Abstract
In monkeys, elevating ambient temperature has been shown to increase sensitivity to MDMA reinforcement. Previous rodent studies have shown that elevations in thyroid status (hyperthyroidism) parallel changes in elevating the ambient temperature on MDMA-induced thermogenesis, but the interaction has not been examined in monkeys. The present study was designed to evaluate the effects of chronic levothyroxine (3.0 or 4.5 μg/kg/day, im; Levo) treatment on MDMA-induced increases in temperature following 1.5 mg/kg (iv) MDMA and self-administration when MDMA (0.03-0.3 mg/kg/inj) and food were available under a concurrent fixed-ratio 30 schedule in rhesus monkeys (n=4). Previous studies had shown that 1.5 mg/kg MDMA did not affect thermoregulation at 24°C. Following chronic Levo treatment, this dose resulted in significant increases in MDMA-induced thermogenesis. In the self-administration experiment, MDMA choice increased with dose, such that food was preferred over saline and a low MDMA dose (0.03 mg/kg/inj), whereas 0.1 and 0.3 mg/kg/inj MDMA was preferred over food. While elevating ambient temperature had been shown to increase MDMA potency, there was no effect of chronic Levo treatment on MDMA choice. These results suggest that changes in thyroxine levels do not parallel the changes in ambient temperature in altering the reinforcing strength of MDMA.
Keywords: MDMA, rhesus monkey, self-administration, drug-choice, thyroid hormones, temperature
INTRODUCTION
3,4-methylenedioxymethamphetamine (MDMA)-induced thermogenesis and reinforcement are influenced by ambient temperature in both rodents and nonhuman primates (Gordon et al., 1991; Cornish et al., 2003; Banks et al., 2007a,b; Von Huben et al., 2007). MDMA-induced thermogenesis in rodents is also thyroid hormone dependent (Sprague et al., 2003, 2007). Clinically, there is a case report suggesting that hyperthyroidism potentiated the hyperthermic effects of ecstasy (Martin et al., 2007). Currently, there is a void in our understanding of the effects of thyroid hormones on MDMA-induced thermogenesis and reinforcement in monkeys. Previously, we reported that elevating the ambient temperature produced changes in MDMA-induced thermodysregulation and increased the potency of MDMA reinforcement (Banks et al., 2007a,b). In the present study, we hypothesized that elevating thyroid hormone levels would have similar effects as elevating the ambient temperature on MDMA-induced thermogenesis and the relative reinforcing strength of MDMA under a concurrent fixed-ratio (FR) schedule of MDMA and food choice. An advantage of concurrent schedules is that the dependent variable, drug choice, is less influenced by the direct effects of the drug on response rates and that response allocation is related to changes in reinforcement magnitude (Katz, 1990; Woolverton and Nader, 1990; Bergman and Paronis, 2006).
METHODS
Subjects
Four individually housed adult male rhesus monkeys (Macaca mulatta), surgically prepared with an indwelling intravenous catheter and a subcutaneous vascular access port (Access Technologies, Skokie, IL; Martelle et al., 2007), served as subjects. All monkeys were previous trained under a concurrent FR 30 schedule of food and MDMA (0.03-0.3 mg/kg/inj)presentation (Banks et al., 2007b). Monkeys were weighed bi-weekly and fed enough food daily (LabDiet #5038 Monkey Chow and fresh fruit) to maintain body weights at approximately 95% of free-feeding levels. Rectal temperatures were taken at least 3 times/week after Levo administration, but before the behavioral session. The number of food pellets earned was calculated into the daily food requirements and subjects were fed at least 2 hours after completion of the experimental sessions. Environmental enrichment was provided as outlined in the Animal Care and Use Committee (ACUC) of Wake Forest University (WFU) Nonhuman Primate Environmental Enrichment Plan. All procedures were approved by the ACUC of WFU and performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Procedure
Experimental sessions were conducted in ventilated and sound-attenuating chambers (Med Associates, East Fairfield, VT) with the ambient temperature inside the chamber maintained at 24 ± 1°C as previously described (Banks et al., 2007b). Briefly, the skin above the venous access port was cleaned with Betadine and 95% ethanol and the port was connected to the infusion pump via a 22-gauge Huber point needle. At the beginning of each session, the pump was operated for approximately 3 sec to fill the port and catheter with the concentration of MDMA or saline available for that session. Sessions began with illumination of lights above both levers. Thirty consecutive responses on a lever resulted in either a 10-sec infusion or delivery of a 1-gram banana-flavored pellet, followed by a 10-sec timeout. The lever designated as food and drug was randomly determined for each monkey and remained constant throughout the experiment. Sessions ended after 30 total reinforcers had been earned or 60 min had elapsed. Each dose was available for at least five consecutive sessions and remained available until responding was deemed stable (percent injection-lever responding ± 20% of the mean of three consecutive sessions with no trend). Doses were tested in mixed order in each monkey.
After completion of the MDMA dose-response curve, the effects of Levo were examined. At the start of Levo (3.0 μg/kg/day, im) treatment and for 10 consecutive days, responding was only maintained by food presentation under an FR 30 schedule of reinforcement prior to reinstituting MDMA self-administration. This dose of Levo was based on pilot data demonstrating behavioral effect as determined by a shift in the MDMA dose-effect curve. Levo treatment continued when the MDMA self-administration dose-response curve was re-determined after 10 days of Levo treatment. In two subjects where the dose-effect curve was not altered after the 3.0 μg/kg/day Levo treatment, the Levo dose was increased to 4.5 μg/kg/day for 10 days and the MDMA dose-effect curve re-determined. During Levo treatment, all behavioral sessions were conducted approximately 60 min after levothyroxine administration.
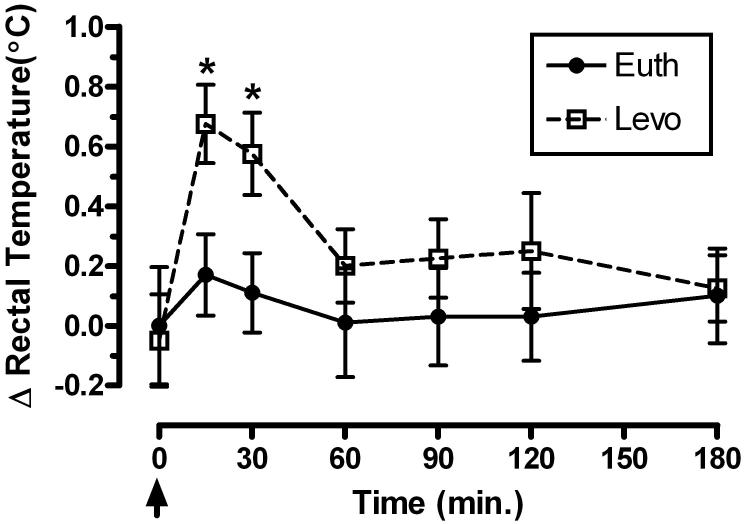
At the conclusion of the behavioral experiments, subjects were challenged with noncontingent MDMA (1.5 mg/kg, iv) approximately 24 hours after the last Levo injection to assess the effects of chronic Levo treatment on MDMA-induced thermogenesis. Rectal temperature was recorded for 3 hours using a RET-1 rectal probe attached to a TH-3 thermocouple (Physitemp, Clifton, NJ). For comparison, the effects of 1.5 mg/kg MDMA on thermogenesis in the absence of Levo treatment (Banks et al., 2007a) were included in Fig. 1.
Figure 1.
Effects of chronic levothyroxine treatment (Levo; open symbols) on MDMA (1.5 mg/kg, iv)-induced changes in rectal temperature. Data (mean ± SEM, n=4) are represented as change (°C) from baseline (38.4±0.2°C; time 0 min.) temperature. * indicates significantly different from baseline (p < 0.05). Closed symbols (Euth; basal thyroid levels) represent effects of MDMA at room temperature (from Banks et al., 2007a). Arrow indicates time of MDMA administration.
Drugs
Levothyroxine sodium (Bedford Laboratories, Bedford, OH) was obtained from the WFU Baptist pharmacy and dissolved in sterile saline. (±) MDMA HCl was provided by the National Institute on Drug Abuse (Bethesda, MD) and dissolved in sterile saline.
Plasma thyroxine levels assessment
Before and 10 days after initiating Levo treatment, venous blood (2 mL) was collected and transferred to a Vacutainer® tube with no additives (Becton-Dickinson, Franklin Lakes, NJ). Blood samples were centrifuged at 3000 rpm for 10 min at 4°C and thyroxine levels were determined by Antech Diagnostics (Lake Success, NY).
Data Analysis
Plasma thyroxine levels at baseline and after Levo were analyzed using a paired t-test. Temperature data were analyzed using a one-way ANOVA with time as the factor followed by a Dunnett post-hoc test comparing each time point back to baseline (time 0). Behavioral data were analyzed with repeated measures mixed linear regression using SAS Proc Mixed (Version 8.2, SAS, Cary, NC) with Levo, MDMA dose and their interaction as factors. For the self-administration experiments, the primary dependent variables examined were MDMA choice (calculated by dividing the total number of MDMA-lever responses by the total responses on both levers), number of MDMA and food reinforcers earned per session. Differences were considered significant at the 95% level of confidence (p<0.05).
RESULTS
Effects of chronic Levo treatment on plasma thyroxine levels
Chronic Levo treatment significantly increased (t = 4.99, p<0.05) plasma thyroxine levels on average 160% from baseline (6.3 ± 0.6 μg/dL; n=4) in the absence of MDMA.
Effects of chronic Levo treatment on MDMA-induced changes in temperature
Chronic Levo treatment did not significantly change basal rectal temperature measured over the 3 month treatment period (38.3±0.1°C versus 38.4±0.2°C). Administration of noncontingent (±) MDMA (1.5 mg/kg, iv) at 24°C significantly increased rectal temperature (F6,27 = 3.16, p < 0.05; Fig. 1). Post-hoc analysis revealed a significant (p < 0.05) change in rectal temperature at 15 and 30 min. post-MDMA that returned to baseline at 60 min.
Effects of chronic Levo treatment on MDMA choice
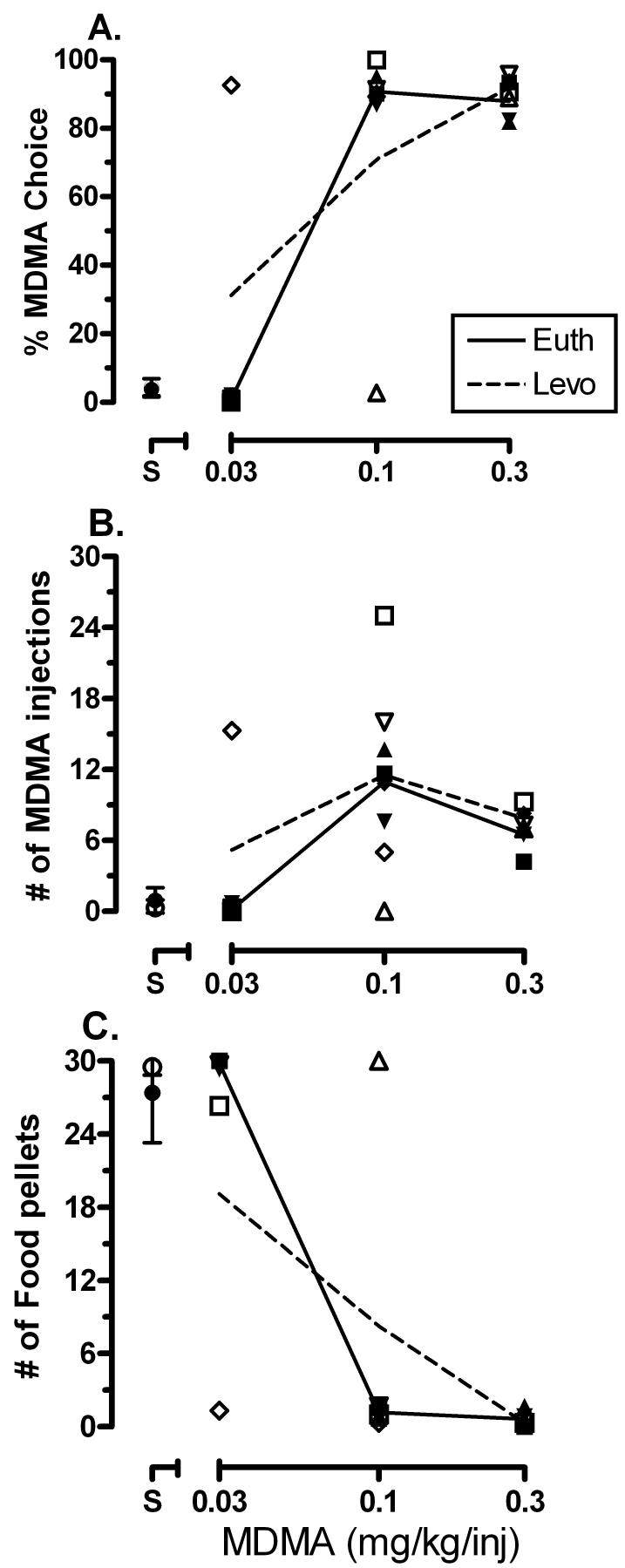
Under baseline conditions, when the choice was between saline or a low dose of MDMA (0.03 mg/kg/inj) and food, monkeys primarily chose food (Fig. 2A; open symbols). Increases in MDMA dose resulted in increases in MDMA choice, such that 0.1 and 0.3 mg/kg/inj MDMA were primarily (p < 0.05) chosen over food. There was a significant main effect of MDMA dose for MDMA choice (F3,9 = 26.85, p < 0.05; Fig. 2A), number of MDMA injections earned (F3,9 = 6.81, p < 0.05; Fig. 2B) and number of food pellets earned (F3,9 = 30.7, p < 0.05; Fig. 2C). Chronic Levo treatment did not significantly alter any dependent measure.
Figure 2.
Effects of chronic levothyroxine (Levo) treatment on MDMA choice (A), MDMA injections (B) and food pellets (C) under a concurrent schedule of MDMA and food availability. Abscissa represents MDMA dose (0.03 - 0.3 mg/kg/inj). Data (mean ± SEM, n=4) above S represent when saline was the alternative to food. Individual subjects are represented by different symbols with filled symbols indicating baseline thyroid levels (Euth) and open symbols indicating (Levo) condition. The solid or dashed lines indicate the group means at baseline (Euth) or during (Levo) treatment, respectively.
DISCUSSION
The rationale for these studies was based on the parallel effect of either elevating the ambient temperature or thyroid hormone levels on potentiating MDMA-induced thermogenesis (Gordon et al., 1991; Sprague et al., 2003, 2007). We have previously shown that noncontingent MDMA administration does not significantly change rectal temperature at 24°C (Banks et al., 2007a). In the present study, after chronic Levo treatment, MDMA administration at 24°C did significantly increase rectal temperature. Although the topography of the thermogenic effect was different (peak thermogenic effect was 15 min.) compared to elevating the ambient temperature under identical conditions (peak thermogenic effect was 90 min.; Banks et al., 2007a), this finding confirms a parallel effect of elevating the ambient temperature and increasing thyroid hormone levels on MDMA-induced thermogenesis and extends the role of thyroid hormones in MDMA-induced thermogenesis to monkeys.
Preclinical research has demonstrated an interaction between ambient temperature and MDMA reinforcement (Cornish et al., 2003; Banks et al., 2007b). For example, elevating ambient temperature increased low-dose MDMA choice in the same monkeys used in the present study under identical schedule conditions (Banks et al., 2007b). However, in contrast to the effects of elevating the ambient temperature, elevating thyroxine levels did not increase the relative reinforcing strength of MDMA despite having an effect on MDMA-induced thermogenesis. Individual differences were noted, unrelated to Levo dose. These findings support the contention that elevating thyroxine levels and elevating ambient temperature, which have parallel effects on MDMA-induced thermodysregulation, do not have similar consequences on MDMA reinforcement. The effects of ambient temperature on reinforcement appear not to be mediated through thermoregulatory mechanisms, such as the hypothalamic-pituitary-thyroid axis.
ACKNOWLEDGEMENTS
We appreciate the insightful comments and statistical assistance of Drs. Linda Porrino and Beth Reboussin. No authors have financial conflicts of interest with the research described in this manuscript.
This research was supported by National Institute on Drug Abuse grants DA-06634 (MAN) and DA-020281 (MLB).
REFERENCES
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects 3,4-methylendioxymethamphetamine (MDMA)-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007a;35:1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Czoty PW, Nader MA. Effects of ambient temperature on the relative reinforcing strength of MDMA using a choice procedure in monkeys. Psychopharmacology. 2007b doi: 10.1007/s00213-007-0932-7. doi:10.1007/s00213-007-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Paronis CA. Measuring the Reinforcing Strength of Abused Drugs. Mol Interv. 2006;6:273–283. doi: 10.1124/mi.6.5.9. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–41. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O’Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol BiochemBehav. 1991;38:339–44. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Grundt P, Ross J, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–82. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Martin TL, Chiasson DA, Kish SJ. Does hyperthyroidism increase risk of death due to the ingestion of ecstasy? J Forensic Sci. 2007;52:951–3. doi: 10.1111/j.1556-4029.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methlyenedioxymethamphetamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:59–66. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Yang X, Sommers J, Gilman TL, Mills EM. Roles of norepinephrine, free fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J Pharmacol Exp Ther. 2007;320:274–280. doi: 10.1124/jpet.106.107755. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia Induced by (±)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–81. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Nader MA. Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowan A, editors. Modern Methods in Pharmacology Volume 6: Testing and Evaluation of Drugs of Abuse. Wiles-Liss, Inc.; New York: 1990. pp. 165–192. [Google Scholar]