Abstract
In a laboratory study, we monitored the lifetime sexual signalling (advertisement) of wild male Mediterranean fruit flies, and we tested the hypothesis that high lifetime intensity of sexual signalling indicates high survival probabilities. Almost all males exhibited signalling and individual signalling rates were highly variable from the beginning of the adults’ maturity and throughout their life span (average life span 62.3 days). Sexual signalling rates after day 10 (peak maturity) were consistently high until about 1 week before death. There was a positive relationship between daily signalling rates and life span, and an increase in signalling level by one unit over all times was associated with an approximately 50% decrease in mortality rate. Signalling rates early in adult life (day 6–20) were higher in the longest-lived than in the shortest-lived flies. These results support the hypothesis that intense sexual signalling indicates longer life span. We discuss the importance of age-specific behavioural studies for understanding the evolution of male life histories.
Keywords: Behavioural demography, Ceratitis capitata, Life span, Lifetime behaviour, Sexual calling
Introduction
Male fitness is directly correlated with several demographic characteristics, such as age of first reproduction, age-schedule of reproduction, survival of the produced offspring and life time mating success (Futuyama 1997). Total lifetime fitness will be an additive and multiplicative combination of these components, and changes in one of the above traits may by definition have a high impact on fitness. The genetic variation in lifetime mating success is very important from an evolutionary perspective, and long term studies may indicate higher variance than short-term studies (Partridge and Halliday 1984). Therefore, lifetime studies are needed to understand the fitness components and the evolution of male life histories. Indeed, there have been increasing number of recent lifetime behavioural studies in insects, including damselflies (Banks and Thompson 1985; Cordero Rivera and Andres Abad 1999; Stoks 2000), mosquitoes (Bock et al. 1983), beetles (Deka and Hazarika 1998), butterflies and moths (Lepidoptera) (Fitzpatrick and McNeil 1989; Suzuki and Matsumoto 1992), and flies (Partridge and Farquhar 1983). Nevertheless, the majority of studies on male sexual behaviour have focussed on young ages. Male mating success in many polygynous species is positively correlated with sexual advertisement, and sexual advertisement has been considered functionally analogous to other forms of reproductive investment and therefore a main element of male fitness (Brooks and Kemp 2001). Most of the lifetime behavioural studies focus on mating per se and only a few on sexual advertisement of males (Cordts and Partridge 1996; Crnokrak and Roff 1995).
Sexual advertisement (signalling, calling) is a main strategy to attract females and to achieve copulations for males of many polygynous species (Alcock 1997). Increased frequency of sexual advertisement and other similar traits that enhance the endurance of male sexual activity can be favoured by sexual selection (Andersson 1994). Intensive sexual advertisement can also be seen as a handicap, which can only be possessed by strong, fit individuals, indicating a good ability to survive (Barnard 1983; Zahavi 1975). Since sexual advertisement is a major component of male mating success, lifetime male fitness in these species should be directly related to lifetime attractiveness (i.e. their ability to exhibit sexual advertisement at high rates). Therefore, in order to estimate lifetime male attractiveness it is important to know whether the ability of males to perform frequent sexual advertisement changes with age.
High rates of sexual advertisement, and probably other sexual behaviours such as mating, may incur a cost in other fitness components such as longevity. Reproductive cost has frequently been demonstrated in females (see Chapman et al. 1998), but it is not clear in male insects. Cost of reproduction in males includes sexual advertisement (signalling), courtship, mating and production of gametes and associated material. Reproductive activities in nature might also increase predation risk and other external hazards. The expression of sexual advertisement and other similar traits are condition dependent, which, according to Rowe and Houle (1996), can be defined as an “internal property of the individual which accounts for a large proportion of fitness”. The ability to bear the cost might be purely environmental or might reflect differences in male quality. If males do not vary their investment in sexual advertisement (signalling) in relation to their ability to bear the cost, there should be a negative relationship between survival and the intensity of signalling (Jennions et al. 2001). On the other hand, a positive relationship is expected when the expressed trait (signalling in our case) is an honest index of male quality. Several studies found a positive relationship between sexually selected traits and male longevity, while others reported the opposite. However, a recent meta-analysis that determines the broader pattern of this phenomenon shows a positive relationship between traits correlated with male mating success and those correlated with life span (Jennions et al. 2001). Of several studies dealing with the cost of reproduction in male insects, only a few examine the relative cost of the different aspects of reproduction (Cordts and Partridge 1996). More studies are needed to clarify whether other aspects of male sexual behaviour such as pheromone production and signalling, which are energetically demanding, may also be costly to males.
The reproductive and sexual behaviour of the Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann), (Diptera: Tephritidae), is relatively well studied (Eberhard 2000; Sivinski et al. 2000; Yuval and Hendrichs 2000). Receptive females are attracted to “signalling” males emitting a sex pheromone. Males are polygamous and females monogamous or oligogamous. In wild populations, sexual maturity is attained in approximately 10 days after emergence (Papadopoulos et al. 1998). Signalling males usually form loose aggregations (leks) on host plant leaves, and defend small territories. Sexual signalling (including biosynthesis and release of sex pheromone) is an energetically demanding activity (Warburg and Yuval 1997). It is also an important determinant of mating success in this fly, since there is a direct relation between female attraction and signalling frequency (Whittier et al. 1994a). There is a complex interaction between the signalling male and the responding female that ends in copulation. Male courtship starts upon female approach and consists of complex activities such as wing fanning, head rocking, abdomen curling and sound production (Féron 1962; Prokopy and Hendrichs 1979). Only a small number of males account for most of the matings, and a large number of males do not mate (Arita and Kaneshiro 1985; Whittier and Kaneshiro 1995; Whittier et al. 1994b). Successful copulation that leads to sperm transfer lasts on average 2–3 h (Whittier et al. 1992). Sexual signalling is intensive in young males.
We initiated this study because virtually nothing is known about the lifetime sexual advertisement in male medflies, and because no behavioural data exist about the sexual behaviour of old medflies (and in general in insects). We tested the hypothesis that intensive signalling indicates high survival probabilities. Our goals were to determine: (1) changes in signalling frequency throughout the life course of individuals acquiring longitudinal data; (2) the relationship between both sexual signalling at young and old ages, and signalling and longevity; (3) whether sexual signalling in young ages can provide a reliable indicator of male life span; and (4) fitness components of sexual signalling and trait-offs with other life history traits.
Materials and methods
Colony source and environmental conditions
This study was conducted in the laboratory at 25±2°C, 65±5% R.H and 14:10 (L:D), with photophase beginning at 0600 hours. Light was provided by daylight tubes, and the light intensity in the experimental room was 1000–1,500 lux.
The Mediterranean fruit fly adults used here were obtained from field-infested apples that were collected during September–October 1999 from the area of Thessaloniki (northern Greece). Fruits were brought to the laboratory and pupae were collected twice a week. Upon emergence, adults were placed in individual cages with adult food (a mixture of yeast hydrolyzate and sugar, 1:4), and water.
Individual cages consisted of a transparent plastic cup, 12-cm high, 5-cm base diameter and 7.5-cm top diameter which was placed upside down, and glued to the lid of a plastic Petri dish (9-cm diameter). Adult food was placed in abundance on the floor of the cage, and water was provided by a cotton wick that went through a small hole in the Petri dish lid to an underlying Petri dish base, which was filled with water. A lateral window covered with mesh was perforated on the cup’s side for ventilation. The base of the plastic cup (top part of the cage) had been removed, and was replaced by a movable small Petri dish base (6 cm in diameter), which served as an entrance to the cage. Individually caged males could visually detect each other in neighbouring cages and they could also sense the pheromone produced by other males. However, they had no contact (visual or other) with females.
Empirical methods
Sexual signalling was recorded for each individual fly at 10-min intervals from 1200 to 1400 hours (total of 12 counts). Observations were conducted daily and continued until each fly died. Each individual fly was observed instantaneously (2–3 s) and its activity was recorded. Signalling males (also “calling males” in the literature) were distinguished easily from non-signalling ones, since they keep their abdomen curled upward, with a bubble-like structure appearing at the end. In fact, this bubble-like structure consists of the extruded anal sac (terminal end of the rectal epithelium), which is inverted in order to release a sexual pheromone, (Arita and Kaneshiro 1986; Féron 1962). The extrusion of the rectal epithelium is often accompanied by wing fanning. Earlier studies conducted in our laboratory under identical conditions and using the same strain (wild flies) of flies showed a clear daily pattern of signalling activity with peak rates exhibited between 1200 and 1500 hours (Papadopoulos et al. 1998). Based on this, we selected the time period from 1200 to 1400 hours for recording signalling frequency, which was determined by the number of times that males signal (from 1200 to 1400 hours) out of 12 counts. Data were collected for 203 males. The experiment was repeated 4 times with about 50 individually caged males (replicates) each time. Since the data for each replicate were not significantly different we combined them in the final analysis. To determine possible age related changes in daily patterns of signalling, hourly records (from 0700 to 2000 hours) of signalling activity were conducted every 10 days from day 10 to day 90.
The size of each individual was determined at death by measuring the length and width of the thorax. Thorax length is correlated with dry weight in this fly (Whittier et al. 1992). Wing length was not used as a size estimate because the wings were usually destroyed in old males. Male size did not differ significantly among the four replicates (see above).
Statistical methods
Statistical analyses included linear models and Cox regression (Klein and Moeschberger 1997; Neter et al. 1996; Sokal and Rohlf 1995). In regression analyses, life span was the dependent variable and regressed against average signalling per day, or male size (independent variables). In order to determine differences between the longest-lived and the shortest-lived flies in terms of signalling frequency at the beginning of adult life, we performed a repeated measures analysis of variance on signalling rates early in life (up to day 20) of the 20 longest- and the 20 shortest-lived males (considering only those individuals that lived beyond day 25). Daily signalling rates from day 6 (beginning of signalling activity) to day 20 were used as the repeated time (age) factor. Thorax length and width were used as covariates, but since they had no significant effect were excluded from the final model. The 20 longest- and 20 shortest-lived males were chosen for this analysis because we anticipated that differences between them in sexual signalling would be detectable. The first 20 days of adult life represent the main part of sexual activity for young males and it is also a period before mortality prevails. Life table parameters were estimated following standard methods described by Carey (1993). We also fitted a Cox proportional hazards model with the signalling level recorded at age t as the time-dependent covariate X(t) to determine the effect of signalling on lifespan. This model is given by λ(t) = λ0(t)eβo+β1X(t), where λ(t) and λ0(t) denote hazard rate conditional on the observed covariate trajectory X(t), respectively the baseline hazard rate (see Cox 1972; Klein and Moeschberger 1997). The effect of the time-dependent (signalling) covariate on life span is measured by the coefficient β1.
Results
Lifetime signalling rates
The average longevity of medfly males was 62.3±22.4 days (±SD). The mean age at the beginning of sexual signalling was 6.9±1.6 days, and the average signalling per day per male 5.7±1.7 times out of 12 observations. The results of over 151,763 observations and nearly 13,000 insect days for 203 medfly males reveal the lifetime rates of sexual signalling for individual medfly males (Fig. 1). Mortality to day 40 was low, increasing abruptly from days 40 to day 75, and then declining again and remaining low at older ages (>day 100; Fig. 1). Approximately 90% of the males lived to day 40, 20% to day 75, and less than 10% beyond day 100, with the oldest fly dying at day 152. Signalling began on average on day ≈7, and almost all males exhibited sexual signalling at least once by day 13 (Fig. 1). The frequency of signalling increased sharply from day 7 to 10 and reached peak levels from day 10 to 40 (an average of >7 per day; Figs. 1, 2a). Age specific signalling rates declined from day 40 to day 70 and then remained at low levels (≈5 signalling per day) increasing again at very old ages (after day 90). Closer inspection of the data in Figs. 1, 2 shows that the decline in signalling frequency from age 40 to day 70 and the low rates up to day 90 are actually due to increasing mortality rates in this time interval. Signalling ceased an average of 8.1±5.3 days before death (Fig. 1). The high proportion of males at high mortality risk between day 40 and day 70 affects the age specific signalling pattern and explains the decreasing signalling rates we recorded in this time interval. Therefore, the differential mortality that affects signalling rates accounts for the decrease recorded between day 40 and day 70. If the cohort is divided in groups (10 percentiles) according to their life span and their age-specific signalling rates are projected this tendency is clear (Fig. 2b). The group of the 10th percentile (longest-lived flies) did not experience any decrease in signalling activity in the time interval from day 40 to day 70. They performed high signalling rates until the time that some individuals entered into the “high mortality risk” period. Same trends were observed in all the other groups of males. Interestingly very old flies (>90 days) exhibited high signalling rates.
Fig. 1.
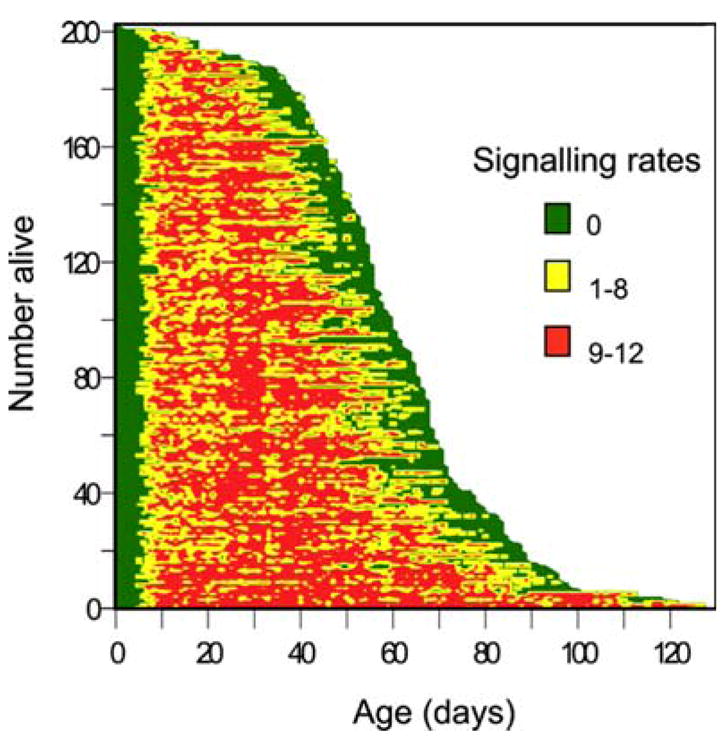
Event history chart for 203 wild Mediterranean fruit fly, Ceratitis capitata, males. Each individual is represented by a horizontal line proportional to his life span. Daily signalling rates are colour-coded in respect to the number that this behaviour exhibited out of 12 observations made within 2-h periods (1200–1400 hours). Green 0 signalling, yellow 1–8, red 9–12
Fig. 2.
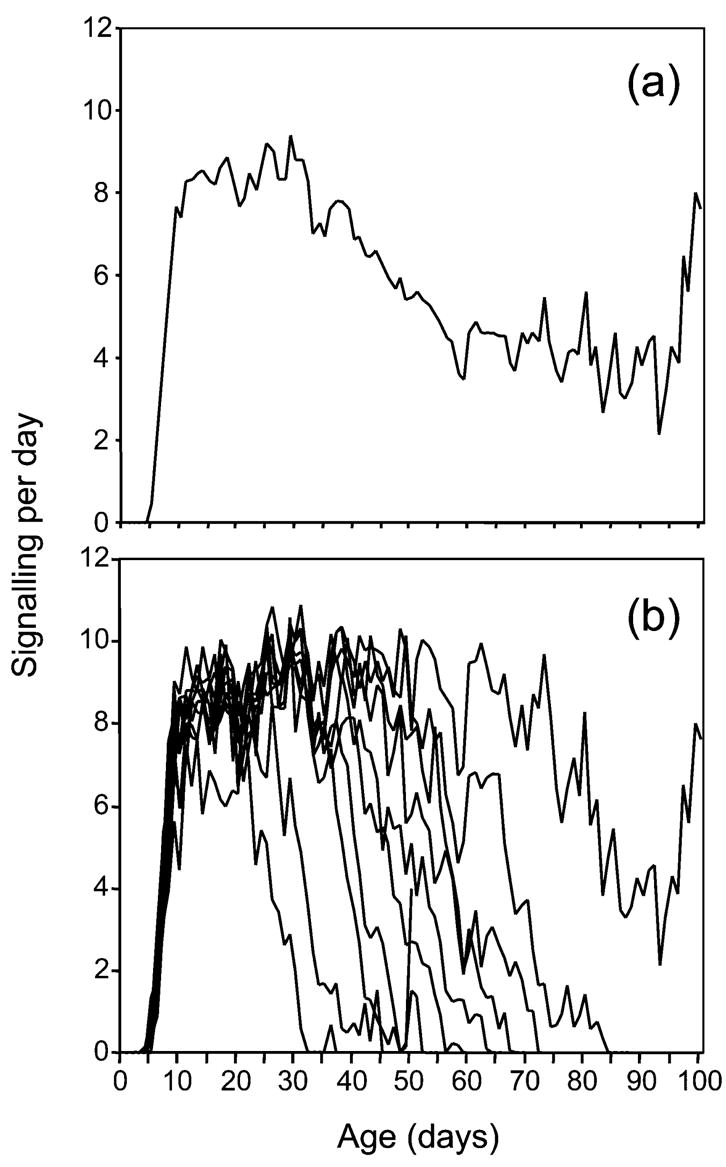
Age-specific signalling rates for the entire cohort (a), and for sub-cohorts of the 10th percentiles (in relation to the life span of individuals) (b)
We used the ages at which males first and last exhibited signalling in order to estimate the sexually active life span. The sexually active life span was almost 15 days shorter than the total life span (47.8 vs 62.3 days). Approximately 100% of the individuals exhibit signalling up to day 40. The percentage of signalling males (sexually active individuals) ranged between 40 and 80% from day 40 to day 100 (Fig. 1). The proportion of the active individuals in the population increased in the older ages.
Age dependent daily rhythm of signalling rates
The daily patterns of signalling were similar for flies of 10, 20 and 30 days old. Signalling exhibited in high rates from the beginning of the photophase until early afternoon (1500 hours), decreased at 1600 hours and ceased at 1700 hours (Fig. 3). Peak rates were recorded around noon (1100–1500 hours). Males older than 40 days displayed similar daily patterns of signalling activity; however, a substantial decrease of signalling was recorded from 0700 to 0900 hours. Peak signalling rates were always recorded from 1200 to 1500 hours independently of male age.
Fig. 3.
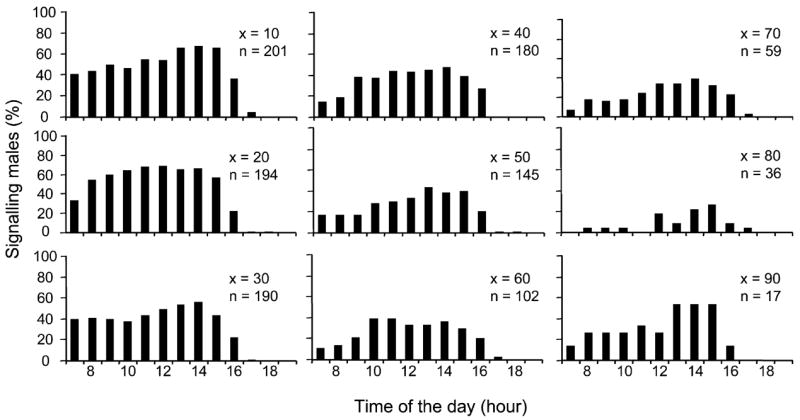
Daily rhythm of signalling activity at different ages (from adult day 10 to adult day 90, x age, n number of individuals alive)
Signalling rates and life span
There was a significant regression relationship between individual signalling per day and total life span (df=1,201; F=120.9; P<0.001; Fig. 4). The higher the frequency of signalling the longer the life span. This relationship accounts for almost 40% of the variability, and indicates that life span and sexual activity are closely associated in male medflies. We also fitted a Cox proportional hazards regression model to the data testing the null hypothesis H0: β1=0 (i.e. signalling does not affect hazard rates and therefore male life span), and it was rejected with P-value of <0.0001. The estimated coefficient β1=−0.5682±0.072 SE indicates that an increase in signalling level by one unit over all times would lead to a factor of e−0.5682 (≈0.5665) for the hazard rate, i.e., an increase in one unit in signalling overall will be accompanied by an approximately 50% decrease in mortality for an individual fly.
Fig. 4.
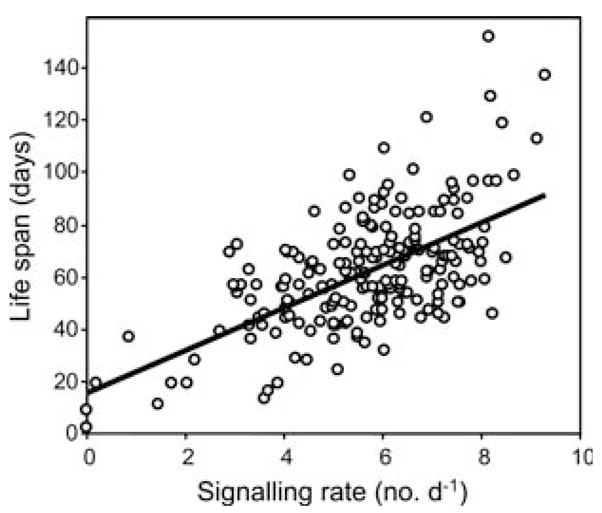
Average daily signalling rates and life span in male medflies (y=15.8+8.2x, r2=0.38)
To predict which of the males in the cohort would be the longest lived we looked for a significant relationship between average signalling rates up to day 40 (before a significant mortality prevailed, see Fig. 1) and the life span of those males who lived beyond day 80 (oldest males in the cohort). A weak (explaining only 24% of the variability) but significant simple linear regression relationship was established (df=1,35; F=7.9; P<0.001). A similar weak but significant relationship was obtained when the life span of the oldest males (males that lived beyond day 80) in the cohort was regressed against the average signalling rates up to day 20 (df=1,35; F=5.7; P=0.02). Finally, we did not find a significant linear relationship between the life span and the age of first signalling (df=1,201; F=0.16; P=0.69).
A repeated measures analysis of variance on signalling rates up to day 20 showed that the longest-lived males (average lifespan 105.9±3.9 SE, n=20) exhibited significantly higher signalling rates (df=1,38; F=16.9, P<0.001) early in adult life than the shortest-lived ones (average lifespan 36.2±1.2 SE, n=20). As expected, age had a significant effect on signalling (df=15,570; F=78.1; P<0.001), while the interaction between the group of males and their age was not significant (df=15,570; F=0.86; P=0.61). Indeed, average signalling rates followed almost the same pattern in the two groups with the longest-lived males performing more signalling in almost all age intervals (Fig. 5).
Fig. 5.
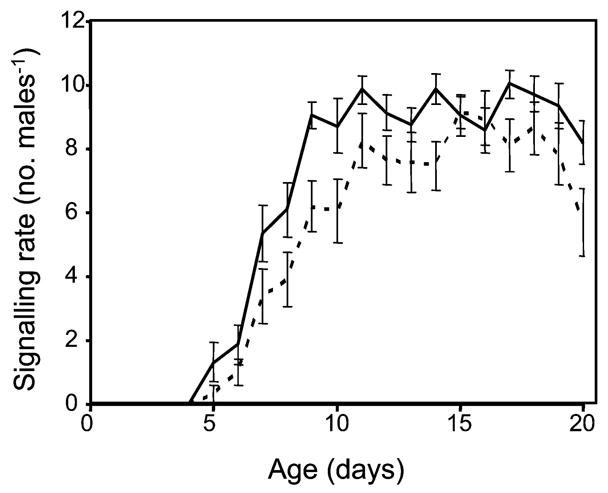
Age specific signalling rates (±SE) to day 20 for the longest-lived (solid line) and the shortest-lived (broken line) males in the cohort (n=20 for both cohorts)
Relationship between adult size and signalling rates
The average thorax length was 2.2±0.1 mm (±SD) (range 1.8–2.4 mm), and thorax width 1.5±0.08 mm (range 1.2–1.7 mm). There was no significant correlation between thorax length, thorax width and either male life span or signalling per day (n=190–193; R<0.11; P>0.05).
Discussion
We report two important findings: (1) the positive relationship between signalling and life span (i.e. negative relationship between signalling and hazard rates); and (2) the significant difference in signalling frequency between the longest- and the shortest-lived flies early in adult life. These results support the hypothesis that intensive sexual signalling (advertisement) is an indicator of longer life span. Since males experienced similar conditions (e.g. environment, high quality food, easy accessibility to food, obtained from the same host and area) this relationship may indicate underlying genetic differences in male quality in terms of life span. Under optimum phenotypic conditions the expression of a trait such as sexual signalling is expected to be higher in fitter individuals. The high variability in signalling frequency among males in this study as well as the differences in signalling frequency between the longest- and the shortest-lived individuals underscore the above suggestion. The difference in signalling rates early in adult life between the longest- and the shortest-lived flies could be also used to predict the longest-lived individuals in the population and also to identify, early in adult life, the fittest males.
Our results that sexual signalling is positively correlated with the life span are in agreement with the recent findings by Jennions et al. (2001), who conducted a meta analysis and found that there is a positive relationship between sexually selected traits (such as sexual signalling) that are correlated with mating success and traits correlated with male life span, and this relationship is stronger in lekking species. Costly sexual advertisement is considered as a life history trait, which is subject to trade-offs with other components of reproductive effort, and future survival (Kokko et al. 2002). Frequency of sexual advertisement and other secondary sexual traits are condition depended and increases with high genetic viability (Rowe and Houle 1996). Theory predicts that if a trait such as sexual signalling in our case is costly its size and life span (viability) should be negatively correlated (Kotiaho et al. 1999). However, a positive correlation between life span and the size of the trait is anticipated if the expression of the trait depends on the phenotypic condition of the individual such that the costs of the trait are less for individuals in good conditions than for individuals in poor condition (Kotiaho et al. 1999 and references therein). Variation in the ability to bear the cost may reflect underlying differences in male quality. In general males of higher quality are more likely to be in better condition and expected to express higher intensity of the respective trait.
Sexual signalling commits medfly males to a high energetic investment, there is a remarkable metabolic cost in biosynthesis and emission of sex pheromone, and only males that can forage successfully can exhibit this behaviour in nature (Warburg and Yuval 1997; Yuval et al. 1998). Here we found that sexual signalling varies a lot among individuals under optimum diet conditions in the laboratory. Therefore, other factors besides successful foraging may be important determinants of signalling frequency. Our results (non-existence of a negative correlation between sexual signalling and life span) show that sexual signalling in the absence of other sexual activities has no obvious effect on male life span under laboratory (predation-free) conditions. However, experimental manipulations in which signalling rates would be disentangled from condition need to be done to address more directly questions regarding the cost of sexual signalling. Metabolic cost, which is increased by courtship activity, is probably important in reducing life span in male Drosophila melanogaster (Cordts and Partridge 1996). Reduced life span due to increased sexual activities might also be related to altered hormonal status in males. Participation in leks and frequent signalling in nature might also be costly in terms of reduced viability since it reduces male opportunities for foraging and exposes them to high predation risk (Hendrichs et al. 1991, 1994; Hendrichs and Hendrichs 1998). The importance of predation and food availability as mortality factors in wild populations has not been investigated in detail in this fly. More field work is needed to clarify issues related with male survival in the wild.
Although females cannot assess male quality based on frequency of signalling, males that signal at higher rates enjoy higher reproductive success (Shelly 2000). There is a direct relationship between signalling frequency, female attraction, number of courtships performed and matings achieved (Whittier et al. 1994a). This indicates that signalling frequency may be sexually selected, most probably through male-male competition. The ability of males in lekking or other mating systems to remain reproductively active for a long period is well appreciated as important in increasing the number of matings (Andersson 1994; Andersson and Iwasa 1996). Sexual signalling is universal in this fly because all males exhibited this behaviour (Fig. 1; see also Whittier et al. 1994a). It is also a large time investment for the individuals since signalling was exhibited on >80% of days of the total life span. Nonetheless, the proportion of non-mating males, estimated in short term studies over an approximately 1-week period (early in adult life) is usually high (≈40–55%) (Shelly 2000; Whittier et al. 1994a). Our results indicate that signalling frequency remained almost constant throughout the life span of individuals and dropped only approximately 1 week before death. We can therefore suggest that high signalling rates in medfly males may confer a lifetime mating advantage to individuals providing a large fitness benefit. However, this suggestion should be tested experimentally in order to elucidate whether mating performance, not just signalling frequency, remains constant throughout the life span, and whether old males can compete with younger ones in attracting females and achieving matings. On the other hand, it is not clear whether females gain benefits of mating with an attractive male that signals frequently. In fact, individual females that mate with males with high mating rates (attractive males) do not have sons with higher attractiveness and mating success (Whittier and Kaneshiro 1995). However, it is not known whether their sons experience higher survival probabilities and higher lifetime mating success through longer reproductive life spans.
Lifetime data of individual flies provide insight into inter-fly variation in sexual signalling, allow between-fly comparisons in lifetime levels of signalling rates, and reveal compositional influences on the cohort averages (Carey et al. 1998). They also provide insights into intra-fly differences in signalling rates connecting the intensity of expression early and late in life. The results of this study show that signalling, and probably other behaviours, have age-specific properties that define the sexually active life span of individuals, which is a major component of male fitness in lekking species. The sexually active life span as it is indicated by signalling is almost 2 weeks shorter than the total life span, and the frequency of signalling remains constantly high for individuals during most of this period. After peak rates have been assumed, a substantial decrease and cessation in daily signalling rates can be used as an index of high mortality risk. In fact, as medfly males approach death, they spend a progressively increasing amount of time in a supine position, which indicates an irreversible morbidity condition and predicts death (Papadopoulos et al. 2002). Sexual signalling and supine behaviour can be used to determine morbidity dynamics and its underlying causes in male medflies, also providing reliable behavioural biomarkers of aging. A biomarker of aging is a behavioural or biological parameter of an organism that can, either alone or in some multivariate composite, better predict functional capacity and/or mortality risk at some later age than the chronological age (Markowska and Breckler 1999). Other behavioural adjustments to increase of the mortality risk, such as alternations of the reproductive behaviour of parasitic wasps (Roitberg et al. 1993), can provide important indicators of individual and cohort life expectancy.
Because studies of different types of insect behaviour such as foraging, mating, and reproductive behaviours have traditionally focused on young or middle-aged individuals, little is known about insect behaviour in older individuals or the relationship of different behaviours at young ages to life history traits in older individuals (Alcock 1997). Likely reasons for ignoring the behaviour of the elderly in nature is because life table information in field populations suggest that most individuals in nature die young, and that the evolutionary theory of aging (Kirkwood and Austad 2000; Partridge 2001 suggests that older individuals contribute little to fitness. Behavioural research on older individuals not only provides a more complete account of behavioural dynamics during all stages of a species’ life course, it also provides data and context for testing evolutionary theories of aging and organisational framework for interdisciplinary research in life history theory. Behavioural traits, not only birth and death rates, can also be characterised as age-specific intensity as we show in the current study. Age-specific behavioural data can then be used to test theories about the cost of reproduction in the context of mating, signalling, and courting as well as juxtaposed with changes in other life history traits.
Acknowledgments
We thank Hugh Dingle and Debra Judge for comments on earlier drafts. The research was funded by PO1-08761 from the U.S. National Institute on Aging.
Contributor Information
Nikos T. Papadopoulos, Department of Agriculture, Laboratory of Applied Zoology and Parasitology, Aristotle University of Thessaloniki, 540 06 Thessaloniki, Greece Department of Entomology, University of California, Davis 95616, California, USA, e-mail: npapadopoulos@ucdavis.edu, Tel.: +1-530-7546209, Fax: +1-530-7521537.
Byron I. Katsoyannos, Department of Agriculture, Laboratory of Applied Zoology and Parasitology, Aristotle University of Thessaloniki, 540 06 Thessaloniki, Greece
Nikos A. Kouloussis, Department of Agriculture, Laboratory of Applied Zoology and Parasitology, Aristotle University of Thessaloniki, 540 06 Thessaloniki, Greece
James R. Carey, Department of Entomology, University of California, Davis 95616, California, USA Centre for the Economics and Demography of Aging, University of California, Berkeley 94720, California, USA.
Hans-Georg Müller, Department of Statistics, University of California, Davis 95616, California, USA.
Ying Zhang, Department of Statistics, University of California, Davis 95616, California, USA.
References
- Alcock J. Animal behavior. 6. Sinauer; Sunderland, Mass: 1997. [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton, N.J: 1994. [Google Scholar]
- Andersson M, Iwasa Y. Sexual selection. Trends Ecol Evol. 1996;11:53–58. doi: 10.1016/0169-5347(96)81042-1. [DOI] [PubMed] [Google Scholar]
- Arita LH, Kaneshiro KY. The dynamics of the lek system and mating success in males of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) Proc Hawaii Entomol Soc. 1985;25:39–48. [Google Scholar]
- Arita LH, Kaneshiro KY. Structure and function of the rectal epithelium and anal glands during mating behavior in the Mediterranean fruit fly male. Proc Hawaii Entomol Soc. 1986;26:27–30. [Google Scholar]
- Banks MJ, Thompson DJ. Lifetime mating success in the damselfly Coenagrion puella. Anim Behav. 1985;33:1175–1183. [Google Scholar]
- Barnard CJ. Animal behaviour: ecology and evolution. Wiley; New York: 1983. [Google Scholar]
- Bock ME, Reisen WK, Milby MM. Lifetime mating pattern of laboratory-adapted Culex tarsalis males. Mosq News. 1983;43:350–354. [Google Scholar]
- Brooks R, Kemp DJ. Can older males deliver the good genes? Trends Ecol Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. [DOI] [PubMed] [Google Scholar]
- Carey JR. Applied demography for biologists with special emphasis on insects. Oxford University Press; New York: 1993. [Google Scholar]
- Carey JR, Liedo P, Müller HG, Wang JL, Vaupel JW. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Funct Ecol. 1998;12:359–363. [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc R Soc Lond B. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero Rivera A, Andres Abad JA. Lifetime mating success, survivorship and synchronized reproduction in the damselfly Ischnura pumilio (Odonata: Coenagrionidae) Int J Odonatol. 1999;2:105–114. [Google Scholar]
- Cordts R, Partridge L. Courtship reduces longevity of male Drosophila melanogaster. Anim Behav. 1996;52:269–278. [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- Crnokrak P, Roff DA. Fitness differences associated with calling behaviour in the two wing morphs of male sand crickets, Gryllus firmus. Anim Behav. 1995;50:1475–1481. [Google Scholar]
- Deka M, Hazarika LK. Lifetime mating pattern of rice hispa, Dicladispa armigera (Oliv.) (Coleoptera: Chrysomelidae) Crop Res Hisar. 1998;16:253–256. [Google Scholar]
- Eberhard W. Sexual behavior and sexual selection in the Mediterranean fruit fly, Ceratitis capitata (Dacinae: Ceratitidini) In: Aluja M, Norrbom A, editors. Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC Press: Boca Raton, Fla; 2000. pp. 457–489. [Google Scholar]
- Féron M. L’instinct de reproduction chez la mouche méditerranéean des fruits Ceratitis capitata Wied. (Dipt. Trypetidae). Comportement sexuel. Comportement de ponte. Rev Path Veg Entomol Agr Fr. 1962;41:1–129. [Google Scholar]
- Fitzpatrick SM, McNeil JN. Lifetime mating potential and reproductive success in males of the true armyworm, Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae) Funct Ecol. 1989;3:37–44. [Google Scholar]
- Futuyama DJ. Evolutionary biology. Sinauer; Sunderland, Mass: 1997. [Google Scholar]
- Hendrichs MA, Hendrichs J. Perfumed to be killed: Interception of Mediterranean fruit fly (Diptera: Tephritidae) sexual signalling by predatory foraging wasps (Hymenoptera: Vespidae) Ann Entomol Soc Am. 1998;91:228–234. [Google Scholar]
- Hendrichs J, Katsoyannos BI, Papaj DR, Prokopy RJ. Sex-differences in movement between natural feeding and mating sites and tradeoffs between food consumption, mating success and predator evasion in Mediterranean fruit flies (Diptera, Tephritidae) Oecologia. 1991;86:223–231. doi: 10.1007/BF00317534. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Katsoyannos BI, Wornoayporn V, Hendrichs MA. Odor mediated foraging by yellowjacket wasps (Hymenoptera, Vespidae): predation on leks of pheromone calling Mediterranean fruit fly males (Diptera, Tephritidae) Oecologia. 1994;99:88–94. doi: 10.1007/BF00317087. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Moller AP, Petrie M. Sexually selected traits and adult survival: a meta-analysis. Q Rev Biol. 2001;76:3–36. doi: 10.1086/393743. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Klein JP, Moeschberger ML. Survival analysis. Springer; New York Berlin Heidelberg: 1997. [Google Scholar]
- Kokko H, Brooks R, McNamara JM, Houston AI. The sexual selection continuum. Proc R Soc Lond B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho JS, Alatalo RV, Mappes J, Parri S. Sexual signalling and viability in a wolf spider (Hygrolycosa rubrofasciata): measurements under laboratory and field conditions. Behav Ecol Sociobiol. 1999;46:123–128. [Google Scholar]
- Markowska AL, Breckler SJ. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. J Gerontol Biol Sci. 1999;12:B549–B566. doi: 10.1093/gerona/54.12.b549. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutneer MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4. McGraw Hill; Boston, Mass: 1996. [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Economopoulos AP, Carrey JR. Effect of adult age, food, and time of day on sexual calling incidence of wild and mass-reared Ceratitis capitata males. Entomol Exper Appl. 1998;89:175–182. [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA, Muller HJ, Liu X. Supine behaviour predicts time-to-death in male Mediterranean fruit flies. Proc R Soc Lond B. 2002;269:1633–1637. doi: 10.1098/rspb.2002.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Evolutionary theories of ageing applied to long-lived organisms. Exper Gerontol. 2001;36:641–650. doi: 10.1016/s0531-5565(00)00232-1. [DOI] [PubMed] [Google Scholar]
- Partridge L, Farquhar M. Lifetime mating success of male fruitflies (Drosophila melanogaster) is related to their size. Anim Behav. 1983;31:871–877. [Google Scholar]
- Partridge L, Halliday T. Mating patterns and mate choice. In: Krebs JR, Davies NB, editors. Behavioural ecology an evolutionary approach. 2. Sinauer; Sunderland, Mass: 1984. [Google Scholar]
- Prokopy RJ, Hendrichs J. Mating behavior of Ceratitis capitata (Diptera, Tephritidae) on a field caged host tree. Ann Entomol Soc Am. 1979;72:642–648. [Google Scholar]
- Roitberg BD, Sircom J, van Alphen JJM, Mangel M. Life expectancy and reproduction. Nature. 1993;364:108. doi: 10.1038/364108a0. [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B. 1996;263:1415–1421. [Google Scholar]
- Shelly TE. Male signalling and lek attractiveness in the Mediterranean fruit fly. Anim Behav. 2000;60:245–251. doi: 10.1006/anbe.2000.1470. [DOI] [PubMed] [Google Scholar]
- Sivinski J, Aluja M, Dodson GN, Freidberg AD, Headrick H, Landolt P. Topics in the evolution of sexual behavior in the Tephritidae. In: Aluja M, Norrbom A, editors. Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC Press; Boca Raton, Fla: 2000. pp. 751–792. [Google Scholar]
- Sokal RR, Rohlf EJ. Biometry. 3. Freedman; New York: 1995. [Google Scholar]
- Stoks R. Components of lifetime mating success and body size in males of a scrambling damselfly. Anim Behav. 2000;59:339–348. doi: 10.1006/anbe.1999.1309. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Matsumoto K. Lifetime mating success of males in a natural population of the papilionid butterfly, Atrophaneura alcinous (Lepidoptera: Papilionidae) Res Popul Ecol. 1992;34:397–407. [Google Scholar]
- Warburg MS, Yuval B. Effects of energetic reserves on behavioral patterns of Mediterranean fruit flies (Diptera: Tephritidae) Oecologia. 1997;112:314–319. doi: 10.1007/s004420050314. [DOI] [PubMed] [Google Scholar]
- Whittier TS, Kaneshiro KY. Intersexual selection in the Mediterranean fruit fly: Does female choice enhance fitness. Evolution. 1995;49:990–996. doi: 10.1111/j.1558-5646.1995.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Whittier TS, Kaneshiro KY, Prescott LD. Mating behavior of Mediterranean fruit flies (Diptera: Tephritidae) in a natural environment. Ann Entomol Soc Am. 1992;85:214–218. [Google Scholar]
- Whittier TS, Nam FY, Shelly TE, Kaneshiro KY. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera, Tephritidae) J Insect Behav. 1994a;7:159–170. [Google Scholar]
- Whittier TS, Nam FY, Shelly TE, Kaneshiro KY. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera: Tephritidae) J Insect Behav. 1994b;7:159–170. [Google Scholar]
- Yuval B, Hendrichs J. Behavior of flies in the genus Ceratitis (Dacinae: Ceratitidini) In: Aluja M, Norrbom A, editors. Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC Press; Boca Raton, Fla: 2000. pp. 429–457. [Google Scholar]
- Yuval B, Kaspi R, Shloush S, Warburg MS. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecol Entomol. 1998;23:211–215. [Google Scholar]
- Zahavi A. Mate selection: selection for a handicap. J Theor Biol. 1975;23:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]


