Abstract
Brother of the regulator of imprinted sites (BORIS) was previously described as a transcription factor for epigenetic reprogramming the expression of which is strictly confined to germ cells of adult testes but is aberrantly activated in the vast majority of neoplastic cells. Considering the critical role of BORIS in cancerogenesis and the fact that its expression pattern may preclude thymic tolerance, we generated DNA- and protein-based mouse BORIS antitumor vaccines using a non-DNA-binding version of the BORIS molecule. Clinical use of BORIS as a vaccine Ag would require that certain safety concerns be met. Specifically, administration of the functional BORIS protein would hypothetically pose a risk of BORIS accelerating the progression of cancer. To alleviate such safety concerns, we have developed vaccines based on the BORIS molecule lacking the DNA-binding zinc fingers domain. To enhance anti-BORIS cellular immune responses, we used a standard molecular adjuvant approach. It consisted of plasmids encoding murine IL-12 and IL-18 for a DNA-based vaccine and conventional Th1 type adjuvant, Quil A, for a protein-based vaccine. Both DNA- and protein-based vaccines induced Ag-specific CD4+ T cell proliferation with Th1 and Th2 cytokine profiles, respectively. Protein-based, but not DNA-based, BORIS vaccine induced a significant level of Ab production in immunized animals. Importantly, potent anticancer CD8+-cytotoxic lymphocytes were generated after immunization with the DNA-based, but not protein-based, BORIS vaccine. These cytolytic responses were observed across a wide range of different mouse cancers including mammary adenocarcinoma, glioma, leukemia, and mastocytoma.
Immunotherapy of cancer offers the hope for a natural non-toxic alternative to current conventional approaches that are characterized by high morbidity, inefficiency against metastasis, and development of drug resistance. Ironically, since the first well-characterized immunotherapy of cancer by William Coley more than a century ago, little clinical progress in treating a wide variety of tumors has been achieved (1). Although numerous anticancer vaccines have been developed utilizing a wide range of tumor-associated Ags (TAAs),4 several obstacles have not been overcome (2). Specifically, a state of tolerance has been ascribed to many tumor-associated self-Ags (3–5). One powerful strategy to overcome this hurdle is the combination of adoptive cell transfer, vaccination, and cytokine treatment. This approach, which induced the complete and long-lasting regression of large B16 tumors, suggests that large numbers of vaccine-activated, cytokine-driven, tumor-specific T cells are necessary for this regression (6). In addition, the antitumor potential of T cells may be increased by ex vivo improvement of their specificity, function, and survival via genetic modifications. However, even when tolerance is broken, tumors often mutate, causing loss of Ag expression and subsequent tumor immune escape (7). Accordingly, regardless of the immunotherapeutic strategy, the availability of an ideal target for an anticancer vaccine is extremely important. Historically, it has been believed that the ideal TAA should meet the following criteria: 1) expression restricted to neoplastic cells; 2) immunization induces therapeutic anticancer immune response; and 3) essentiality of the Ag for the function of the tumor such that tumor loss of the Ag would result in loss of tumor activity.
Numerous recent findings in the molecular biology of cancer suggest that the neoplastic phenotype arises and is maintained through genome-wide modifications associated with epigenetic changes (8, 9). Accordingly, several ongoing clinical trials are targeting histone deacetylases and DNA methyltransferases that modulate global gene expression (10, 11). We originally identified the CCCTC-binding factor (CTCF) as a transcription factor regulating c-myc expression (12, 13), which was subsequently found to act as a tumor suppressor (14–16) regulating expression of a wide variety of genes that are often involved in cancer development through regulation of cell growth and apoptosis (17, 18). For instance, CTCF target genes include members of the INK4a locus (19), hTERT (20), Rasgfr (21), IGFII, and H19 (22, 23) as well as a number of cell cycle- and cell death-modulating genes including p53 or p21 (24).
In addition to the direct regulation of gene promoters, the modus operandi of CTCF constitutively present in all somatic cells and lineage-specific regulated enhancer-blocking elements. These elements are universally characterized by clustered high affinity CTCF-binding sites found in all known vertebrate chromatin insulators, which maintain structure-functional autonomy of differentially expressed genomic loci. Moreover, imprinting control regions are regulated by methylation-dependent binding of CTCF to a subset of its target sites responsible for a parent-of-origin-dependent monoallelic expression of imprinted genes (17, 18).
More recently, we have cloned mouse and human brother of the regulator of imprinted sites (BORIS) gene, encoding a germ line testis-specific CTCF paralog containing same central DNA-binding domain but differing in N and C termini, thereby suggesting that BORIS could play a role of the interfering mutation of CTCF-driven regulatory pathways if it is abnormally expressed in somatic cells (18, 25). Indeed, we and others have found abnormal activation of BORIS in various human cancers, including both primary tumors and cancer cell lines (Refs. 23, 26, and 27 and our unpublished data). Moreover, it was shown that aberrantly expressed BORIS competes with CTCF for shared DNA target sites and can tether epigenetic modifications to and around such sites, thus resulting in modulation of gene expression (26, 27) and transformation of normal rodent fibroblasts in standard oncogenicity assays (our unpublished data). Therefore, BORIS can be classified as a unique cancer-testis (CT) gene with cell-transforming activity that is most likely mediated by competition with somatic tumor suppressor CTCF and by the function of BORIS in targeting epigenetic modifications (26).
Given the important role of BORIS in neoplastic cell transformation and in regulation of the other CT genes, we sought to investigate whether a vaccine based on the BORIS Ag can be an effective cancer treatment modality. To evaluate the anticancer immunity of BORIS Ag, we used a plasmid encoding the truncated mouse BORIS molecule (pmBORIS) (28) or the recombinant truncated BORIS (mBORIS) protein purified from Escherichia coli. Herein, we demonstrate that immunization with pmBORIS leads to Ag-specific Th1-type immune responses that can mediate cytotoxicity to a wide range of histologically unrelated tumors in an MHC class I-dependent manner, whereas immunization with mBORIS protein induces preferentially anti-BORIS Ab response. Cytotoxicity data indicating that this Ag is naturally processed and presented by tumor cells suggests the practicality of using this Ag that may be critical for the tumorogenicity and/or maintaining the oncogenic phenotype as a cancer vaccine. Thus, to our knowledge, we demonstrate for the first time the feasibility of using the tumor-promoting transcription factor BORIS for anticancer vaccine.
Materials and Methods
Mice
Female 8- to 10-wk-old BALB/c mice were purchased from The Jackson Laboratory. All animals were housed in a temperature- and light cycle-controlled facility, and their care was under the guidelines of the National Institutes of Health and the approved Institutional Animal Care and Use Committee protocol at the University of California (Irvine, CA).
Plasmids
pmBORIS encodes mBORIS with a deleted DNA-binding motif of the BORIS molecule to prevent possible adverse events due to inhibition of BORIS functional activity (28). Plasmids encoding molecular adjuvants IL-12 (pIL12; Invivogen Life Technologies) and IL-18 (pIL18; gift from Dr. R. Xiang, The Scripps Research Institute, La Jolla, CA) and pmBORIS were isolated using the EndoFree Plasmid maxikit (Qiagen). The purity of the plasmid DNA was assayed and confirmed via UV spectrophotometry and gel electrophoresis.
Purification of recombinant mBORIS protein
To prepare recombinant mBORIS, we subcloned the mBORIS gene into the E. coli expression vector pXi, kindly provided by Dr. P. Felgner (University of California). The mBORIS gene was amplified by PCR and inserted into the pXi vector in frame with N-terminal His tag by homologous recombination in the E. coli DH5α strain. For in vivo expression and large scale purification, an E. coli BL21(DE3) strain, transformed with the pXi-mBORIS plasmid, was grown in Luria broth with kanamycin (28°C, OD600 0.8). Protein synthesis was induced by isopropyl-β-d-thiogalactopyranoside (Calbiochem; 1 mM, 3–5 h). mBORIS protein were purified by affinity chromatography on a TALON metal affinity resin column (BD Biosciences; Clontech), and fractions were analyzed (10% Tris-SDS-PAGE). Positive fractions were combined, dialyzed against PBS, and concentrated by Centricon centrifugal filter devices (50,000 MWCO; Millipore). Purified protein has been used for immunization (protein vaccine), detection of anti-BORIS Abs, and proliferation assays.
Expression of BORIS in mouse cancer cell lines
Expression of BORIS protein in four different cancer cell lines and normal splenocytes was analyzed by FACS using affinity-purified chicken anti-BORIS Abs (25). These Abs have been previously well characterized, and the specificity of these Abs was demonstrated by blocking with peptide derived from BORIS protein (25). Briefly, cancer cells and splenocyte cultures from BALB/c mice were fixed and permeabilized using Perm/Fix Buffer (BD Pharmingen). After a washing, cells were stained first with primary Abs and then with FITC-conjugated goat anti-chicken secondary Abs (Abcam). Flow cytometry analyses were performed using a FACScan flow cytometer (BD Biosciences), and data were analyzed by CellQuest software (BD Biosciences).
Immunohistochemistry
Immunohistochemical analysis was performed essentially as described in Ref. 25. Briefly, paraffin-embedded tumor sections were boiled in a high pressure cooker for 4 min in citrate buffer. Then slides were treated with 3% hydrogen peroxide 20–30 min before blocking with 3% BSA. The primary Ab (affinity-purified chicken IgY anti-BORIS ap-2-Ab; dilution, 1/25) was applied on the tumor sections (overnight, 4°C). For negative control, a step with the primary Abs was omitted and replaced by 3% blocking solution in every set of slides stained. Sections were washed in PBS three times and incubated with biotinylated goat anti-chicken IgG (Vector Laboratories; dilution, 1/200; 1 h). The sections were washed, and the Vectastain ABC Elite kit was used for detection of positive staining as recommended by the manufacturer (Vector Laboratories).
Immunizations
Gold beads were coated with the purified pmBORIS or mixture of pmBORIS with plasmids encoding IL-12 and IL-18 molecular adjuvants (pIL12 and pIL18) and used for immunization with the gene gun (pmBORIS; 6 μg/mouse; pIL12 and pIL18, 3 μg/mouse each), as we described previously (28, 29). Two groups of mice were injected four times biweekly with pmBORIS or pmBORIS mixed with pIL12 and pIL18 (n = 3). One group of mice was immunized by s.c. injection with mBORIS protein formulated in QuilA four times biweekly (n = 3). Control groups (n = 3) were injected with pIL12 and pIL18, QuilA alone or nonimmunized. All experiments were repeated twice more with three mice in each group; representative data for each assay are shown in Results.
Analysis of anti-BORIS Ab responses
Total anti-BORIS Abs were detected as described previously (30, 31). Briefly, the wells of 96-well plates (Immulon II, Dynatech) were coated with 2.5 μM recombinant mBORIS protein (pH 9.7, 2 h, 37°C). The wells were washed and blocked, and serial dilutions of the primary sera from experimental and control mice were added to the wells. After incubation and washing, HRP-conjugated anti-mouse IgG was added as recommended by the manufacturer (The Jackson Laboratory). The reaction was developed by adding 3,3′,5,5′-tetramethylbenzidine (Pierce Chemical) substrate solution and stopped by 2 M H2SO4. All plates were analyzed spectrophotometrically at 450 nm (Thermo max; Molecular Devices). Amyloid-β peptide was used as an irrelevant Ag (2.5 μM) for coating of wells, and background absorption was subtracted from experimental wells. The isotypes of anti-mBORIS Abs were analyzed using HRP-conjugated Abs specific to mouse IgG1, IgG2a, IgG2b, and IgM Igs, and the IgG1-IgG2a ratio was calculated.
Analysis of T cell proliferation and production of cytokines
CD4+ T cell proliferation was analyzed by FACS. Briefly, to detect Ag-specific proliferation of CD4+ T cells, we stained splenocyte cultures with 2.5 μM succinimidyl ester of CFSE (Molecular Probes) for 10 min at 37°C. After a washing, cells were incubated for 3 days in AIM-V medium alone or with recombinant mBORIS protein (2 μM). After incubation, cultures were stained with PE-labeled rat anti-mouse CD4 mAbs (BD Pharmingen). Because dead cells might fluoresce nonspecifically, these cells were excluded from the assay using a nucleic acid dye (7-aminoactinomycin D; BD Pharmingen), and proliferation of viable cells was analyzed by a FACScan flow cytometer (BD Biosciences) according to the manufacturer's instructions. The CD4+ population was analyzed using CellQuest software (BD Biosciences). After overlaying of histograms generated with in vitro restimulated or nonstimulated splenocyte cultures, we calculated the δ (Δ = percent of proliferating CD4+ T cells in restimulated culture minus that in nonstimulated culture). Proliferation of total splenocytes was also analyzed using a [3H]thymidine incorporation assay as we described previously (30, 31).
ELISPOT was used to detect production of IFN-γ and IL-4 in restimulated splenocyte cultures from experimental mice as previously described by us (30, 31). Spots were counted using a dissecting microscope (Olympus) by two independent observers while blinded with respect to experiment condition, and the results were examined for differences between Ag-stimulated and nonstimulated cultures as described earlier (31). FACS was used to detect the intracellular cytokines by subsets of T cells as described (32). Briefly, the cultures of splenocytes from experimental and control animals were restimulated for 3–4 days with mBORIS protein (2 μM) and then for 4–6 h with PMA and ionophore (ionomycin), both Sigma-Aldrich. In addition we added brefeldin A (BD Biosciences) to block cytokine secretion, which increases intracellular accumulation. Surface staining was performed using FITC-labeled anti-mouse CD4 and CD8 mAb (BD Pharmingen); after a washing, cells were fixed and permeabilized, and IL-4 or IFN-γ production by CD4+ and CD8+ T cell subsets was detected using the appropriate PE-labeled anti-mouse Abs (BD Pharmingen). The percentages of CD4+IFN-γ+, CD4+IL-4+, and CD8+IFN-γ+ cells were calculated in populations of CD4+ or CD8+ T cell subsets using CellQuest software (BD Biosciences).
Detection of lymphocyte-mediated cytotoxicity
Flow cytometry was used to assess direct CTL activity in immunized mice as described (33). Briefly, immune spleens from experimental and control mice were isolated on day 8 after the last immunization, and splenocyte cultures were prepared in RPMI 1640 (Invitrogen Life Technologies) supplemented with 10% FBS, 100 U penicillin G, 100 μg/ml streptomycin sulfate, and 2 mM l-glutamine. To test the effector cell function, the freshly isolated splenocytes (effector cells) were mixed with target tumor cells labeled with CellTracker Green dye at E:T ratios of 50:1, 25:1, 12.5:1, and 6.25:1. As targets, we used four histologically different mouse tumor cell lines: mammary carcinoma (4T1); mastocytoma (P815); glioma (GL261); and T cell lymphoma (EL4). After a 3-h coincubation, the E:T mixtures were washed, fixed, and permeabilized before staining with PE-labeled anti-caspase-3 Abs (BD Pharmingen). After incubation (20 min, 4°C) and washing, the number of activated caspase-3-positive apoptotic cells was detected in CellTracker Green-positive target cells population, and then the percentage of apoptotic cells was calculated using CellQuest software.
Statistical analysis
All statistical parameters (average values, SD, significant differences between groups) were calculated using GraphPad Prism 3.0 Software. Statistical significance between groups was determined by one-way ANOVA with Tukey's multiple comparison posttest (p < 0.05 was considered significant).
Results
Induction of CD4+T cell proliferation and Ab responses in mice immunized with DNA and protein-based BORIS vaccines
Clinical use of BORIS as a vaccine Ag would require certain safety concerns to be met. Specifically, administration of the functional BORIS protein would hypothetically pose the risk of BORIS accelerating progression of cancer, especially if DNA vaccines were used. To alleviate such safety concerns, we have developed DNA (38) and protein vaccines based on mutant BORIS lacking the DNA-binding zinc fingers domain, but maintaining aa 1–258 and 572–636. Furthermore, to enhance anti-BORIS cellular immune responses, we have used a standard molecular adjuvant approach consisting of plasmids encoding murine IL-12 and IL-18 for DNA-based vaccine and conventional adjuvant QuilA for protein-based vaccine. In our experiments, we used these molecular adjuvants, because previously it was demonstrated that: 1) IL-12 and IL-18 cytokines are differentially effects on IFN-γ production in primary T cells at the transcriptional level; 2) although IL12 induces substantial levels of IFN-γ, the presence of IL-18 significantly enhanced production of this important Th1 cytokine; and 3) only a small amount of IL-12 is required for effective protection against different pathogens in the presence of IL-18 (34–37).
BALB/c mice were immunized with pmBORIS, pmBORIS mixed with pIL12/pIL18, or mBORIS protein formulated in conventional Th1-type adjuvant QuilA, and cellular and humoral immune responses were analyzed. Control mice were injected with empty vector mixed with pIL12 and pIL18 or QuilA alone, or they remained nonimmunized. As demonstrated in Fig. 1A, immunization with pmBORIS, pmBORIS mixed with pIL12/IL18, or mBORIS protein induced strong Ag-specific proliferation of CD4+ subset of T cells. In splenocytes isolated from mice immunized with pmBORIS vaccine mixed with molecular adjuvants, we detected higher levels of CD4+ T cell proliferation than in immune splenocytes isolated from mice immunized with pmBORIS alone (Fig. 1A). Strong proliferation of immune splenocytes isolated from mice immunized with DNA or protein-based vaccines was confirmed in a separate experiment by traditional thymidine incorporation assay (Fig. 1B). However, analysis of humoral immune response demonstrated that only mice immunized with protein induced a high titer of BORIS-specific Ab (Fig. 1C). In addition to the magnitude of humoral immune responses, we also analyzed the isotype of anti-BORIS Abs, given that this characteristic has previously been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines to the immune response (38–40). Immunizations with mBORIS protein or pmBORIS/pIL12/pIL18 did not induce significant levels of IgM or IgG2b Abs (data not shown). At the same time mBORIS protein generated predominantly a Th2 phenotype (IgG1-IgG2a ratio, 9.6) in experimental Th2-prone mice (41), whereas DNA immunization shifted it toward Th1 phenotype (IgG1-IgG2a ratio, 2.1). Thus, despite the fact that the IgG1-IgG2a ratio was still >2, this ratio declined ∼5-fold in DNA-immunized mice compared with animals injected with mBORIS protein, primarily due to suppression of the IgG1 response in these Th2-prone mice.
FIGURE 1.
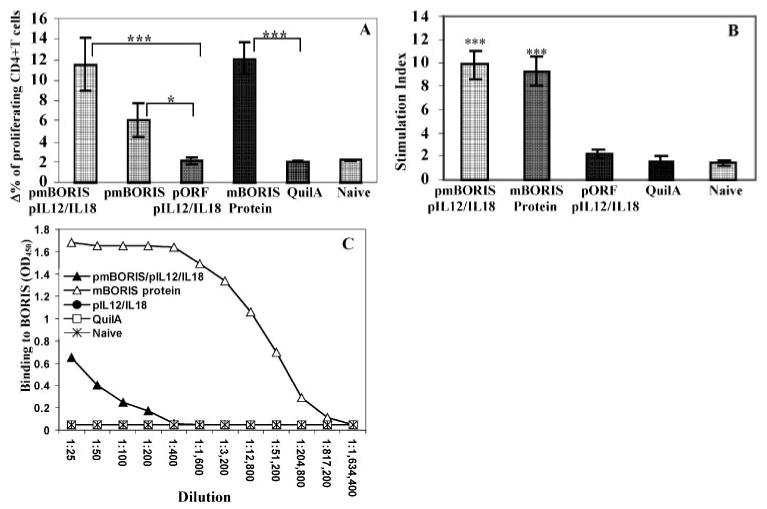
Mice immunized with pmBORIS/pIL12/IL18, pmBORIS, or mBORIS protein formulated in QuilA induced Ag-specific proliferation of immune CD4+ cells detected by flow cytometry (A) and immune splenocytes detected by [3H]thymidine assay (B). Only mice immunized with mBORIS formulated in conventional adjuvant QuilA induced high titers of BORIS-specific Ab (C). Data are the representative results from one experiment (n = 3 for each group), and similar results were obtained in two other experiments (n = 3 for each group in each experiment). Bars, SD. ***, p < 0.001; * p < 0.05.
DNA vaccine encoding mBORIS protein induces Th1 type immune responses
To detect Th1 and Th2 profile of cytokines we analyzed production of IFN-γ and IL-4 lymphokines in the splenocyte cultures from immunized and control mice using ELISPOT. Groups of mice injected with DNA-based vaccine formulated in molecular adjuvant induced significantly higher IFN-γ than IL-4 responses (p > 0.001), whereas equal numbers of cells producing both cytokines were detected in cultures of splenocytes isolated from mice immunized with mBORIS protein (Fig. 2). As expected, splenocytes from naive mice or animals immunized only with adjuvants did not generate significant levels of IL4 or IFN-γ cytokines (Fig. 2). These data support results described in Fig. 1 and suggest that DNA- and protein-based vaccines induced different subsets of Th cells.
FIGURE 2.
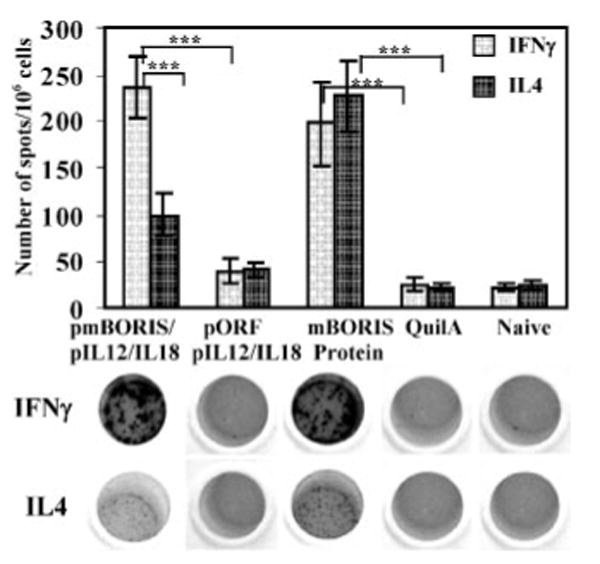
mBORIS-based DNA vaccine activated significantly higher numbers of IFN-γ- than IL-4-producing splenocytes, whereas protein vaccine activated an equal number of cells producing IFN-γ or IL-4. Representative photographs of ELISPOT wells are presented. Experiments were performed three times (n = 3 for each group in each experiment). Data are the average results from three independent experiments. Bars, SD. ***, p < 0.001.
Because Th1 responses have been implicated in antitumor immunity, whereas Th2 type responses attenuate cell-mediated immunity and enhance humoral immunity, we decided to further analyze production of cytokines by CD4+ T cells only in mice immunized with DNA-based mBORIS vaccine. We demonstrated that both pmBORIS and pmBORIS/pIL12/IL18 Ags induced robust production of Th1 cytokine (IFN-γ) by CD4+ T cells (Fig. 3), although immunization of mice with pmBORIS mixed with pIL12/IL18 induced higher numbers of IFN-γ producing CD4+ T cells than immunization with pmBORIS alone. We also analyzed production of the Th2 cytokine (IL-4) by CD4+ T cells from mice immunized with pmBORIS or pmBORIS plus pIL12/IL18. It was apparent that both groups of mice possessed significantly lower numbers of CD4+T cells producing IL-4 than IFN-γ (p < 0.001), indicating that both formulations of BORIS-based vaccine induced Th1-type immune responses even in Th2-prone BALB/c mice. Next, we demonstrated that immunizations with pmBORIS and pmBORIS/pIL12/IL18 induced a significant number of CD8+ T cells producing this Th1 cytokine. pmBORIS mixed with pIL12/IL18 was more potent in inducing higher numbers of IFN-γ-producing CD8+ T cells than immunization with pmBORIS Ag without molecular adjuvant (Fig. 4).
FIGURE 3.
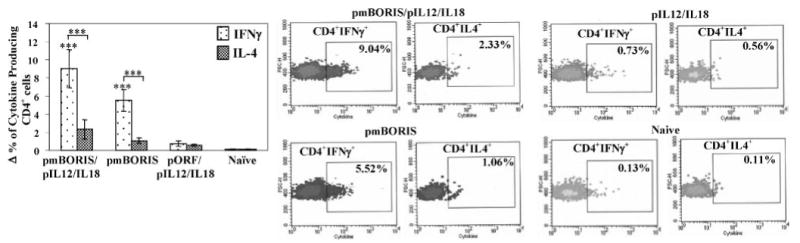
mBORIS-based DNA vaccine induces production of IFN-γ and IL-4 by Ag-specific CD4+ cells isolated from immune mice. Cytokine production by CD4+ cells was detected by flow cytometry (only gated populations of CD4+ cells are presented). The percent of CD4+ IFN-γ+, CD4+ IL-4, cells was calculated by subtraction of background (Δ) (percent of cytokine-producing CD4+ cells in nonrestimulated splenocyte culture). Experiments were performed three times (n = 3 for each group in each experiment). Data are the average results from three independent experiments. Bars, SD. ***, p < 0.001.
FIGURE 4.
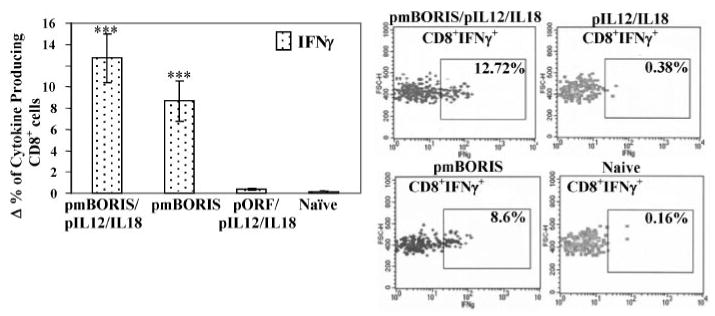
mBORIS-based DNA vaccine induces production of IFN-γ by Ag-specific CD8+ cells isolated from immune mice. The percent of CD8+IFN-γ+ cells was calculated by subtraction of background (Δ) (percent of cytokine-producing CD8+ cells in nonrestimulated splenocyte culture). Experiments were performed three times (n = 3 for each group in each experiment). Data are the average results from three independent experiments. Error bars, SD. ***, p < 0.001.
DNA vaccine encoding mouse mBORIS induces cytolytic cells capable of killing histologically distinct tumor cell lines by MHC class I-restricted manner
Previously we reported the expression of BORIS transcript in 4T1 mammary carcinoma, GL261 glioma and P815 mastocytoma cell lines (28); however, expression of BORIS protein in these cells was not demonstrated. To use these histologically unrelated tumor cells as targets in CTL assays, we first analyzed the expression of BORIS protein in all these types of mouse tumor cells of the H-2d immune haplotype and in the T cell lymphoma line (EL4) of H-2b immune haplotype. Using FACS and affinity-purified chicken anti-BORIS Abs, which bind to both human and mouse BORIS molecules (25), we demonstrated intracellular expression of mouse BORIS protein in all types of cancer cell lines, but not in normal splenocytes (Fig. 5).
FIGURE 5.
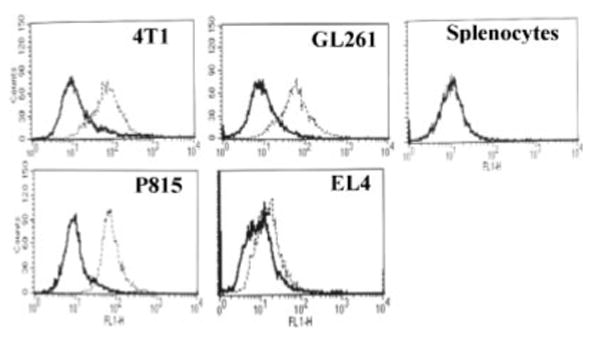
Detection of BORIS protein in histologically unrelated tumor cell lines by flow cytometry using anti-BORIS chicken Ab. Dotted line, anti-BORIS primary Abs followed by secondary FITC-anti-chicken Abs; bold line, secondary Abs only. FL1-H, Fluorescence.
To assess the cytolytic activity of immune splenocytes, we used the recently developed flow cytometry assay to detect effector cell-induced caspase activation within individual target cells (33). Target cells used consisted of four histologically distinct mouse tumor cell lines: mammary carcinoma; mastocytoma; glioma; and T cell lymphoma. Notably, all cell lines, except EL4 have MHC class I matching with effector cells (H2d), whereas the EL4 T cell lymphoma line has the H-2b background. Freshly isolated immune splenocytes from mice vaccinated with pmBORIS plus pIL12/IL18 or pmBORIS killed 25 and 12% of 4T1 target cells, respectively, at E:T 50:1. Importantly, immune lymphocytes isolated from mice immunized with pmBORIS/pIL12/pIL18 killed 4T1 target cells even at a E:T 25:1. Immunization with pmBORIS alone induced a lower number of cytolytic cells than vaccination with pmBORIS mixed with pIL12/IL18 (Fig. 6A). The same effector cells from immune mice killed only target cells of H-2d haplotype, but not EL-4 cells of H-2b haplotype (Fig. 6B). In other words, the killing of unmodified target cells endogenously expressing BORIS molecules was MHC class I restricted. However, one may argue that the immune splenocytes did not kill EL4 cells, because of lower level of BORIS expression by this cell line compared with that of other mouse tumor cell types (Fig. 5). Thus, to directly demonstrate that the E:T interaction is MHC class I restricted, we blocked the MHC class I interaction with TCR using anti-mouse H-2Dd mAbs. At concentrations of 1 μg/106 cells, this Ab completely inhibited the killing of 4T1 tumor cells (Fig. 6C), indicating that the cytolytic activity observed was mediated in a MHC class I-restricted manner. As we expected from our data described in this article, protein-based mBORIS vaccine that induced the Th2 type of immune response did not induce cytolytic responses at all (Fig. 6D).
FIGURE 6.
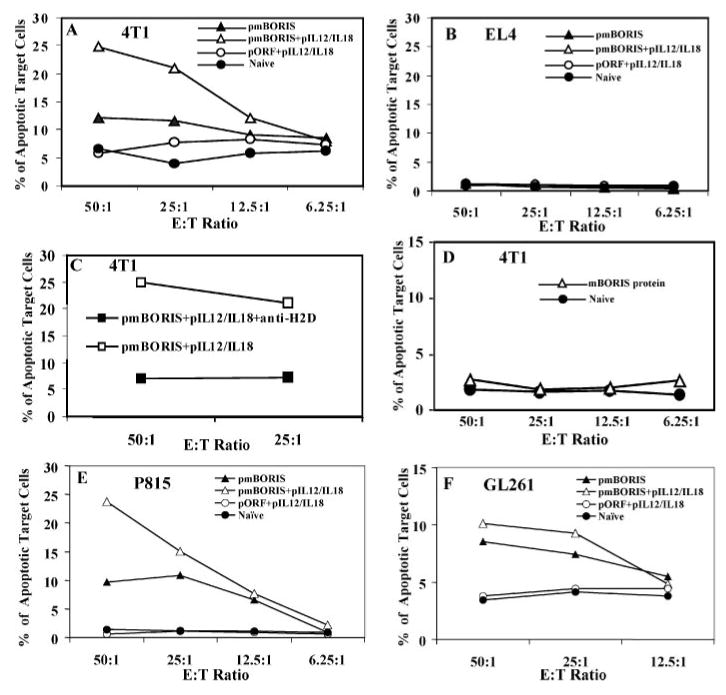
Immunizations with pmBORIS but not protein vaccines induced Ag-specific and MHC I-restricted direct cytotoxicity. Immune splenocytes (effector cells) isolated from mice (H-2d) immunized with pmBORIS/pIL12/IL18 or pmBORIS killed only MHC-class I-matched 4T1 (H-2d) (A) but not non-matched EL4 (H-2b) (B) target tumor cells. Blocking of MHC class I decreased the percent of target cells killing to background level (C). Immune splenocytes isolated from mice immunized with mBORIS protein did not kill MHC-class I-matched 4T1 tumor cells (D). Immune splenocytes from immunized mice killed also MHC class I-matched (H-2d) P815 mastocytoma (E) and GL261 glioma (F) target tumor cells as well. Experiments were performed three times (pools of splenocytes from three mice for each group in each experiment were analyzed). Data are the representative results from one experiment (similar results were obtained in two other experiments (n = 3 for each group in each experiment)).
Next we hypothesized that because BORIS is expressed in various types of mouse cancer cells, immune splenocytes from vaccinated mice should be able to kill all MHC-matched tumor cells. Accordingly, we coincubated immune splenocytes isolated from mice vaccinated either with pmBORIS or pmBORIS plus pIL12/IL18 with P815 or GL261 lines and demonstrated that these target cells can be killed by effector cells (Fig. 6, E and F). We did not find mouse tumors that lack expression of the BORIS molecule; however, because control nonimmunized mice or animals injected with pIL12/pIL18 did not possess cytolytic activity, we argue that our vaccination-induced Ag-specific CD8+ T cells.
Discussion
In this study, we described for the first time the immunogenic potential of a cancer vaccine based on the unique CT Ag BORIS which has the following important properties: 1) BORIS, being a paralog of a widely expressed cell growth suppressor CTCF gene, represents an instant CTCF-interfering mutant because both factors share the same central DNA-binding domain (which in both proteins accounts for one-third of the total length of each polypeptide), flanked by completely distinct N and C termini. Accordingly, to avoid competition resulting in malfunctions of CTCF-binding sites, the expression of BORIS is normally restricted to CTCF-deficient germ cells of adult testicular tissue (25); 2) in various histologically distinct neoplastic cells, BORIS is abnormally activated (23, 26, 27); 3) BORIS functionally acts as a factor that induces derepression of many genes associated with malignancy, including other CT genes such as MAGE-A1, NY-ESO-1, as well as the Oct-4 gene (26, 27); 4) because aberrant expression of this downstream BORIS target gene, Oct-4, is considered to mark so-called cancer stem cells (42–44), BORIS is likely to be expressed very early in the carcinogenesis process, i.e., upstream of the Oct-4 activation; 5) conditional expression of BORIS in normal rodent fibroblasts (cells were forced to express ectopic BORIS) results in attaining all classic features for cell-transformation (S. Pack et al. manuscript in preparation). Thus, because of the important role of BORIS in initiating and possibly maintaining of the oncogenic phenotype, as well as on the fact that its expression may preclude thymic tolerance, we initiated this study and generated DNA and protein vaccines based on truncated non-DNA-binding BORIS (mBORIS).
Immunization with protein induced high titers of BORIS-specific Ab (Fig. 1C) and Th2-type cytokine responses (Fig. 2), but not cytolytic activity (Fig. 6). In contrast, vaccination with pmBORIS induced potent Ag-specific proliferative (Fig. 1, A and B), Th1-type cytokine (Fig. 2), and cytolytic responses (Fig. 6). Previously, it was shown that immunization of mice with recombinant SV40 protein or DNA encoding this viral Ag induced Th2- and Th1-type responses, respectively (45, 46). Our results with a novel CT-Ag BORIS support this observation. Additionally, as it was previously shown with other immunogens (34–37) the mixture of pIL12 and pIL18 molecular adjuvants augmented Ag-specific cellular immune responses in mice immunized with pmBORIS (Figs. 1–4 and 6). More importantly, cytolytic responses were observed across a wide range of histologically unrelated mouse tumors (Fig. 6) including mammary adenocarcinoma (4T1), glioma (GL261), and mastocytoma (P815), which naturally expressed BORIS molecule (Fig. 5). Effector cells generated after vaccination with both pmBORIS and pmBORIS/pIL12/pIL18 was CD8+CTL because they killed target cells in an MHC class I-restricted manner (Fig. 6). This observation is very important because it is known that induction of the Th1-type CD4+ T cell and/or CD8+CTL responses are critical to achieve effective therapeutic antitumor vaccination.
Although a majority of TAAs are expressed in tumor cells in usual or aberrant forms, TAA are typically also expressed on a variety of normal cells. Thus, they are recognized by the immune system as self-molecules, and the immune system has protective mechanisms for preventing self-tissue Ag recognition and autoimmune responses. Therefore, to successfully induce antitumor cellular immune responses, the breaking of immune tolerance to such tumor TAAs is essential (47). Secondly, even in cases when tolerance can be broken by immunization, tumors often mutate immunogenic epitopes so as to evade immune attack or in some cases completely lose expression of the Ag itself resulting in immune escape (7, 48). Finally, another limitation of vaccines based on BrCA-relevant TAAs is that such Ags may induce potentially harmful autoimmunity through cross-reactivity with self-Ags. Because current data indicate that immune protection against cancers requires the generation of strong cellular responses against TAAs expressed by the malignant cells, it is not surprising that peptide, viral, DNA, and even dendritic loaded anticancer vaccines based on different self-Ags were not very effective (49–52). An ideal TAA should be strongly immunogenic, expressed in tumor cells but not in normal tissues, be crucial for the maintenance of the oncogenic phenotype of cancer cells, such that tumor loss of the specific Ag would result in loss of tumor activity. Several Ags, expressed by the novel class of so-called CT genes, meet to these criteria (53). One such CT gene, BORIS, recently described by us (25) indeed appears to be very immunogenic (Figs. 1–6) even in the Th2-prone BALB/c mice (54, 55). As we mentioned above, the expression of BORIS is normally restricted to germ cells of adult testicular tissue, and therefore T cells specific to BORIS should not be tolerized in females. At the same time, although a testis is an immune-privileged organ we presume that BORIS-specific T cells are not tolerized also in males.
The transcription of BORIS not only was increased abnormally in many different human cancers (25–27), but it also induced demethylation and subsequent expression of many other CT genes, as well as Oct-4, indicating that it is expressed very early in malignancy (26, 27). Although we did not detect BORIS transcript in any tested normal tissues (25–27), Scanlan et al. (56) were still able to find traces of cDNA in pancreas (0.03 μg), prostate (0.01 μg), thymus (0.01 μg), and kidney (0.01 μg), which were >100 times lower than those found in the testis (>2 μg) (56). We suggest that the presence of such small amounta of transcripts need not necessarily correlate with synthesis of protein. To confirm this suggestion, we immunostained several different normal and cancerous tissues and showed that BORIS protein was expressed only in lung, breast, prostate, and pancreas cancers, but not in normal tissues (Fig. 7). Although low levels of BORIS protein undetectable by immunohistochemistry may present in normal cells, we suggest that this is not enough for presentation of immune peptide to the T cells in primary lymphoid organs or in thymus. In other words, expression pattern of BORIS protein may preclude thymic tolerance. Interestingly, the same results was described with MAGE-A1 Ag (presence of traces of cDNA but not protein in pancreas; Refs. 53 and 56) and authors came to the same conclusion, indicating that extremely low levels of CT Ag is unlikely to result in the presence of immunologically relevant levels of MHC/CT peptide complexes.
FIGURE 7.
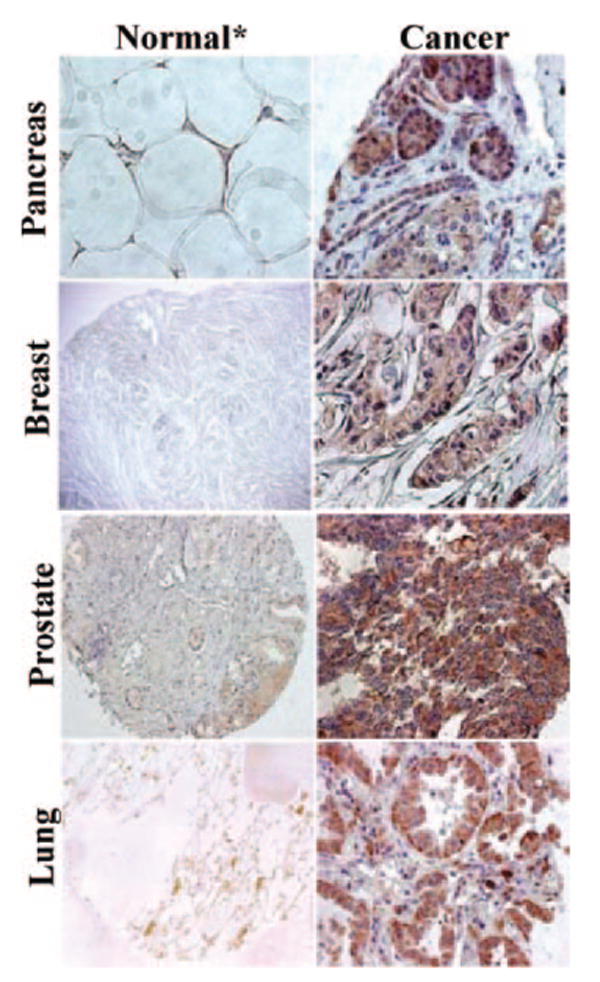
Immunohistochemical patterns of BORIS expression in different human normal and cancer tissues. BORIS protein was detected by using chicken anti-BORIS Abs visualized by secondary biotinylated goat anti-chicken Abs and diaminobenzidine chromogen detection. *, In prostate panel, benign prostatic hyperplasia (BPH) tissue was used instead of normal.
In conclusion, our immunological analysis demonstrated that the novel BORIS CT Ag is highly immunogenic and is expressed in all types of human and mouse tumor cells analyzed thus far (25) but not in normal tissues. Thus, our results demonstrate for the first time the feasibility of vaccination with a cancer-associated epigenetic regulator for induction of T cytotoxic immunity against histologically unrelated tumors. Because DNA vaccines have traditionally induced lower immune responses in large animals and humans than that in mice, we are also evaluating additional strategies based on mBORIS. We believe that highly efficient antitumor CTLs may be generated using dendritic cells (DC) pulsed with mBORIS protein fused with protein transduction domain (PTD). Recently, it was demonstrated that another CT Ag, NY-ESO-1, fused with PTD and purified from E. coli was taken up by monocyte-derived DC and presented to human T cells through the MHC class I pathway (57). Authors showed that DC loaded with PTD-NY-ESO-1 induced CD8+ cellular antitumor immunity superior to that induced by DC loaded with NY-ESO-1 protein. Thus, we suggest that PTD will deliver fused mBORIS across the DC membrane directly into the cell cytosol, and this immunogen will be processed and presented to CD8+ CTL through MHC class I-restricted pathway as demonstrated for PTD-NY-ESO-1 (57).
We suggest that the strategy based on using the whole mBORIS Ag instead of a small peptide will not be restricted to specific HLA types and that the selection of T cell epitopes in vivo will occur naturally. Additionally, we believe that the identification of a highly immunogenic target Ag is extremely important not only for anticancer vaccination but also for ex vivo generation of tumor-specific CD8+ CTL for adoptive cell transfer (58, 59) currently successfully used on the clinical side. Collectively, our immunological data along with other published results on the BORIS molecule indicate that it is an attractive candidate for the immunotherapy of cancer.
Acknowledgments
We thank A. Karapetyan for technical help.
Footnotes
This work was supported by National Institutes of Health Grants RO1 AI-44809, RO1 AG-20241, and RO1 NS-050895.
Abbreviations used in this paper: TAA, tumor-associated Ag; BORIS, brother of the regulator of imprinted sites; CTCF, CCCTC-binding factor; pmBORIS, plasmid encoding the truncated mouse BORIS molecule; mBORIS, mouse mutated BORIS; CT, cancer-testis; DC, dendritic cell; PTD, protein transduction domain.
Disclosures: M. G. Agadjanyan has stock in OncoMune Incorporated, and together with A. Ghochikyan, they have patent no. US2004/027856/WO2005/021029. V. V. Lobanenkov and D. Loukinov have patent No. WO2003/072799.
References
- 1.Hoption Cann SA, van Netten JP, van Netten C. Dr. William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- 2.Graziano DF, Finn OJ. Tumor antigens and tumor antigen discovery. Cancer Treat Res. 2005;123:89–111. doi: 10.1007/0-387-27545-2_4. [DOI] [PubMed] [Google Scholar]
- 3.Cheng F, Gabrilovich D, Sotomayor EM. Immune tolerance in breast cancer. Breast Dis. 2004;20:93–103. doi: 10.3233/bd-2004-20111. [DOI] [PubMed] [Google Scholar]
- 4.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 5.Ruffini PA, Biragyn A, Kwak LW. Recent advances in multiple myeloma immunotherapy. Biomed Pharmacother. 2002;56:129–132. doi: 10.1016/s0753-3322(02)00169-5. [DOI] [PubMed] [Google Scholar]
- 6.Overwijk WW. Breaking tolerance in cancer immunotherapy: time to ACT. Curr Opin Immunol. 2005;17:187–194. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Kerkmann-Tucek A, Banat GA, Cochlovius B, Zoller M. Antigen loss variants of a murine renal cell carcinoma: implications for tumor vaccination. Int J Cancer. 1998;77:114–122. doi: 10.1002/(sici)1097-0215(19980703)77:1<114::aid-ijc18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.El-Osta A. Mechanisms of abnormal gene expression in tumor cells. EXS No. 2006;96:351–361. doi: 10.1007/3-7643-7378-4_15. [DOI] [PubMed] [Google Scholar]
- 9.Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst. 2005;97:1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 10.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 11.Murgo AJ. Innovative approaches to the clinical development of DNA methylation inhibitors as epigenetic remodeling drugs. Semin Oncol. 2005;32:458–464. doi: 10.1053/j.seminoncol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, Polotskaja AV, Goodwin GH. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. [PubMed] [Google Scholar]
- 13.Filippova GN, Fagerlie S, Klenova EM, Myers C, Dehner Y, Goodwin G, Neiman PE, Collins SJ, Lobanenkov VV. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol. 1996;16:2802–2813. doi: 10.1128/mcb.16.6.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippova GN, Lindblom A, Meincke LJ, Klenova EM, Neiman PE, Collins SJ, Doggett NA, Lobanenkov VV. A widely expressed transcription factor with multiple DNA sequence specificity, CTCF, is localized at chromosome segment 16q22.1 within one of the smallest regions of overlap for common deletions in breast and prostate cancers. Genes Chromosomes Cancer. 1998;22:26–36. [PubMed] [Google Scholar]
- 15.Rasko JE, Klenova EM, Leon J, Filippova GN, Loukinov DI, Vatolin S, Robinson AF, Hu YJ, Ulmer J, Ward MD, et al. Cell growth inhibition by the multifunctional multivalent zinc-finger factor CTCF. Cancer Res. 2001;61:6002–6007. [PubMed] [Google Scholar]
- 16.Filippova GN, Qi CF, Ulmer JE, Moore JM, Ward MD, Hu YJ, Loukinov DI, Pugacheva EM, Klenova EM, Grundy PE, et al. Tumor-associated zinc finger mutations in the CTCF transcription factor selectively alter its DNA-binding specificity. Cancer Res. 2002;62:48–52. [PubMed] [Google Scholar]
- 17.Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 18.Klenova EM, Morse HC, 3rd, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol. 2002;12:399–414. doi: 10.1016/s1044-579x(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 19.Filippova GN, Thienes CP, Penn BH, Cho DH, Hu YJ, Moore JM, Klesert TR, Lobanenkov VV, Tapscott SJ. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat Genet. 2001;28:335–343. doi: 10.1038/ng570. [DOI] [PubMed] [Google Scholar]
- 20.Renaud S, Loukinov D, Bosman FT, Lobanenkov V, Benhattar J. CTCF binds the proximal exonic region of hTERT and inhibits its transcription. Nucleic Acids Res. 2005;33:6850–6860. doi: 10.1093/nar/gki989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon B, Herman H, Hu B, Park YJ, Lindroth A, Bell A, West AG, Chang Y, Stablewski A, Piel JC, et al. Rasgrf1 imprinting is regulated by a CTCF-dependent methylation-sensitive enhancer blocker. Mol Cell Biol. 2005;25:11184–11190. doi: 10.1128/MCB.25.24.11184-11190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 23.Ulaner GA, Vu TH, Li T, Hu JF, Yao XM, Yang Y, Gorlick R, Meyers P, Healey J, Ladanyi M, Hoffman AR. Loss of imprinting of IGF2 and H19 in osteosarcoma is accompanied by reciprocal methylation changes of a CTCF-binding site. Hum Mol Genet. 2003;12:535–549. doi: 10.1093/hmg/ddg034. [DOI] [PubMed] [Google Scholar]
- 24.Qi CF, Martensson A, Mattioli M, Dalla-Favera R, Lobanenkov VV, Morse HC., 3rd CTCF functions as a critical regulator of cell-cycle arrest and death after ligation of the B cell receptor on immature B cells. Proc Natl Acad Sci USA. 2003;100:633–638. doi: 10.1073/pnas.0237127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, et al. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci USA. 2002;99:6806–6811. doi: 10.1073/pnas.092123699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H, 3rd, Schrump DS, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–7762. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 27.Hong JA, Kang Y, Abdullaev Z, Flanagan PT, Pack SD, Fischette MR, Adnani MT, Loukinov DI, Vatolin S, Risinger JI, et al. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–7774. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- 28.Loukinov D, Ghochikyan A, Mkrtichyan M, Ichim TE, Lobanenkov VV, Cribbs DH, Agadjanyan MG. Antitumor efficacy of DNA vaccination to the epigenetically acting tumor promoting transcription factor BORIS and CD80 molecular adjuvant. J Cell Biochem. 2006;98:1037–1043. doi: 10.1002/jcb.20953. [DOI] [PubMed] [Google Scholar]
- 29.Ghochikyan A, Vasilevko V, Petrushina I, Movsesyan N, Babikyan D, Tian W, Sadzikava N, Ross TM, Head E, Cribbs DH, Agadjanyan MG. Generation and characterization of the humoral immune response to DNA immunization with a chimeric β-amyloid-interleukin-4 minigene. Eur J Immunol. 2003;33:3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLADR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 32.Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–124. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Packard BZ, Brown MJ, Komoriya A, Feinberg MB. Assessment of lymphocyte-mediated cytotoxicity using flow cytometry. Methods Mol Biol. 2004;263:125–140. doi: 10.1385/1-59259-773-4:125. [DOI] [PubMed] [Google Scholar]
- 34.Triccas JA, Sun L, Palendira U, Britton WJ. Comparative affects of plasmid-encoded interleukin 12 and interleukin 18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol Cell Biol. 2002;80:346–350. doi: 10.1046/j.1440-1711.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 35.Tapia E, Perez-Jimenez E, Lopez-Fuertes L, Gonzalo R, Gherardi MM, Esteban M. The combination of DNA vectors expressing IL-12 + IL-18 elicits high protective immune response against cutaneous leishmaniasis after priming with DNA-p36/LACK and the cytokines, followed by a booster with a vaccinia virus recombinant expressing p36/LACK. Microbes Infect. 2003;5:73–84. doi: 10.1016/s1286-4579(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Gierynska M, Eo SK, Kuklin N, Rouse BT. Influence of DNA encoding cytokines on systemic and mucosal immunity following genetic vaccination against herpes simplex virus. Microbes Infect. 2003;5:571–578. doi: 10.1016/s1286-4579(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 37.Hanlon L, Argyle D, Bain D, Nicolson L, Dunham S, Golder MC, McDonald M, McGillivray C, Jarrett O, Neil JC, Onions DE. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J Virol. 2001;75:8424–8433. doi: 10.1128/JVI.75.18.8424-8433.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mossmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 39.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 40.Hasbold J, Hong JS, Kehry MR, Hodgkin PD. Integrating signals from IFN-γ and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J Immunol. 1999;163:4175–4181. [PubMed] [Google Scholar]
- 41.Miura T, Mizuki D, Sasaki S, Hasegawa S, Sashinami H, Nakane A. Host resistance to Listeria monocytogenes infection is enhanced but resistance to Staphylococcus aureus infection is reduced in acute graft-versus-host disease in mice. Infect Immun. 2000;68:4340–4343. doi: 10.1128/iai.68.7.4340-4343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 43.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 45.Bright RK, Beames B, Shearer MH, Kennedy RC. Protection against a lethal challenge with SV40-transformed cells by the direct injection of DNA-encoding SV40 large tumor antigen. Cancer Res. 1996;56:1126–1130. [PubMed] [Google Scholar]
- 46.Bright RK, Shearer MH, Kennedy RC. Immunization of BALB/c mice with recombinant simian virus 40 large tumor antigen induces antibody-dependent cell-mediated cytotoxicity against simian virus 40-transformed cells: an antibody-based mechanism for tumor immunity. J Immunol. 1994;153:2064–2071. [PubMed] [Google Scholar]
- 47.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 48.Makki A, Weidt G, Blachere NE, Lefrancois L, Srivastava PK. Immunization against a dominant tumor antigen abrogates immunogenicity of the tumor. Cancer Immun. 2002;2:4. [PubMed] [Google Scholar]
- 49.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timmerman JM, Levy R. Cancer vaccines: pessimism in check. Nat Med. 2004;10:1279. doi: 10.1038/nm1204-1279a. author reply 1279–1280. [DOI] [PubMed] [Google Scholar]
- 51.Hsu FJ, Caspar CB, Czerwinski D, Kwak LW, Liles TM, Syrengelas A, Taidi-Laskowski B, Levy R. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma: long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 52.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 53.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Hosiawa KA, Min W, Yang J, Zhang X, Garcia B, Ichim TE, Zhou D, Lian D, Kelvin DJ, Zhong R. Cytokines regulate the pattern of rejection and susceptibility to cyclosporine therapy in different mouse recipient strains after cardiac allografting. J Immunol. 2003;171:3823–3836. doi: 10.4049/jimmunol.171.7.3823. [DOI] [PubMed] [Google Scholar]
- 55.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- 56.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 57.Batchu RB, Moreno AM, Szmania SM, Bennett G, Spagnoli GC, Ponnazhagan S, Barlogie B, Tricot G, van Rhee F. Protein transduction of dendritic cells for NY-ESO-1-based immunotherapy of myeloma. Cancer Res. 2005;65:10041–10049. doi: 10.1158/0008-5472.CAN-05-1383. [DOI] [PubMed] [Google Scholar]
- 58.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101:14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]


