Abstract
Neurotransmitter receptors that inhibit the release of opioid peptides in the spinal cord may play an important role in modulating pain. Serotonin is an important neurotransmitter in bulbospinal descending pathways, and 5-HT1 receptors have been shown to inhibit synaptic transmission. Our goal was to determine whether 5-HT1A receptors inhibit opioid release in the spinal cord. Opioid release was evoked from rat spinal cord slices by electrically stimulating one dorsal horn, and measured in situ through the internalization of μ-opioid receptors in dorsal horn neurons. Stimulation with 1000 square pulses at 500 Hz produced internalization in 60% of the μ-opioid receptor neurons in the stimulated dorsal horn, but not in the contralateral one. The selective 5-HT1A receptor agonist 8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT) inhibited the evoked μ-opioid receptor internalization by about 50%, with an approximate IC50 of 50 nM. The effect of 8-OH-DPAT was attributed to inhibition of opioid release and not of the receptor internalization process, because 8-OH-DPAT did not inhibit the internalization induced by incubating the slices with a μ-opioid receptor agonist (endomorphin-2, 100 nM). The selective 5-HT1A receptor antagonist WAY100135 (10 μM) blocked the inhibition produced by 1 μM 8-OH-DPAT. These results show that 5-HT1A receptors inhibit opioid release in the spinal dorsal horn, probably from a subpopulation of enkephalin-containing presynaptic terminals. Therefore, 5-HT1A receptors likely decrease the analgesia produced by endogenously released opioids.
Keywords: 5-HT1A receptor, dorsal horn, enkephalin, internalization, mu-opioid receptor, serotonin
1. Introduction
Morphine and other alkaloid opiates acting on μ-opioid receptors (MORs) are the most powerful analgesics available, but their use can lead to tolerance and dependence. Since MORs are activated physiologically by endogenous opioid peptides, increasing the availability of opioid peptides should produce analgesia. Indeed, inhibiting opioid degradation produced analgesia (Chou et al., 1984; Fournie-Zaluski et al., 1992; Noble et al., 1992b) and had the added benefit of producing little tolerance and dependence (Noble et al., 1992a; Noble et al., 1992c). This suggests that endogenous opioids may not present the notorious drawbacks of the alkaloid opiates.
Another possible way to increase opioid availability is by blocking neurotransmitter receptors that inhibit opioid release. The internalization of MORs can serve as an in situ measure of opioid release, an approach that has been successfully used in the spinal cord (Marvizon et al., 1999, Trafton et al., 2000; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005), the brain (Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001) and the intestine (Patierno et al., 2005). MOR internalization is a valid measure of MOR activation by released opioids, because MOR internalization correlated well with several measures of MOR activation, including adenylyl cyclase inhibition, [γ-35S]GTP binding and analgesia (Marvizon et al., 1999; Trafton et al., 2000).
Using MOR internalization to measure opioid release in the spinal cord, we have shown that it is not affected by GABAA, GABAB, δ-opioid or cholecystokinin receptors, but it is inhibited by NMDA receptors acting in conjunction with large conductance Ca2+-sensitive K+ channels (Song and Marvizon, 2005). In another study (Song and Marvizon, 2003b), we observed that electrical stimulation of the dorsolateral funiculus, which contains numerous opioid-containing axons, failed to induce opioid release in the dorsal horn. We attributed this failure to the recruitment of other descending fibers in the dorsolateral funiculus that inhibited opioid release. One important bulbospinal descending pathway is serotonergic. Serotonin, acting on presynaptic 5-HT1 receptors, has been shown to inhibit synaptic transmission (Wu et al., 1991). Indeed, 5-HT1 receptors couple to αi/o G proteins (Pauwels, 2000) and therefore can inhibit neurotransmitter release by inactivating voltage-gated Ca2+ channels (Dolphin, 2003). Accordingly, we decided to investigate whether 5-HT1 receptors inhibit opioid release in the spinal cord.
2. Results
To evoke opioid release, we stimulated one dorsal horn of spinal cord slices with 1000 square electrical pulses delivered at 500 Hz. In a previous study (Song and Marvizon, 2003b) we determined that this stimulus maximized the amount of opioid released while minimizing substance P release. The release of endogenous opioids was measured in situ by the internalization of MORs. This stimulus produced MOR internalization in about 60 % of the MOR neurons (located in laminae I and II) of the stimulated dorsal horn (Fig. 1). Almost none of the MOR neurons of the contralateral dorsal horn showed MOR internalization (Fig. 1), indicating that the electrical current did not spread there. Confocal images of dorsal horn neurons with and without MOR internalization have appeared in several of our previous publications (Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005).
Figure 1. MOR internalization evoked by electrical stimulation was inhibited by 8-OH-DPAT.
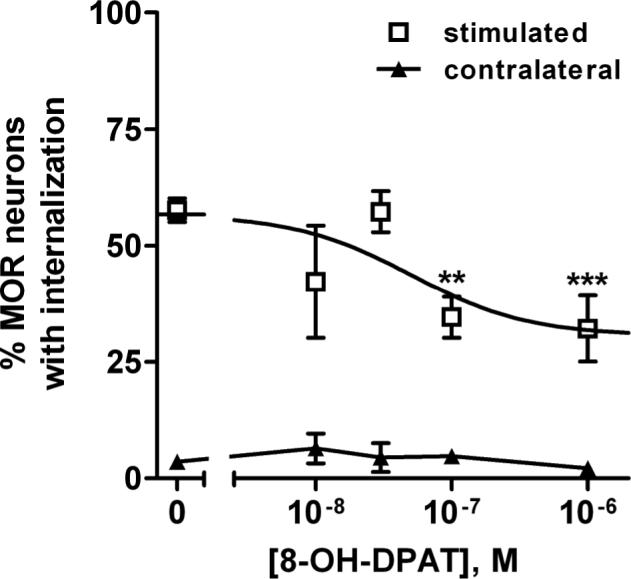
MOR internalization was evoked by dorsal horn stimulation with 1000 electrical pulses at 500 Hz. 8-OH-DPAT inhibited the internalization with Log IC50 = −7.30 ± 0.55 (IC50 = 50 nM). Data are the mean ± SEM of 3−12 replicates. The inhibition was partial, decreasing MOR internalization from 57 ± 4 % (“top”) to 31 ± 6 % (“bottom”). The data were fitted significantly better by a dose-response function than by a horizontal line (p=0.003, F-test). Two-way ANOVA revealed significant effects of stimulation (p<0.0001) and drug (p=0.002). Bonferroni's post-test: ** p < 0.01, *** p<0.001 compared with control.
The selective agonist of 5-HT1A receptors, 8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT) (Claustre et al., 1991; Crick and Wallis, 1991; Crick et al., 1994; Raiteri et al., 1991; Sprouse, 1991), produced a concentration-dependent inhibition of the MOR internalization evoked by the electrical stimulation (Fig. 1). The inhibition was only partial, leveling off at about 50 % of the control value with concentrations of 8-OH-DPAT of 0.1 μM and 1 μM. The effect of 8-OH-DPAT was statistically significant, because it significantly reduced MOR internalization at these two concentrations. Moreover, the data were fitted significantly better by the dose-response function than by a horizontal line (p=0.003, F-test). The IC50 of 8-OH-DPAT was 50 nM (95% confidence interval 4−677 nM, Log IC50 = −7.30 ± 0.55). No effect of 8-OH-DPAT on MOR internalization was observed in the contralateral dorsal horn (Fig. 2).
Figure 2. A 5-HT1A receptor antagonist reversed the inhibition produced by 8-OH-DPAT.
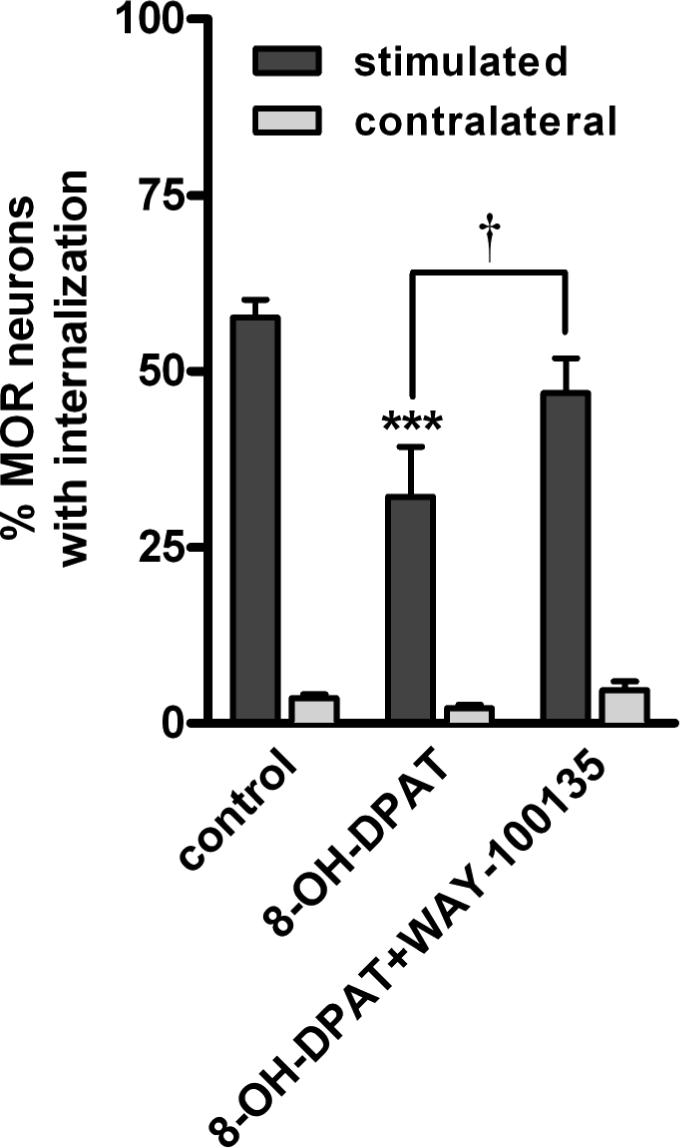
MOR internalization was evoked by dorsal horn stimulation with 1000 electrical pulses at 500 Hz. 8-OHDPAT (1 μM) decreased the evoked MOR internalization. This effect was reversed by the 5-HT1A antagonist WAY-100135 (10 μM). Data are the mean ± SEM of 3−12 replicates. Two-way ANOVA revealed a significant effect of stimulation (p<0.0001) and drugs (p<0.001). Bonferroni's post-test: *** p<0.001 compared with control, † p<0.05 compared to 8-OH-DPAT.
To determine whether the decrease produced by 8-OH-DPAT of the evoked MOR internalization was caused by inhibition of opioid release or by inhibition of MOR internalization itself, we studied the effect of 8-OH-DPAT on MOR internalization induced by the MOR agonist endomorphin-2. Spinal cord slices were incubated for 10 min at 35 °C with 100 nM endomorphin-2 in the absence or presence of 1 μM 8-OH-DPAT. A previous study (Song and Marvizon, 2003a) showed that 100 nM is the lowest concentration of endomorphin-2 that produces maximal MOR internalization. With endomorphin-2 alone, MOR internalization was present in 99.0 ± 1.0 % of the MOR neurons (n=3 slices); with endomorphin-2 and 8-OH-DPAT it was present in 99.5 ± 0.2 % of the MOR neurons (n=3 slices). Therefore, 8-OH-DPAT did not have any affect the MOR internalization induced by an exogenous agonist, and its inhibition of the MOR internalization evoked by electrical stimulation has to be attributed to inhibition of opioid release.
To confirm that the inhibition of opioid release produced by 8-OH-DPAT was mediated by 5-HT1A receptors, we determined whether it could be blocked by the selective 5-HT1A antagonist WAY-100135 (Fletcher et al., 1993a; Fletcher et al., 1993b; Fletcher et al., 1996). Indeed, 10 μM WAY-100135 superfused to the slices together with 1 μM 8-OH-DPAT brought the evoked MOR internalization back to control levels (Fig. 2). MOR internalization in the presence of 8-OH-DPAT plus WAY-100135 was significantly larger (p<0.05) than in the presence of 8-OH-DPAT alone and not significantly different from control. WAY-100135 could not have increased MOR internalization itself because it did not change MOR internalization in the contralateral dorsal horn (Fig. 2).
3. Discussion
This study shows that 5-HT1A receptors inhibit the release of opioid peptides in the spinal cord. We found that 8-OH-DPAT, a highly selective agonist of 5-HT1A receptors (Middlemiss and Fozard, 1983), inhibits MOR internalization evoked by electrical stimulation of the dorsal horn, which we used to measure opioid release (Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005). 8-OH-DPAT inhibits opioid release and not the internalization process itself, because it did not inhibit MOR internalization induced by the application of an agonist, endomorphin-2.
Because 8-OH-DPAT inhibited the evoked MOR internalization only partially, its concentration-response curve was quite shallow, which prevented us from calculating its IC50 with accuracy. Still, the IC50 for 8-OH-DPAT we obtained, 50 nM, was in the range of its IC50 values found in other studies of inhibitory effects mediated by 5-HT1A receptors. These included its inhibition of the neuronal firing rate of serotonergic dorsal raphe neurons (IC50= 7 nM) (Sprouse, 1991), the carbachol-stimulated phosphoinositide response in rat hippocampal slices (IC50 = 11 nM) (Claustre et al., 1991) and the monosynaptic reflex in the neonatal rat spinal cord (IC50= 850 nM) (Crick et al., 1994). The involvement of 5-HT1A receptors in the inhibition of opioid release was confirmed by the reversal of the effect of 8-OH-DPAT by WAY100135, a highly selective 5-HT1A antagonist active both pre- and postsynaptically (Fletcher et al., 1993a).
The fact that 8-OH-DPAT produced a partial inhibition of the evoked MOR internalization suggests that 5-HT1A receptors inhibit opioid release only from a subpopulation of opioid-containing terminals. Indeed, MOR internalization is likely induced by the release of both enkephalins and dynorphins (Song and Marvizon, 2003a), which are present in different presynaptic terminals in the dorsal horn (Standaert et al., 1986). Since mRNA for the 5-HT1A receptor colocalizes with enkephalin in the dorsal horn (Zhang et al., 2002), it is possible that 5-HT1A receptors inhibit enkephalin release but not dynorphin release. 5-HT1 receptors signal through Gi/o (Pauwels, 2000), which can inhibit opioid release by inactivating voltage-gated Ca2+ channels (Dolphin, 2003).
Serotonergic terminals in the spinal cord are of supraspinal origin (Stamford, 1995), with a substantial fraction of the serotonin content of the dorsal horn originating in the nucleus raphe magnus (Oliveras et al., 1977). Although for some time it was believed that the bulbospinal pain inhibitory pathway was serotonergic, it is now clear that the serotonergic neurons in the nucleus raphe magnus are different from the ON and OFF cells that mediate descending facilitation and inhibition, respectively, of pain responses (Gao and Mason, 2000). By inhibiting spinal opioid release, 5-HT1A receptors would decrease opioid-mediated analgesia, resulting in an overall pro-algesic effect. Previous studies on the effect of 5-HT1A receptors on pain responses have provided conflicting results, including both analgesic (Danzebrink and Gebhart, 1991; Eide et al., 1990; el-Yassir et al., 1988; el-Yassir and Fleetwood-Walker, 1990; Gjerstad et al., 1996; Oyama et al., 1996; Xu et al., 1994) and hyperalgesic effects (Alhaider and Wilcox, 1993; Ali et al., 1994; Crisp et al., 1991; Murphy and Zemlan, 1990; Solomon and Gebhart, 1988; Zemlan et al., 1988). Therefore, it is likely that 5-HT1A receptors produce multiple actions in the spinal cord with opposite results on pain modulation. Our results show that one of the pro-algesic actions of 5-HT1A receptors is the inhibition of opioid release from a subpopulation of enkephalin-containing presynaptic terminals.
4. Experimental Procedures
Animal procedures were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System, and conform to NIH guidelines. Efforts were made to minimize the number of animals and their suffering.
4.1. Spinal cord slices
Coronal spinal cord slices were prepared as previously described (Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005). Media used for the slices were: aCSF, containing (in mM) 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose; K+-aCSF, containing 5 mM of KCl, and sucrose-aCSF, the same as K+-aCSF except that NaCl was iso-osmotically replaced with sucrose. The spinal cord was extracted from 3−4 weeks old male Sprague-Dawley (Harlan, Indianapolis, IND) rats under isoflurane anesthesia. Coronal slices (400 μm) were cut with a Vibratome (Technical Products International, St. Louis, MO) in ice-cold sucrose-aCSF. Up to six slices from each animal were cut sequentially in the L1-L4 region.
4.2. Slice stimulation
To evoke opioid release, slices were stimulated electrically at the dorsal horn as described (Song and Marvizon, 2003b; Song and Marvizon, 2005). Slices were placed in a custom-made chamber and superfused with aCSF at 35 °C containing peptidase inhibitors (10 μM actinonin, captopril and phosphoramidon, with 6 μM dithiothreitol). The slice was held vertically with stainless steel insect pins, and a stimulating electrode was placed with its poles on either side of one dorsal horn. The shape and size of the stimulating electrode was such that it completely covered one of the dorsal horns (“hook” shape with 1 mm diameter, made from two 0.25 mm platinum/iridium parallel wires separated 1 mm; purchased from Frederick Haer & Co., Bowdoin, ME). The contralateral side of the slice was marked with a round hole in the ventral horn in order to recognize it in the histological sections. Electrical stimulation consisted of 1000 square pulses (30 V, 0.4 ms) delivered at 500 Hz, which was found to be optimal to evoke opioid release and MOR internalization (Song and Marvizon, 2003b). Peptidase inhibitors and other compounds were superfused to the slices starting 5 min before and ending 5 min after the stimulation, at which point the slices were put in ice-cold fixative (4 % paraformaldehyde, 0.18 % picric acid in 0.1 M sodium phosphate buffer).
2.3. Immunohistochemistry
Histological sections of 25 μm were cut from the slices and labeled as previously described (Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005). To label MORs we used a rabbit antiserum (1:7000 dilution) raised against amino acids 384−398 of the cloned rat MOR-1 (DiaSorin, Stillwater, MN, catalog no. 24216). This antiserum has been characterized (Arvidsson et al., 1995) and shown to label dorsal horn neurons (Spike et al., 2002). Pre-absorption of the MOR antibody with its immunizing peptide (10 μg/ml) abolished the staining. The secondary antibody was Alexa-488 goat anti-rabbit IgG (Molecular Probes, Eugene, OR), used at 1:2000 dilution for 2 hr at room temperature. Sections were mounted in Prolong (Molecular Probes).
2.4. Quantification of MOR internalization
MOR internalization was quantified as previously described (Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Song and Marvizon, 2005). MOR neurons were counted visually using a fluorescence microscope with a 100× objective, classifying them as with or without MOR internalization. Counting was done blind to the treatment. Neuronal somata with five or more endosomes were considered as having internalization. All MOR neurons of the stimulated and the contralateral dorsal horns were counted for each histological section, and 3−5 sections per slice (chosen randomly) were used. This amounted to 60−200 MOR neurons counted per hemi-slice (stimulated or contralateral side of a slice). Data from one hemi-slice was considered as one replicate measure for statistical purposes.
2.5. Data analysis
Data were analyzed using Prism 4.3 (GraphPad Software, San Diego, CA). Statistical analyses consisted of two-way ANOVA and Bonferroni's post-test, with significance set at 0.05. The first variable of the two-way ANOVA, to which the Bonferroni's post-test was applied, was “drugs”, and the second variable was “stimulation” (stimulated vs. contralateral side of the slice). Concentration-response data were fitted by the logistic dose-response function: Y= bottom + (top-bottom) / (1 + 10^(Log IC50-Log X)), where “top” and “bottom” are the maximum and minimum values, respectively, of the response, and IC50 is the dose that produces half of the maximum inhibition. Parameter constraints were bottom > 0 %. An F-test (Motulsky and Christopoulos, 2003) was used to determine whether the data were fitted significantly better by the dose-response function or a horizontal line.
Acknowledgements
Supported by grant 2 R01-DA012609 to J.C.M. from NIDA. We thank Kendrick Che and Orlando A. Perez for their help.
Abbreviations
- 5-HT
5-hydroxytriptamine
- 8-OH-DPAT
8-hydroxy-2-dipropylaminotetralin
- MOR
μ-opioid receptor
References
- Alhaider AA, Wilcox GL. Differential roles of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor subtypes in modulating spinal nociceptive transmission in mice. J. Pharmacol. Exp. Ther. 1993;265:378–385. [PubMed] [Google Scholar]
- Ali Z, Wu G, Kozlov A, Barasi S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Brain Res. 1994;661:83–90. doi: 10.1016/0006-8993(94)91184-3. [DOI] [PubMed] [Google Scholar]
- Chou J, Tang J, Del Rio J, Yang HY, Costa E. Action of peptidase inhibitors on methionine5-enkephalin-arginine6-phenylalanine7 (YGGFMRF) and methionine5-enkephalin (YGGFM) metabolism and on electroacupuncture antinociception. J. Pharmacol. Exp. Ther. 1984;230:349–352. [PubMed] [Google Scholar]
- Claustre Y, Benavides J, Scatton B. Potential mechanisms involved in the negative coupling between serotonin 5-HT1A receptors and carbachol-stimulated phosphoinositide turnover in the rat hippocampus. J. Neurochem. 1991;56:1276–1285. doi: 10.1111/j.1471-4159.1991.tb11422.x. [DOI] [PubMed] [Google Scholar]
- Crick H, Wallis DI. Inhibition of reflex responses of neonate rat lumbar spinal cord by 5-hydroxytryptamine. Br. J. Pharmacol. 1991;103:1769–1775. doi: 10.1111/j.1476-5381.1991.tb09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick H, Manuel NA, Wallis DI. A novel 5-HT receptor or a combination of 5-HT receptor subtypes may mediate depression of a spinal monosynaptic reflex in vitro. Neuropharmacology. 1994;33:897–904. doi: 10.1016/0028-3908(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Spanos LJ, Uram M, Perni VC, Donepudi HB. Analgesic effects of serotonin and receptor-selective serotonin agonists in the rat spinal cord. Gen. Pharmacol. 1991;22:247–251. doi: 10.1016/0306-3623(91)90441-8. [DOI] [PubMed] [Google Scholar]
- Danzebrink RM, Gebhart GF. Evidence that spinal 5-HT1, 5-HT2 and 5-HT3 receptor subtypes modulate responses to noxious colorectal distension in the rat. Brain Res. 1991;538:64–75. doi: 10.1016/0006-8993(91)90377-8. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol. Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J. Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide PK, Joly NM, Hole K. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536:195–200. doi: 10.1016/0006-8993(90)90025-7. [DOI] [PubMed] [Google Scholar]
- el-Yassir N, Fleetwood-Walker SM, Mitchell R. Heterogeneous effects of serotonin in the dorsal horn of rat: the involvement of 5-HT1 receptor subtypes. Brain Res. 1988;456:147–158. doi: 10.1016/0006-8993(88)90356-3. [DOI] [PubMed] [Google Scholar]
- el-Yassir N, Fleetwood-Walker SM. A 5-HT1-type receptor mediates the antinociceptive effect of nucleus raphe magnus stimulation in the rat. Brain Res. 1990;523:92–99. doi: 10.1016/0006-8993(90)91639-x. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, Haskins JT, Jones D, Mansell HL, Reilly Y. WAY100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur. J. Pharmacol. 1993a;237:283–291. doi: 10.1016/0014-2999(93)90280-u. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Cliffe IA, Dourish CT. Silent 5-HT1A receptor antagonists: utility as research tools and therapeutic agents. Trends Pharmacol. Sci. 1993b;14:41–48. doi: 10.1016/0165-6147(93)90185-m. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Fournie-Zaluski MC, Coric P, Turcaud S, Lucas E, Noble F, Maldonado R, Roques BP. “Mixed inhibitor-prodrug” as a new approach toward systemically active inhibitors of enkephalin-degrading enzymes. J. Med. Chem. 1992;35:2473–2481. doi: 10.1021/jm00091a016. [DOI] [PubMed] [Google Scholar]
- Gao KM, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J. Neurophysiol. 2000;84:1719–1725. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- Gjerstad J, Tjolsen A, Hole K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Waszak-McGee J, Mayer EA. Internalization of μ-opioid receptors in rat spinal cord slices. Neuroreport. 1999;10:2329–2334. doi: 10.1097/00001756-199908020-00020. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur. J. Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-Induced μ-Opioid Receptor Internalization in the Medial Preoptic Nucleus Is Mediated via Neuropeptide Y-Y1 Receptor Activation in the Arcuate Nucleus of Female Rats. J. Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Zemlan FP. Selective serotonin1A/1B agonists differentially affect spinal nociceptive reflexes. Neuropharmacology. 1990;29:463–468. doi: 10.1016/0028-3908(90)90168-q. [DOI] [PubMed] [Google Scholar]
- Noble F, Coric P, Fournie-Zaluski MC, Roques BP. Lack of physical dependence in mice after repeated systemic administration of the mixed inhibitor prodrug of enkephalin-degrading enzymes, RB101. Eur. J. Pharmacol. 1992a;223:91–96. doi: 10.1016/0014-2999(92)90822-l. [DOI] [PubMed] [Google Scholar]
- Noble F, Soleilhac JM, Soroca-Lucas E, Turcaud S, Fournie-Zaluski MC, Roques BP. Inhibition of the enkephalin-metabolizing enzymes by the first systemically active mixed inhibitor prodrug RB 101 induces potent analgesic responses in mice and rats. J. Pharmacol. Exp. Ther. 1992b;261:181–190. [PubMed] [Google Scholar]
- Noble F, Turcaud S, Fournie-Zaluski MC, Roques BP. Repeated systemic administration of the mixed inhibitor of enkephalin-degrading enzymes, RB101, does not induce either antinociceptive tolerance or cross-tolerance with morphine. Eur. J. Pharmacol. 1992c;223:83–89. doi: 10.1016/0014-2999(92)90821-k. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Bourgoin S, Hery F, Besson JM, Hamon M. The topographical distribution of serotoninergic terminals in the spinal cord of the cat: biochemical mapping by the combined use of microdissection and microassay procedures. Brain Res. 1977;138:393–406. doi: 10.1016/0006-8993(77)90680-1. [DOI] [PubMed] [Google Scholar]
- Oyama T, Ueda M, Kuraishi Y, Akaike A, Satoh M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neuroscience Res. 1996;25:129–135. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- Patierno S, Zellalem W, Ho A, Parsons CG, Lloyd KC, Tonini M, Sternini C. N-Methyl-d-aspartate receptors mediate endogenous opioid release in enteric neurons after abdominal surgery. Gastroenterology. 2005;128:2009–2019. doi: 10.1053/j.gastro.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ. Diverse signalling by 5-hydroxytryptamine (5-HT) receptors. Biochem. Pharmacol. 2000;60:1743–1750. doi: 10.1016/s0006-2952(00)00476-7. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Maura G, Barzizza A. Activation of presynaptic 5-hydroxytryptamine1-like receptors on glutamatergic terminals inhibits N-methyl-D-aspartate-induced cyclic GMP production in rat cerebellar slices. J. Pharmacol. Exp.Ther. 1991;257:1184–1188. [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J. Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RE, Gebhart GF. Mechanisms of effects of intrathecal serotonin on nociception and blood pressure in rats. J. Pharmacol. Exp. Ther. 1988;245:905–912. [PubMed] [Google Scholar]
- Song B, Marvizon JC. Peptidases prevent μ-opioid receptor internalization in dorsal horn neurons by endogenously released opioids. J. Neurosci. 2003a;23:1847–1858. doi: 10.1523/JNEUROSCI.23-05-01847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce μ-opioid receptor internalization in the rat spinal cord. J. Neurosci. 2003b;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. NMDA receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce μ-opioid receptor internalization in the rat spinal cord. Neuroscience. 2005;136:549–562. doi: 10.1016/j.neuroscience.2005.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse JS. Inhibition of dorsal raphe cell firing by MDL 73005EF, a novel 5-HT1A receptor ligand. Eur.J. Pharmacol. 1991;201:163–169. doi: 10.1016/0014-2999(91)90340-v. [DOI] [PubMed] [Google Scholar]
- Stamford JA. Descending control of pain. Br. J. Anaesth. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Watson SJ, Houghten RA, Saper CB. Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J. Neurosci. 1986;6:1220–1226. doi: 10.1523/JNEUROSCI.06-05-01220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the mu-opioid receptor: Responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J. Neurosci. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Wang MY, Dun NJ. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991;554:111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu XC, Han JS. Serotonin receptor subtypes in spinal antinociception in the rat. J. Pharmacol. Exp. Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]
- Zemlan FP, Behbehani MM, Murphy RM. Serotonin receptor subtypes and the modulation of pain transmission. Prog. Brain Res. 1988;77:349–355. doi: 10.1016/s0079-6123(08)62801-0. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Gao X, Ji GC, Huang YL, Wu GC, Zhao ZQ. Expression of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain. 2002;98:287–295. doi: 10.1016/S0304-3959(02)00026-X. [DOI] [PubMed] [Google Scholar]


