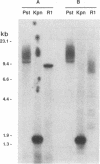
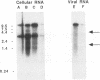
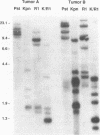
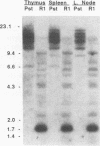
Abstract
We analyzed viral recombination events that occur during the preleukemic period in AKR mice. We tagged a molecular chimera between the nonleukemogenic virus Akv and the leukemogenic mink cell focus-inducing (MCF) virus MCF 247 with an amber suppressor tRNA gene, supF. We injected the supF-tagged chimeric virus that contains all of the genes of MCF 247 except the envelope gene, which in turn is derived from Akv, into newborn AKR mice to evaluate its pathogenic potential. Approximately the same percentage of animals developed leukemia with similar latent periods when injected with either the tagged or nontagged virus. DNA from tumors induced in AKR mice by the tagged chimeric virus was analyzed by Southern blotting with the supF gene as a probe. One set of tumors contained the injected supF-tagged virus. Two kinds of supF-tagged proviruses were found in a second set of tumors. One group of supF-tagged viruses had a restriction map consistent with that of the injected virus, while the other group of proviruses had restriction maps that suggested that the proviruses had acquired an MCF virus-like envelope gene by recombination with endogenous viral sequences. These results demonstrate that injected viruses recombine in vivo with endogenous viral sequences. Furthermore, the progression to leukemia was accelerated in mice that develop tumors containing proviruses with an MCF virus env gene, emphasizing the importance of the role of the MCF virus env gene product in transformation.
Full text
PDF


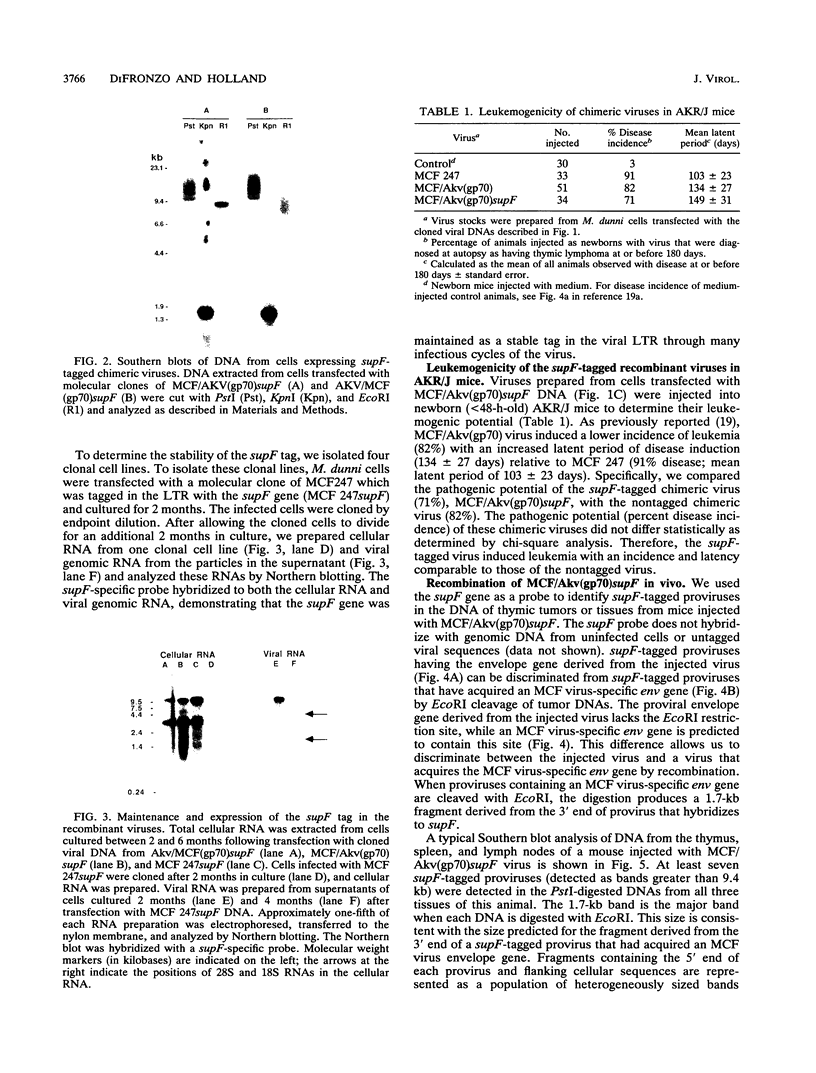
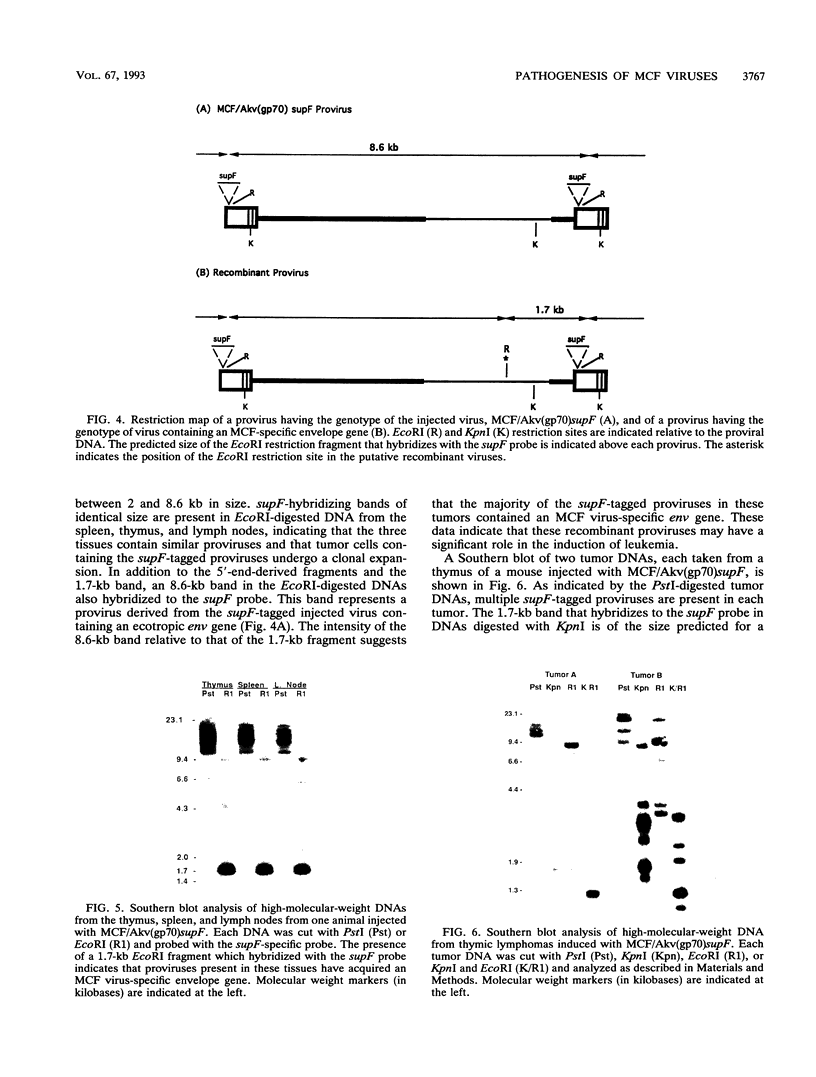



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Thompson M. M., Hartley J. W. Host range of mink cell focus-inducing viruses. Virology. 1985 Jan 30;140(2):239–248. doi: 10.1016/0042-6822(85)90362-9. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H. Characterization of polytropic MuLVs from three-week-old AKR/J mice. Virology. 1986 Aug;153(1):122–136. doi: 10.1016/0042-6822(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotides that segregate with tropism and with properties of gp70 in recombinants between N- and B-tropic murine leukemia viruses. J Virol. 1978 Apr;26(1):153–158. doi: 10.1128/jvi.26.1.153-158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groenink M., Andeweg A. C., Fouchier R. A., Broersen S., van der Jagt R. C., Schuitemaker H., de Goede R. E., Bosch M. L., Huisman H. G., Tersmette M. Phenotype-associated env gene variation among eight related human immunodeficiency virus type 1 clones: evidence for in vivo recombination and determinants of cytotropism outside the V3 domain. J Virol. 1992 Oct;66(10):6175–6180. doi: 10.1128/jvi.66.10.6175-6180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Thomas C. Y., Chattopadhyay S. K., Koehne C., O'Donnell P. V. Influence of enhancer sequences on thymotropism and leukemogenicity of mink cell focus-forming viruses. J Virol. 1989 Mar;63(3):1284–1292. doi: 10.1128/jvi.63.3.1284-1292.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Chatis P. A., Hopkins N., Hartley J. W. Construction of recombinants between molecular clones of murine retrovirus MCF 247 and Akv: determinant of an in vitro host range property that maps in the long terminal repeat. J Virol. 1985 Jan;53(1):152–157. doi: 10.1128/jvi.53.1.152-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Joyner A., Keller G., Phillips R. A., Bernstein A. Retrovirus transfer of a bacterial gene into mouse haematopoietic progenitor cells. Nature. 1983 Oct 6;305(5934):556–558. doi: 10.1038/305556a0. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Ihle J. N. Mechanisms of C-type viral leukemogenesis. I. Correlation of in vitro lymphocyte blastogenesis to viremia and leukemia. J Immunol. 1979 Nov;123(5):2351–2358. [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991 May;65(5):2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Li Q. X., Fan H. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J Virol. 1990 Aug;64(8):3701–3711. doi: 10.1128/jvi.64.8.3701-3711.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hering C., Hartley J. W., Rowe W. P., Hopkins N. Analysis of the genomes of mink cell focus-inducing murine type-C viruses: a progress report. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1269–1274. doi: 10.1101/sqb.1980.044.01.138. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Oliff A., Signorelli K., Collins L. The envelope gene and long terminal repeat sequences contribute to the pathogenic phenotype of helper-independent Friend viruses. J Virol. 1984 Sep;51(3):788–794. doi: 10.1128/jvi.51.3.788-794.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R., Ghosh A. K., Kumar D. V., Bachman B. A., Shibata D., Roy-Burman P. Recombination between feline leukemia virus subgroup B or C and endogenous env elements alters the in vitro biological activities of the viruses. J Virol. 1991 Dec;65(12):6495–6508. doi: 10.1128/jvi.65.12.6495-6508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Genes affecting mink cell focus-inducing (MCF) murine leukemia virus infection and spontaneous lymphoma in AKR F1 hybrids. J Exp Med. 1983 Aug 1;158(2):353–364. doi: 10.1084/jem.158.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Janesch N. J., Chakraborti A., Sawyer S. T., Hankins W. D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990 Mar;64(3):1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets R. L., Pandey R., Klement V., Grant C. K., Roy-Burman P. Biologically selected recombinants between feline leukemia virus (FeLV) subgroup A and an endogenous FeLV element. Virology. 1992 Oct;190(2):849–855. doi: 10.1016/0042-6822(92)90924-e. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Anderson G. R., Tronick S. R., Aaronson S. A. Evidence for genetic recombination between endogenous and exogenous mouse RNA type C viruses. Cell. 1974 Jun;2(2):87–94. doi: 10.1016/0092-8674(74)90096-8. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C., Coffin J. M. Virological events leading to spontaneous AKR thymomas. J Virol. 1991 Mar;65(3):1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Berg P. Homologous recombination of copackaged retrovirus RNAs during reverse transcription. J Virol. 1992 Apr;66(4):2378–2388. doi: 10.1128/jvi.66.4.2378-2388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Bear S. E. Infection by mink cell focus-forming viruses confers interleukin 2 (IL-2) independence to an IL-2-dependent rat T-cell lymphoma line. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4611–4615. doi: 10.1073/pnas.88.11.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- York-Higgins D., Cheng-Mayer C., Bauer D., Levy J. A., Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990 Aug;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Salameh A. M., Cloyd M. W. Oncogenicity of AKR mink cell focus-inducing murine leukemia virus correlates with induction of chronic phosphatidylinositol signal transduction. J Virol. 1992 Oct;66(10):6125–6132. doi: 10.1128/jvi.66.10.6125-6132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]