 h and then storing at −80°C. The stock solution was diluted with saline at a ratio of 1:20 and adjusted to pH 7.2 before use. The vehicle solution was prepared in the same way without simvastatin. For pretreatment studies, mice were given 40mg/kg simvastatin or vehicle solution by oral gavage for 3 consecutive days before the surgery; Simvastatin was not given after surgery. For post-treatment studies, mice were given 40mg/kg or vehicle solution subcutaneously once at 6 hrs, or twice at 6 and 18 hrs after surgery.
TNF-alpha neutralizing antibody (R&D Systems, Inc. MN, USA) was administered as a single i.p. injection at a dose of 800 μg/kg body weight [61] immediately after CLP. Normal mouse IgG was used as a control.
Survival study
Survival was assessed every 6-12 hrs starting 6 hrs after surgery. Antibiotic injection and fluid resuscitation were started 6 hrs after surgery by subcutaneous injection, and then repeated every 12 hrs for 4 days.
Renal histology analysis
Tissue was fixed in 10% formalin and embedded in paraffin. 4 μm sections were stained with periodic acid-Schiff (PAS) reagent. Histologic changes in the cortex and the OSOM were assessed by quantitative measurements of tissue damage. As tubular damage was mainly vacuolization, the damage was defined as tubular vacuolar degeneration. The degree of kidney damage was estimated at 400X magnification using more than 100 randomly selected tubules for each animal by following the criteria: 0, normal;1, area of damage<25% of tubules; 2, damage involving 25% to 50% of tubules; 3, damage involving 50% to 75% of tubules; 4, 75% to 100% of tubules being affected.
Assessment of renal microvascular protein leak using Evans blue dye
The microvascular leakage of Evans blue dye was assessed as previously [62-64] with slight modifications. Thirty minutes before sacrifice, mice were injected intravenously with Evans blue dye (Sigma-Aldrich, MO, USA) 2 mL/kg at 1 % in 0.9 % sodium chloride via a tail vein. At sacrifice, mice were perfused with PBS through the left ventricle until blood was totally eliminated. The kidneys were weighed, snap-frozen in liquid nitrogen, and stored at −80°C. The kidneys were homogenized in 1mL formamide and incubated 55°C for 18 hr. The supernatant was collected after centrifugation at 10,000Xg for 30 min. The amount of Evans blue dye in the supernatant was analyzed by measuring absorbance at 620 nm. Results were calculated from a standard curve of Evans blue dye and expressed as micrograms of Evans blue dye per gm of kidney (wet weight).
Assessment of renal hypoxia
Renal hypoxia was assessed by pimonidazole immunohistochemistry. Pimonidazole (Chemicon International, Inc. CA, USA) was administrated intraperitoneally at the dose of 60 mg/kg 2hrs before sacrifice. Kidneys were bisected and immersed, and gently agitated in 10% formalin at room temperature for 24 hrs, and processed for paraffin embedding. 4μm sections were processed by the manufacture's procedure. All procedures were conducted at room temperature. Briefly, the sections were incubated with 0.01% pronase (Biomeda, CA, USA) for 5 min after deparaffinization and peroxidase quenching. After washing with PBS, the sections were incubated with 10% normal donkey serum in 1% bovine serum albumin (BSA) in PBS for 20 min. The sections were sequentially incubated with Hypoxyprobe-1 Mab1 (1:100 dilution with PBS containing 1% BSA) for 2 hrs, biotin-SP-conjugated donkey anti-mouse IgG (1:1000 dilution, Jackson Immunolaboratory, PA, USA) for 30 min, peroxidase-conjugated streptavidin for 30 min, 3,3′-diaminobenzaidine (DAB), then counterstatined with hematoxylin. No staining was detected without Mab1 (not shown). Renal hypoxia was evaluated by semiquantitative measurements of pimonidazole staining on tubules of the cortex and the outer stripe of the outer medulla. The degree of pimonidazole staining was estimated at 400X magnification using more than 5 randomly selected fields for each animal by the following criteria according to the intensity and the extent of positive cells: 0, no staining; 1, slight, 2; moderate, 3; severe staining.
Analysis of renal microvasculature by FITC-labeled lectin perfusion
To identify vessels with intact blood flow, the renal vasculature was identified with a lectin that binds uniformly to the luminal surface of endothelial cells [65-67]. 30μg of FITC-Lycopersicon Esculentum lectin (Vector Laboratories, CA, USA) was injected via a tail vein 5 min before sacrifice. The vasculature was perfused with PBS via the left ventricle, and the kidneys were harvested after intracardiac perfusion of PBS and fixed with 2% paraformaldehyde for 1 hour followed by freezing in OCT compound. 5μm cryosections were evaluated with a Leica DMRXE fluorescence microscope.
Statistical analysis
Differences between groups were examined for statistical significance by analysis of variance (ANOVA) with a multiple comparison correction. Comparisons between survival curves were made using a log-rank test (Prism 4.0, Graphpad Software, Inc., CA, USA). A P value < 0.05 was accepted as statistically significant.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDDK.
References
- 1.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama. 1995;273:117–123. [PubMed] [Google Scholar]
- 4.Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 5.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. Jama. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 6.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 7.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 8.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.De Vriese AS. Prevention and treatment of acute renal failure in sepsis. J Am Soc Nephrol. 2003;14:792–805. doi: 10.1097/01.asn.0000055652.37763.f7. [DOI] [PubMed] [Google Scholar]
- 10.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Yoo P, Zhou M, et al. Reduction in vascular responsiveness to adrenomedullin during sepsis. J Surg Res. 1999;85:59–65. doi: 10.1006/jsre.1999.5634. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Zhou M, Rana MW, et al. Differential alterations in microvascular perfusion in various organs during early and late sepsis. Am J Physiol. 1992;263:G38–43. doi: 10.1152/ajpgi.1992.263.1.G38. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Chung CS, Ayala A, et al. Differential alterations in cardiovascular responses during the progression of polymicrobial sepsis in the mouse. Shock. 2002;17:55–60. doi: 10.1097/00024382-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Dear JW, Kobayashi H, Jo SK, et al. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005;67:2159–2167. doi: 10.1111/j.1523-1755.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 15.Miyaji T, Hu X, Yuen PS, et al. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 16.Mason JC. The statins--therapeutic diversity in renal disease? Curr Opin Nephrol Hypertens. 2005;14:17–24. doi: 10.1097/00041552-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis. 2005;45:2–14. doi: 10.1053/j.ajkd.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Khanal S, Attallah N, Smith DE, et al. Statin therapy reduces contrast-induced nephropathy: An analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Pierre-Paul D, Gahtan V. Noncholesterol-lowering effects of statins. Vasc Endovascular Surg. 2003;37:301–313. doi: 10.1177/153857440303700501. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 21.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 22.Liappis AP, Kan VL, Rochester CG, et al. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 23.Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 24.Merx MW, Liehn EA, Janssens U, et al. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 25.Merx MW, Liehn EA, Graf J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 26.Wang le F, Patel M, Razavi HM, et al. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–1639. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 27.Whittle BJ, Morschl E, Pozsar J, et al. Helicobacter pylori lipopolysaccharide provokes iNOS-mediated acute systemic microvascular inflammatory responses in rat cardiac, hepatic, renal and pulmonary tissues. J Physiol Paris. 2001;95:257–259. doi: 10.1016/s0928-4257(01)00035-3. [DOI] [PubMed] [Google Scholar]
- 28.Carrithers M, Tandon S, Canosa S, et al. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 31.Franko AJ, Chapman JD. Binding of 14C-misonidazole to hypoxic cells in V79 spheroids. Br J Cancer. 1982;45:694–699. doi: 10.1038/bjc.1982.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36:288–295. doi: 10.1345/aph.1A289. [DOI] [PubMed] [Google Scholar]
- 33.Schrier RW. Cancer therapy and renal injury. J Clin Invest. 2002;110:743–745. doi: 10.1172/JCI16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonetti PO, Lerman LO, Napoli C, et al. Statin effects beyond lipid lowering--are they clinically relevant? Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 35.Yokota N, O'Donnell M, Daniels F, et al. Protective effect of HMG-CoA reductase inhibitor on experimental renal ischemia-reperfusion injury. Am J Nephrol. 2003;23:13–17. doi: 10.1159/000066301. [DOI] [PubMed] [Google Scholar]
- 36.Zager RA, Shah VO, Shah HV, et al. The mevalonate pathway during acute tubular injury: selected determinants and consequences. Am J Pathol. 2002;161:681–692. doi: 10.1016/S0002-9440(10)64224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zager RA, Johnson AC, Hanson SY. Sepsis syndrome stimulates proximal tubule cholesterol synthesis and suppresses the SR-B1 cholesterol transporter. Kidney Int. 2003;63:123–133. doi: 10.1046/j.1523-1755.2003.00735.x. [DOI] [PubMed] [Google Scholar]
- 38.Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 39.Klingebiel T, von Gise H, Bohle A. Morphometric studies on acute renal failure in humans during the oligoanuric and polyuric phases. Clin Nephrol. 1983;20:1–10. [PubMed] [Google Scholar]
- 40.Vollmar B, Glasz J, Leiderer R, et al. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 41.Basile DP, Fredrich K, Weihrauch D, et al. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol. 2004;286:F893–902. doi: 10.1152/ajprenal.00328.2003. [DOI] [PubMed] [Google Scholar]
- 42.Sutton TA, Kelly KJ, Mang HE, et al. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F91–97. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- 43.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 44.Goto T, Fujigaki Y, Sun DF, et al. Plasma protein extravasation and vascular endothelial growth factor expression with endothelial nitric oxide synthase induction in gentamicin-induced acute renal failure in rats. Virchows Arch. 2004;444:362–374. doi: 10.1007/s00428-004-0977-5. [DOI] [PubMed] [Google Scholar]
- 45.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 46.Naidu BV, Woolley SM, Farivar AS, et al. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2003;126:482–489. doi: 10.1016/s0022-5223(03)00699-8. [DOI] [PubMed] [Google Scholar]
- 47.Sutton TA, Mang HE, Campos SB, et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 48.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 49.Chiao H, Kohda Y, McLeroy P, et al. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 51.Singer M, De Santis V, Vitale D, et al. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 52.Mital S, Zhang X, Zhao G, et al. Simvastatin upregulates coronary vascular endothelial nitric oxide production in conscious dogs. Am J Physiol Heart Circ Physiol. 2000;279:H2649–2657. doi: 10.1152/ajpheart.2000.279.6.H2649. [DOI] [PubMed] [Google Scholar]
- 53.Mital S, Magneson A, Loke KE, et al. Simvastatin acts synergistically with ACE inhibitors or amlodipine to decrease oxygen consumption in rat hearts. J Cardiovasc Pharmacol. 2000;36:248–254. doi: 10.1097/00005344-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Yamakuchi M, Greer JJ, Cameron SJ, et al. HMG-CoA reductase inhibitors inhibit endothelial exocytosis and decrease myocardial infarct size. Circ Res. 2005;96:1185–1192. doi: 10.1161/01.RES.0000170229.49776.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knotek M, Rogachev B, Wang W, et al. Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 2001;59:2243–2249. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham PN, Dyanov HM, Park P, et al. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817–5823. doi: 10.4049/jimmunol.168.11.5817. [DOI] [PubMed] [Google Scholar]
- 57.Remick DG, Newcomb DE, Bolgos GL, et al. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 58.Echtenacher B, Weigl K, Lehn N, et al. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun. 2001;69:3550–3555. doi: 10.1128/IAI.69.6.3550-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Yuen PS, Dunn SR, Miyaji T, et al. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286:F1116–1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 61.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi WI, Quinn DA, Park KM, et al. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1627–1632. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- 63.Carattino MD, Cueva F, Zuccollo A, et al. Renal ischemia-induced increase in vascular permeability is limited by hypothermia. Immunopharmacology. 1999;43:241–248. doi: 10.1016/s0162-3109(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 64.Green TP, Johnson DE, Marchessault RP, et al. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin Med. 1988;111:173–183. [PubMed] [Google Scholar]
- 65.Ezaki T, Baluk P, Thurston G, et al. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manotham K, Tanaka T, Matsumoto M, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto M, Tanaka T, Yamamoto T, et al. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol. 2004;15:1574–1581. doi: 10.1097/01.asn.0000128047.13396.48. [DOI] [PubMed] [Google Scholar]