Abstract
Purpose
To evaluate the therapeutic efficacy of two antiepileptic compounds, RWJ-333369 and RWJ-333369-A in a well-established experimental model of lateral fluid percussion (FP) traumatic brain injury (TBI) in the rat.
Methods
Anethestized Male Sprague-Dawley rats (n = 227) were subjected to lateral FP brain injury or sham-injury. Animals were randomized to receive treatment with RWJ-333369 (60 mg/kg, p.o.) or its analog RWJ-333369-A (60 mg/kg, p.o.), or vehicle (equal volume) at 15 minutes, 4, 8, and 24 hours post-injury. In Study I, animals were assessed at 48 hours for acute motor and cognitive function and then sacrificed to evaluate regional cerebral edema. In Study II, animals were evaluated post-injury for motor function at 48 hours and weekly thereafter from 1 to 4 weeks. Post-traumatic learning ability was assessed 4 weeks post-injury, followed by evaluation of hemispheric tissue loss.
Results
In Study I, no improvement in acute memory or motor function was observed following administration of either RWJ-333369 or RWJ-333369-A in brain-injured animals compared to vehicle-treated, brain-injured animals. However, brain-injured animals receiving treatment with RWJ-333369-A had a significant reduction in post-traumatic cerebral edema in both injured and contralateral hippocampus compared to brain-injured, vehicle-treated controls (p < 0.05). In Study II, treatment with either compound did not result in any improvement of neuromotor function, learning ability or change in lesion volume following brain injury.
Conclusions
These results indicate that the novel antiepileptic compound RWJ-333369-A reduces post-traumatic hippocampal edema without affecting neurobehavioral or histological outcome. It remains unclear whether this small effect on hippocampal edema is related to the ability of this compound to attenuate seizure activity.
Keywords: Antiepileptic drugs (AEDs), brain swelling, head injury, neuroprotetion, post-traumatic epilepsy
1. Introduction
Approximately 1.4 million individuals sustain a traumatic brain injury (TBI) in the United States annually, resulting in 5.3 million Americans currently living with long-term disabilities (Centers for Disease Control and Prevention; Centers for Disease Control and Prevention). The difficulty in finding a “cure” for TBI is due, in part, to the heterogeneous nature of injuries that are sustained following TBI due to differences in age, sex, injury site, force of impact, as well as the complexity of the molecular and cellular response to injury. The physical or primary injury causes a number of secondary or delayed processes to occur at the cellular level ultimately leading to acute neuronal death (Graham, Gennarelli & McIntosh, 2002; Lowenstein, Thomas, Smith & McIntosh, 1992). These pathological cascades include the release of excitatory amino acids (EAAs), such as glutamate (Faden, Demediuk, Panter & Vink, 1989; Hayes, Jenkins & Lyeth, 1992; Katayama, Becker, Tamura & Ikezaki, 1990). The subsequent influx of calcium into neurons via activation of N-methyl-D-aspartate (NMDA) channels, results in the breakdown ofthe cytoskeleton (Hayes, Yang, Whitson, et al. 1995; Saatman, Bozyczko-Coyne, Marcy, Siman & McIntosh, 1996), secondary axotomy (Maxwell, Donnelly, Sun, Fenton, Puri & Graham, 1999; Povlishock & Pettus, 1996), release of reactive oxygen species (ROS) (Marklund, Clausen, Lewander & Hillered, 2001; Povlishock & Kontos, 1992) and sustained mitochondrial damage (Lifshitz, Friberg, Neumar, et al. 2003; Sullivan, Keller, Mattson & Scheff, 1998). The release of glutamate also contributes to the accumulation of water (cytotoxic and vasogenic edema) in different brain regions by contributing to the increased permeability of the blood brain barrier (BBB) (Mayhan & Didion, 1996). The secondary response to the TBI also involves the release of pro-inflammatory cytokines such as IL-6, IL-1 and TNF-alpha (Fan, Young, Barone, Feuerstein, Smith & McIntosh, 1995; Fan, Young, Barone, Feuerstein, Smith & McIntosh, 1995; Morganti-Kossmann, Rancan, Stahel & Kossmann, 2002). These cellular and molecular changes are known to be associated with prolonged and continuing cell death, which has been documented up to 1 year in experimental models (Kaplanski, Pruneau, Asa, et al. 2002; Pierce, Smith, Trojanowski & McIntosh, 1998; Smith, Chen, Pierce, et al. 1997).
These data suggest that there is a window of opportunity during which the administration of neuroprotective drugs can act on secondary or delayed processes to improve outcome following TBI, by either attenuating excitotoxicity or acting on other deleterious downstream pathways. A number of strategies have been pursued to develop a novel neuroprotective treatment for TBI. Previous studies have reported that administration of the Ca2+-channel blockers, (R,S)-(3,4-dihydro-6,7-dimethoxy-isoquinoline-1-yl)-2-phenyl-N, N-di[2-(2,3,4-trimethoxyphenyl)ethyl]-acetamide (LOE 908) or (S)-emopamil, following lateral fluid percussion (FP) injury in rats can improve motor function at acute time points in rats (Cheney, Brown, Bareyre, et al., 2000; Okiyama, Smith, Thomas, McIntosh, 1992). Several glutamate antagonists have also been shown to be efficacious in a variety of experimental TBI models (for review see (Royo, Schouten, Fulp, et al. 2003). When assessed acutely, the NMDA receptor blockers CP-98,113; CP-101,581; and CP-101,606 significantly attenuated the effects of injury on spatial memory while CP-98,113 significantly reduced cerebral edema following lateral FP brain injury in rats (Okiyama, Smith, White, Richter & McIntosh, 1997).
In addition to contributing to cell death after TBI, the activation of excitotoxic mechanisms has been associated with the induction of seizures and epileptogenesis (Urbanska, Czuczwar, Kleinrok & Turski, 1998; Yeh, Bonhaus, Nadler & McNamara, 1989). To date, only a few studies have evaluated the potential beneficial effects of antiepileptic drugs (AEDs) in animal models of TBI despite the finding that many of these compounds exert effects on those mechanisms known to mediate the deleterious consequences of TBI, including agents that antagonize various types of glutamate receptors, increase GABAergic inhibition, or block Na+ or Ca2+ channels (for review see (Czapinski, Blaszczyk & Czuczwar, 2005). Enhancement of GABA-A receptor function by diazepam has been demonstrated to decrease excitotoxic effects in a model of midline FP injury in rats (O'Dell, Gibson, Wilson, DeFord & Hamm, 2000). Topiramate, which blocks sodium channels (DeLorenzo, Sombati & Coulter, 2000; Zona, Ciotti & Avoli, 1997), inhibits KA/AMPA receptors (Shank, Gardocki, Streeter & Maryanoff, 2000), and increases GABA (White, Brown, Woodhead, Skeen & Wolf, 2000), was found to improve gross motor function (4 weeks) and rotating pole scores (1 and 4 weeks) when administered after lateral FP brain injury in rats (Hoover, Motta, Davis, et al., 2004). Remacemide hydrochloride, an NMDA receptor antagonist that also acts on sodium channels, has also been reported to decrease cortical lesion following a parasagittal FP brain injury in rats (Smith, Perri, Raghupathi, Saatman & McIntosh, 1997).
Hernandez (Hernandez, 1997) reported that early administration of many AEDs after TBI could compromise the patients' recovery. Therefore, information about both the beneficial and harmful effects of novel AEDs on post-traumatic recovery is of critical importance before they are administered to patients to treat early clinical post-traumatic seizures. In the present study, two novel antiepileptic compounds, RWJ-333369 (S-2-O-carbamoyl-1-o-chlorophenyl-ethanol) and its derivative RWJ-333369-A, were assessed for their therapeutic utility using the lateral FP model of experimental brain injury to produce injuries similar to human TBI (Graham, Raghupathi, Saatman, Meaney & McIntosh, Thompson, Lifshitz, Marklund, et al., 2005). The novel compound RWJ-333369 has recently been shown to protect the hippocampal CA1 region, thalamus, amygdala and ventral cortices in the lithium-pilocarpine model of epilepsy and delay or prevent recurrent seizures (Francois, Ferrandon, Koning & Nehlig, 2005). Nehlig et al. (2005) also reported that RWJ-333369 displays potent antiepileptic properties in genetic models of both absence and audiogenic epilepsy. Although these results demonstrate the ability of this compound to protect against seizures and cell death in models of epilepsy, nothing is known regarding their potential neuroprotective and/or behavioral efficacy in TBI. For this study, we hypothesized that acute administration of the antiepileptics RWJ-333369 or its structurally-related compound, RWJ-333369-A, would attenuate behavioral deficits, regional brain swelling and/or protect selectively vulnerable brain regions from cell death following experimental TBI in rats.
2. Methods
2.1. Animals
Adult male Sprague-Dawley rats (324−403 g, n = 227; Harlan, Inc., Indianapolis, IN) were used. The animals were housed in humidity/temperature-regulated colony, two animals per cage. The colony lights were on a 12-hour light/dark cycle and the rats were supplied food and water ad libitum. All investigators were blinded to injury and treatment status during behavioral testing. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and were conducted according to standards published by the National Research Council (1996).
2.2. Surgery and fluid percussion injury: (Study I & Study II)
All animals were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and placed in a stereotaxic frame. The scalp and temporal muscle were reflected over the left parietal lobe and a five-mm craniectomy was made centered between lambda and bregma. The dura remained intact. A stainless steel screw was placed anterior to the craniectomy to increase the fixation of the luer-lok to the skull. A modified luer-lok cap was fixed over the craniectomy opening using dental acrylic (DentSply®, Dentsply International Inc., York, PA, USA) and then filled with saline. Sham-injured (control) animals (n = 83 in total for Study I and II) received anesthesia and surgery without injury and were placed on heating pads to maintain normothermia while the cement was allowed to dry at which time the cap was removed and the skin sutured. A second group of animals were subjected to lateral FP brain injury (n = 144 in total for Study I and II) of moderate-to-severe level (2.36 ± 0.12 atm). The brain injuries were induced by a rapid bolus of saline (22 ms) striking the dura and causing brain deformation as originally described (McIntosh, Smith, Voddi, Perri & Stutzmann, 1996). The peak pressure in atmospheres was noted for each injury along with the seizure and apnea of each animal immediately following injury. Animals were excluded from the study if their apnea exceeded 60 seconds. The caps were removed and the incisions sutured. Sham-injured and brain-injured animals remained on heating pads until they were able to walk and hold their heads erect.
2.3. Drug Dosing and Administration
Brain-injured and sham-injured animals for both Study I and Study II were randomly assigned to receive either one of the two antiepileptic compounds or vehicle. RWJ-333369-A (60 mg/kg, n = 35 brain-injured, n = 28 sham-injured) or RWJ-333369 (60 mg/kg, n = 34 brain-injured, n = 27 sham-injured), kind gifts of R.W. Johnson Pharmaceutical Research Institute, Spring House, PA, or vehicle [equal volume of 5% Solutol® HS 15 (BASF, Germany) in 5% Methylcellulose (n = 35 brain-injured, n = 28 sham-injured) via oral gavage (p.o.)] were administered at 15-minutes post-injury followed by additional oral doses of drug or vehicle at 4, 8, and 24 hours post-injury. Dose and timing of administration were determined from previous studies showing efficacy of these compounds in experimental models of epilepsy (Francois, et al., 2005; Nehlig, et al., 2005).
2.4. Study I: Evaluation of acute post-traumatic motor and cognitive deficits and brain water content in the acute post-traumatic period
2.4.1. Morris water maze: Assessment of acute post-traumatic retrograde memory
To examine the cognitive effects of RWJ-333369-A and RWJ-333369 in the acute post-traumatic period, animals (sham-injured, vehicle-treated n = 15; sham-injured, RWJ-333369-A-treated n = 14; and sham-injured, RWJ-333369-treated n = 14; brain-injured vehicle-treated n = 17, brain-injured, RWJ-333369-A-treated n = 16, and brain-injured, RWJ-333369-treated n = 16) were assessed for post-injury visuospatial memory deficits at 48 hours using a 1-meter Morris water maze (MWM) test, following the paradigm originally described by Smith et al. (1991). This paradigm has been used successfully to detect both injury and treatment effects on cognition following experimental brain injury in rats (Hernandez, 1997; Marklund, Clausen, McIntosh & Hillered, 2001; Okiyama, Smith, White & McIntosh, 1998). The animals were trained prior to injury over 2 consecutive days, 10 trials a day (maximum 60 seconds per trial), to locate the platform using external visual cues. The rats were placed into a black-painted, circular pool measuring 1 meter across filled with water (20−24°C), from which they could escape onto a hidden platform (11.5 cm × 11.5 cm) sub-merged one centimeter below the water surface. The training was performed in the afternoon on the day before surgery/injury and then the morning on the day of surgery/injury. Following the last trial, the animals were given a fixed 90-minute interval before the first pentobarbital injection. At 48 hours post-injury, the animals were placed again in the MWM for two separate trials, but without the platform. The animals swim patterns were tracked over a 60 second time period using a computerized video system (Accutrak®, San Diego, CA, USA) and a score was calculated based on the set divided zones as previously described (Smith, et al., 1991). Following each trial, the animals were placed in bins with overhead ceramic heating lamps to help them maintain normothermia.
2.4.2. 48 hour Composite Neuroscore: Assessment of acute post-traumatic neurologic motor function
Immediately following cognitive testing at 48 hours post-injury animals were assessed for acute post-traumatic motor function using a previously reported composite neuroscore test (Cheney, Weisser, Bareyre, et al., 2001; McIntosh, Vink, Noble, Yamakami, Fernyak & Faden, 1989; Sanderson, Raghupathi, Martin, Millerm & McIntosh, 1999). These tests have been shown to be sensitive indicators of the severity of injury (Dixon, Lyeth, Povlishock, et al., 1987; Faden, O'Leary, Fan, Bao, Mullins & Movsesyan, 2001). The neuroscore test consisted of seven separate tests, all scored using a four point system (zero being severly impaired and four being normal) for a maximum total score of 28. The seven tests included were: left and right forelimb flexion, left and right hindlimb flexion, left and right lateral pulsion, and angle board. The angle board test assessed the animals' ability to remain standing on an incline in both the horizontal and vertical positions. For this test, the score of zero through four was calculated by first recording the maximum angle that the animal can remain on the incline for five seconds. This score was compared to a pre-surgery baseline and the final score was calculated using the degree of change when a score of four equals no change and one point is subtracted from four for each 2.5 degree change from baseline with zero being the lowest possible score.
2.4.3. Acute Edema: Assessment of regional brain water content
Following cognitive and motor function testing, all brain-injured and sham-injured animals were euthanized for measurement of cerebral edema formation at 48 hours post-injury, the optimal time to measure brain water content following experimental FP injury (Soares, Thomas, Cloherty & McIntosh, 1992). The animals were anesthetized with sodium pentobarbital (65 mg/kg, i.p.), decapitated, and the brains were removed from the skull as quickly as possible. A three mm coronal section was removed from the occipital-parietal level (Bareyre, Wahl, McIntosh & Stutzmann, 1997), which included the injury site over the left parietal cortex. First, the different brain areas were dissected out on the ipsilateral side (side of injury) on a cold, glass plate in the following order: (I) hippocampus, (2) injured parietal cortex, (3) adjacent parietal cortex, and (4) thalamus. The contralateral side was then dissected out in the same order. Edema was measured using the wet weight-dry weight technique (Soares, et al., 1992). After each individual part was dissected out, sections were weighed (scale used: Denver Instrument Company, TR-104) on aluminum foil to obtain the wet weight of the tissue. The tissue was then placed in a 104°C oven (Fisher Scientific, Isotemp Oven) for 24 hours. The tissue was then reweighed to obtain the dry weight of each brain area. The percentage of water content was calculated using the following equation: [% of water [(wet weight – dry weight)/wet weight]/× 100].
2.5. Study II: Evaluation of long-term (48 hours to 4 weeks) post-traumatic vestibular motor function and visuospatial learning and neuroprotection
2.5.1. Composite Neuroscore: Assessment of gross neurologic motor function
The animals (sham-injured, vehicle-treated n = 13; sham-injured, RWJ-333369-A-treated n = 14; and sham-injured, RWJ-333369-treated n = 13; brain-injured vehicle-treated n = 18, brain-injured, RWJ-333369-A-treated n = 19, and brain-injured, RWJ-333369-treated n = 18) were evaluated for motor function at 48 hours and weekly for 1 to 4 weeks post-injury. Composite neuroscore testing was performed at 48 hours and weekly from one to four weeks as previously described for Study I.
2.5.2. Beam Balance: Assessment of vestibulomotor function
The assessment of vestibulomotor function was conducted at 72 hours and weekly from one to four weeks post-injury using the beam balance test. This test has previously been shown to be a good measure of deficits in vestibulomotor function following lateral FP injury (Dixon, Ma, Kline, et al., 2003; Wagner, Willard, Kline, et al., 2004). Animals were trained on the task prior to injury, which involved placing the animal on the beam for 60 second intervals until the animal was able to meet criteria: ability to maintain posture (gripping with all four limbs) and balance (using tail) for a full 60 seconds. At testing, the sham-injured and brain-injured rats posture and ability to balance on a 35 centimeter long, 1.5 centimeter wide wooden beam for up to 60 seconds was scored on a zero (severely impaired) to four (normal) scale as follows: 4 = balances with steady posture and exhibits exploratory behavior, 3 = balances with steady posture, no exploratory activity, 2 = shaky movements, exhibiting strength in limbs, 1 = shaky, using belly to balance, flaccid limbs, 0 = immediately slips and begins hanging with forelimbs from beam. The paradigm consists of three trials. A score was given for each trial, which were then averaged together for one final score.
2.5.3. Rotating Pole: Assessment of coordination and integration of movement
Evaluation of the rats' motor capabilities on the rotating pole test was performed at 72 hours and weekly from one to four weeks post-injury. This test has been shown to be a sensitive measure of motor deficits induced by the lateral FP injury (Mattiasson, Philips, Tomasevic, Johansson, Wieloch & McIntosh, 2000). Animals were acclimated with two daily trials, prior to surgery. On the first day, the animals were trained on a non-rotating, wooden pole (four centimeters diameter, 150 centimeters long). The second day of acclimation, the animals were trained on a pole that was rotating to the left direction. Following the sham or brain-injury, the animal was assessed in its ability to walk across the wooden pole, rotating at five rpm in the left direction. A score was determined based on the rats' ability to traverse the pole. For each of three trials, the animals were scored using a grading system based on whether and where the animal fell off of the pole (score 0 or 1 is given depending on the area on the pole where the animal fell off) or the number of footfaults (slips and hops) where a score of 2 = able to traverse the 150 centimeters pole with 4 or more footfaults, a score of 3 = 2−3 footfaults while traversing the entire pole and a score of 4 = 0−1 footfaults while traversing the 150 centimeters distance. The average score for the three trials is presented.
2.5.4. Assessment of visuospatial learning
Learning was evaluated in the 1.83 meter MWM at four weeks post-injury. The rats were placed into a black-painted, circular pool measuring 1.83 meters across filled with water (20−24°C), from which they could escape onto a hidden platform (11.5 cm × 11.5 cm) submerged one cm below the water surface. There was no pretraining of the animals on the task to locate the platform. The learning paradigm consisted of eight trials per day for three days. The amount of time each animal took to locate the platform was recorded, with the maximum time being 60 seconds. Animals that did not find the platform in that time period were recorded as having a 60-second latency and were placed on the platform by the experimenter for 15 seconds. Following each trial, the animals were warmed and dried using ceramic thermal lamps anchored above a wire mesh cage in between each trial.
2.5.5. Assessment of neuroprotection
After finishing the behavioral testing at four weeks post-injury, rats were deeply anesthetized with sodium pentobarbital (65 mg/kg, i.p.). The thoracic cavity and peritoneum were opened, aorta clamped, and animals were transcardially perfused with heparinized 0.9% saline followed by 10% formalin (total volume 200 ml at room temperature). The brains were removed and immersed in 10% formalin and then stored at 4°C until processed for histology.
Brains were embedded in paraffin and cut on a coronal plane (40 μm thick sections) by using a rotary microtome (ThermoShandon, Pittsburgh, PA). All sections between the coronal planes −0.3 mm to −7.3 mm posterior from bregma (atlas coordinates according to (Paxinos & Watson, 1990) were collected and mounted on Superfrost slides. The sections were stained with hemotoxylin and eosin (H & E). For measurement of the lesion volume, 8 H&E stained sections were chosen at one mm intervals beginning at −0.3 mm from Bregma (Paxinos & Watson, 1990). Pre-review of the H&E stained preparations indicated that the entire extent of the cortical lesion was confined within these 8 rostrocaudal levels. The sections were photographed using a computerized image analysis system (MCID M4, St. Catherines, Ontario, Canada). For each section, the investigator drew an outline around the ipsilateral hemisphere. Thereafter, the contralateral hemisphere was outlined as described previously by Hoover et al. (2004). The lesion volume as a percent was calculated using [(contralateral hemispheric volume – ipsilateral hemispheric volume)/(contralateral hemispheric volume) × 100% (Hoover, et al., 2004). All histological analyses were performed by a researcher blinded to animal injury and treatment status.
2.6. Statistical analyses
For both studies, all data were analyzed using Statistica '98 (StatSoft, Tulsa, OK). A p-value <0.05 was considered to be statistically significant; correction was made for multiple tests where appropriate. In Study I, 48-hour neuroscore ordinal, nonparametric data were assessed using Kruskall-Wallis ANOVA, followed by the Mann-Whitney U-test. The median values of these scores are presented. Parametric data including 48-hour MWM memory testing and 48 hour regional cerebral edema are presented as a mean ± standard deviation (SD) and were analyzed using a two-way ANOVA, followed by Student Neuman-Keuls post-hoc if appropriate. For all tests, we analyzed one drug versus vehicle for both sham and injured groups.
In Study II, ordinal, nonparametric data including neuroscore, rotating pole, and beam balance were analyzed using a Kruskall-Wallis ANOVA. If the p-value was <0.05 then a Mann-Whitney U-test was performed. The learning latencies (parametric data) were analyzed using two-way repeated measures ANOVA, if the p-value was <0.05 then the data were analyzed using a Student Neuman-Keuls post-hoc test. The latencies are presented as a mean ± SD. Lesion volume was assessed using a one-way ANOVA.
3. Results
3.1. Mortality
Of the 152 brain-injured animals (both studies I and II), 40 died immediately following injury, resulting in an acute injury-related mortality of 26% which was consistent with the moderate-to-severe level of injury previously reported from our laboratory (Hoover, et al., 2004). Brain-injured animals that experienced weight loss of more than 30% of their pre-injury weights (n = 8 for Studies I and II; 1 received RWJ-333369-A, four received RWJ-333369, three received vehicle) were euthanized and excluded from the behavioral data analyses. No mortality was observed subsequent to the immediate post-injury/pre-drug period (after 24-hours postinjury) and therefore long-term changes in mortality rates following administration of the experimental compounds could not be assessed.
3.2. Study I
3.2.1. Acute post-traumatic motor (neuroscore) and cognitive (MWM) function
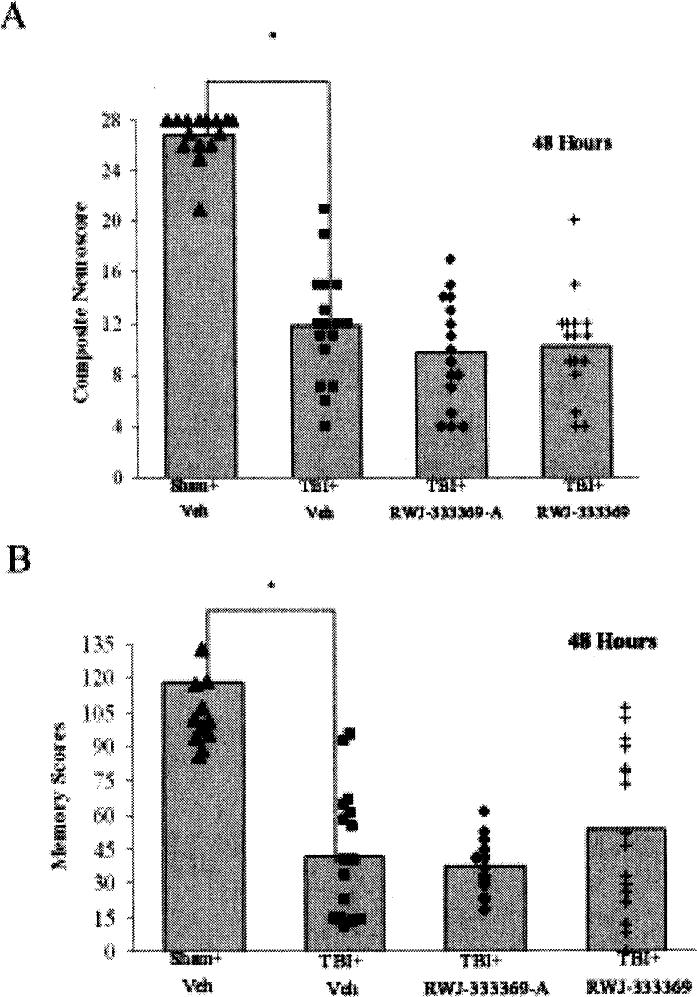
At 48 hours post-injury, brain-injured, vehicle-treated animals were observed to have both motor and memory deficits in comparison to the sham-injured, vehicle-treated controls (p < 0.05; Figss 1A and 1B). After treatment with either RWJ-333369-A or RWJ-333369 (60 mg/kg, p.o.), there was no attenuation of the motor deficit observed at the 48 hour time point (p = n.s.; Fig. 1A). Moreover, treatment with either RWJ-333369-A or RWJ-333369 did not attenuate the injury-induced cognitive deficits in comparison to the brain-injured animals given vehicle at the 48 hour time point (p = n.s.; Fig. 1B).
Fig. 1.
(A) Effects of RWJ-333369-A and RWJ-333369 on 48-hour post-injury neurological inotor function. For both RWJ333369-A vs. vehicle groups and RWJ-333369 vs. vehicle groups, there were significant differences among groups. On post hoc analysis, there was an injury effect at 48 hours between sham-injured, vehicle-treated animals and brain-injured, vehicle-treated animals (p < 0.05*); however, there were no treatment effects (p = n.s.) between either RWJ-333369-A or RWJ-333369 and vehicle-treated animals in brain-injured groups. (B) Effects of RWJ-333369-A and RWJ-333369 on 48-hour post-injury cognitive function. There was a significant brain-injury impairment of retrograde memory in the 1 M Morris water maze at 48 hours post-injury (sig ANOVA p < 0.05, post hoc p < 0.05* sham-injured vehicle versus brain-injured vehicle). There was no improvement in memory seen with the administration of RWJ-333369-A or RWJ-333369 (p = n.s.) in brain-injured animals compared to vehicle. Morris water maze scores are presented as mean.
3.2.2. Acute post-traumatic regional cerebral edema
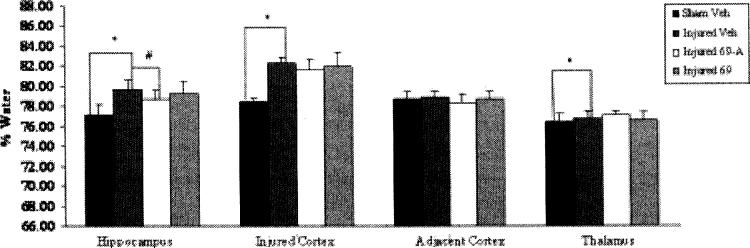
In sham-injured, vehicle-treated animals, the average brain water content in the ipsilateral hippocampus was 77.08%, in the ipsilateral injured cortex 78.41%, and in the ipsilateral thalamus 76.35% (Fig. 2A). Lateral FP brain injury increased brain water content of vehicle-treated animals in the ipsilateral hippocampus to 79.64% (p < 0.05), in the injured cortex to 82.24% (p < 0.05), and in the ipsilateral thalamus to 76.73% (p < 0.05; Fig. 2A). These numbers were consistent with previous sham and injured values obtained in our laboratory (Bareyre, et al., 1997; Hoover, et al., 2004; Soares, et al., 1992). Treatment with RWJ-333369-A significantly reduced brain water content measured at 48 hours in both the injured ipsilateral hippocampus (drug 78.61 ± 1.14% vs. vehicle 79.64 ± 0.95%; p < 0.05; Fig. 2A) and contralateral hippocampus (drug 76.69 ± 0.81% vs. vehicle 78.31 ± 0.83%; p < 0.05; Fig. 2B). Treatment with RWJ-333369 at 48 hours post-injury had no effect on water content in any brain region (p = n.s.; Fig. 2C).
Fig. 2.
Effects of RWJ-333369-A and RWJ-333369 on 48 hour post-injury ipsilateral edema. At 48 hours post-injury, there were significant injury effects (p < 0.05*) in the ipsilateral hippocampus, ipsilateral injured cortex, and the ipsilateral thalamus. In addition, brain water content was significantly reduced in the ipsilateral and contralateral hippocampi of brain-injured animals treated with RWJ-333369-A compared to injured vehicle-treated animals (p < 0.05#). Edema values are presented as mean ± SD.
3.3. Study II
3.3.1. Composite Neuroscore: Assessment of gross neurologic motor function
At 48 hours post-injury through the 4-week time point, brain-injured, vehicle-treated animals were observed to have significant motor deficits in comparison to the sham-injured, vehicle-treated animals (p < 0.05; Fig. 3A). Neither treatment with RWJ-333369-A nor RWJ-333369 attenuated motor deficits at any time point compared to the brain-injured groups administered with vehicle (p = n.s.; Fig. 3A).
Fig. 3.
(A) Effects of RWJ-333369-A and RWJ-333369 on 48 hour, 1, 2, 3, and 4 weeks post-injury on composite neuroscore test. For both RWJ-333369-A vs. vehicle groups and RWJ-333369 vs. vehicle groups, there were significant differences among groups. On post hoc analysis, there was an injury effect at all time points (p < 0.05*); however, there were no treatment effects (p = n.s.) between neither RWJ-333369-A or RWJ-333369 and vehicle-treated animals in the brain-injured groups. (B) Effects of RWJ-333369-A and RWJ-333369 on 72-hour, 1, 2, 3, and 4 weeks post-injury on the beam balance test. For both RWJ-333369-A vs. vehicle groups and RWJ-333369 vs. vehicle groups, there were significant differences among groups. On post hoc analysis, there was an injury effect at all time points (p < 0.05*); however, there were no treatment effects (p = n.s.) between neither RWJ-333369-A or RWJ-333369 and vehicle-treated animals in the brain-injured groups. (C) Effects of RWJ-333369-A and RWJ-333369 on 72-hour, 1, 2, 3, and 4 weeks post-injury on the rotating pole test, left rotation. For both RWJ-333369-A vs. vehicle and RWJ-333369 vs. vehicle groups, there were significant differences among groups. On post hoc analysis, there was an injury effect at all time points (p < 0.05*). There were no significant treatment effects for RWJ-333369-A (p = n.s). At all time points, 72 hours up until four weeks post-injury, injured animals treated with RWJ-333369 performed worse in comparison to brain-injured, vehicle-treated animals. At the 4 week time point; the difference between the two groups was statistically significant (p < 0.05#).
3.3.2. Beam Balance: Assessment of vestibulomotor function
Regardless of treatment status, brain-injured animals exhibited a decrease in beam balance function in comparison to sham-injured animals at all time points (p < 0.05; Fig. 3B). Treatment with either drug did not improve vestibulomotor function of brain-injured animals (Fig. 3B). By the fourth week of testing, all injured groups median scores were equivalent (Fig. 3B).
3.3.3. Rotating Pole: Assessment of coordination and integration of movement
Brain-injured, vehicle-treated animals performed worse on the rotating pole task than sham-injured, vehicle-treated animals at all time points (p < 0.05; Fig. 3C). No treatment effect was observed for either compound (p = n.s.; Fig. 3C), although injured animals treated with RWJ-333369 performed significantly worse on the rotating pole in comparison to brain-injured, vehicle-treated animals (p < 0.05; Fig. 3C).
3.3.4. Assessment of visuospatial learning
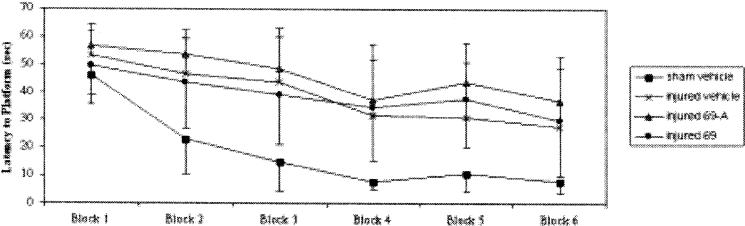
At four weeks post-injury, brain-injured, vehicle-treated animals were observed to have a cognitive deficit when compared to sham-injured, vehicle-treated animals (p < 0.05; Fig. 4). Overall, regardless of group (treatment/vehicle, injured/sham), all animals were able to learn (p < 0.05; Fig. 4). Treatment with either RWJ-333369-A or RWJ-333369 did not attenuate the cognitive deficits observed in brain-injured animals at four weeks post-injury when compared with vehicle-treated, brain-injured groups (p = n.s.; Fig. 4).
Fig. 4.
Effects of RWJ-333369-A and RWJ-333369 on 4 weeks post-injury 1.83 M Morris water maze leading test. For both RWJ-333369-A vs. vehicle and RWJ-333369 vs. vehicle groups there was significant brain-injury related impairment on learning in the Morris water maze (p < 0.05). Overall, all groups showed significant learning (p < 0.05). There was no improvement (decrease) in learning latencies seen with administration of RWJ-333369-A or RWJ-333369 (p = n.s.) in brain-injured animals compared to vehicle. Latencies are presented as mean ± SD.
3.3.5. Assessment of neuroprotection
Measurements at four weeks post-injury of hemispheric tissue volume showed that brain-injured animals treated with either RWJ-333369-A or RWJ-333369 did not have a significant decrease in lesion volume in comparison to brain-injured, vehicle-treated animals (p = n.s.).
4. Discussion
In designing this study, we hypothesized that acute administration of the novel antiepileptic compounds, RWJ-333369 and RWJ-333369-A, would improve functional outcome, reduce regional brain edema and cell death following experimental brain injury in rats. Our observations demonstrate that although acute administration of RWJ-333369-A produced a small but significant reduction in acute cerebral edema in the ipsilateral and contralateral hippocampus in the brain-injured animals, no behavioral efficacy of either compound was observed on motor and cognitive deficits measured during the first four weeks post-injury. Moreover, rats treated with RWJ-333369 showed a slight but significant worsening of motor deficits at four weeks post-injury on the rotating pole task. Neither treatment attenuated cortical cell death (lesion volume) measured at four weeks post-injury. Although a reduction of 1% water content in the hippocampus is not apparently profound, it is, in fact, highly significant and reductions of this magnitude have been previously shown to be temporally associated with highly beneficial effects on cognitive outcome (Okiyama, et al., 1992; Okiyama, et al., 1997; Zhang, Raghupathi, Saatman, Smith & Stutzmann, 1998). Since the mechanistic association between a reduction in regional hippocampal swelling and cognitive improvement has not been established in experimental models of TBI, it is possible that beneficial cognitive effects of RWJ-333369-A may be detectable in the more chronic (later than 1 month) post-injury period.
RWJ-333369-A and RWJ-333369 were administered systemically over the first 24 hours post-injury. The half-life of RWJ-333369 has been determined to be approximately 12 hours in healthy volunteers (B. Zhou, personal communication). Previous studies have demonstrated that administration of RWJ-333369 at escalating doses of 60 mg/kg, 90 mg/kg or 120 mg/kg showed a dose-response neuroprotective effect in reducing the amount of regional cell death in the lithium-pilocarpine model of status epilepticus (Francois, et al., 2005). The two higher doses of RWJ-333369 when administered during status epilepticus, and thereafter for 6 days, delayed or prevented the occurrence of spontaneous, recurrent seizures. Since the present study evaluated these compounds at only a single dose of 60 mg/kg, it is possible that future use of higher doses of these antiepileptic compounds could reveal neurobehavioral and/or neuroprotective efficacy in models of experimental TBI.
The administration of RWJ-333369-A decreased regional edema in the injured and contralateral hippocampus measured at 48 hours post-injury. Following lateral FP brain injury, edema formation can be classified as vasogenic (caused by the breakdown of the BBB) or cytotoxic (associated with neurotoxic cascades) (Unterberg, Stover, Kress & Kiening, 2004). Using this model, cyotoxic edema formation has been shown to begin as early as one hour post-injury in the ipsilateral hippocampus (Soares, et al., 1992). Edema formation peaks at 48 hours post-injury in the ipsilateral brain following lateral FP injury with hippocampal swelling returning to normal by three days post-injury (Soares, et al., 1992). Since the half-life of RWJ-333369-A is approximately 12 hours with the last dose being at 24 hours post-injury, the compound was likely remaining in the central nervous system (CNS) at 48 hours. The positive efficacy of RWJ-333369-A on attenuation of hippocampal edema could be due to an anti-excitotoxic action of this compound or some currently unknown mechanism. Although a trend towards reduced cortical swelling was observed, the lack of significant effect on regional edema in the injured/peri-injured cortex is not surprising, given that a component of this swelling is likely related to intracerebral hemorrhage and vasogenic edema.
Interestingly, the effect of RWJ-333369-A on hippocampal edema was bilateral. Previous studies investigating the effects of anticonvulsant drugs on edema in the lateral FP model in rats have also shown positive results in both injured and contralateral hemispheres. The NMDA receptor blocker, CP-98,113, significantly reduced bilateral cerebral edema in rats at 48 hours post-injury (Okiyama, et al., 1998; Okiyama, et al., 1997), while the Ca2+ channel blocker, (S)-emopamil caused a bilateral reduction in edema at 48 hours following injury (Katayama, et al., 1990). Following closed head trauma in rats, Kaplanski et al. (2002) showed that administration of bradykinin B2 receptor antagonist LF 16−0687 Ms during the first 4 hours post-injury reduced edema in both the ipsilateral and contralateral hemispheres. The finding that RWJ-333369-A, at the dose employed, significantly reduces hippocampal edema, despite the lack of effect of a large magnitude, may be of importance in terms of clinical outcome since post-traumatic edema is associated with increased intracranial pressure and secondary injury cascades. In humans, acute reduction of edema in individuals that suffer from a head injury decreases mortality and improves clinical outcome (Unterberg, et al., 2004).
Although a reduction in hippocampal edema would be unlikely to produce changes in post-traumatic motor dysfunction, we were not able to observe corresponding improvements in cognitive outcome associated with small but significant bilateral reductions in hippocampal edema. The possibility exists that the animals may not have been followed long enough to detect cognitive improvements associated with acute reduction of regional hippocampal edema. Future studies employing increased doses and extended time points for edema assessment may reveal more positive efficacy on neurobehavioral outcome. The mechanisms underlying the reduction of post-traumatic hippocampal edema by RWJ-333369-A remain to be elucidated.
Administration of either RWJ-333369-A or RWJ-333369 did not result in improvement in motor function. Previous studies with other anticonvulsants have, however, shown positive effects on post-traumatic neuromotor deficits following TBI. Administration of topiramate attenuated neuromotor deficits at one week and one month post injury in the lateral FP model of brain injury (Hoover, et al., 2002). Following lateral FP brain-injury in rats, animals treated with the NMDA antagonist, CP-98,113, were found to have an improvement of gross motor function for up to two weeks following injury (Okiyama, et al., 1998). The sodium channel blocker, Riluzole, has also been shown to be effective in attenuating gross motor deficits following lateral FP brain injury in rats up to two weeks post-injury (McIntosh, et al., 1996).
Administration of either RWJ-333369-A or RWJ-333369, at the doses employed, also did not result in a significant decrease in post-traumatic cortical cell loss (lesion volume). Following lateral FP injury, tissue loss due to excitotoxicity, inflammation and other secondary mechanisms results in a decreased hemispheric tissue volume in the injured hemisphere (Bramlett & Dietrich, 2002; Hicks, Soares, Smith & McIntosh, 1996; Scheff & Sullivan, 1999; Soares, et al., 1992). Neuronal cell death begins almost immediately following the injury and has been shown to continue for up to a year (Pierce, et al., 1998; Smith, Chen, Pierce, et al., 1997). Previous research has shown that AEDs and similar compounds can attenuate cortical cell death due to their neuroprotective properties. Belayev et al. (2001). reported that talampanel, a noncompetitive AMPA antagonist, administered acutely following lateral FP brain-injury, reduced lesion area through its neuroprotective properties. In an earlier study, remacemide hydrochloride, administered acutely, was reported to decrease cortical lesion volume at 48 hours following a lateral FP brain injury in rats (Smith, et al., 1997). While both RWJ-333369-A and RWJ-333369 had no positive effect on motor deficits in brain-injured animals, the lack of effect on cortical/thalamic edema and the absence of neuroprotective efficacy with respect to cortical cell death suggests that, at the doses employed, these compounds would not likely improve functional motor outcome. Based on the recent studies of Francois et al. (2005) and Nehlig et al. (2005), it is possible that the administration of higher doses of RWJ-333369 or RWJ-333369-A would demonstrate neuroprotection and associated neurobehavioral efficacy on motor function in experimental models of TBI.
The observation that RWJ-333369-A did not negatively impact behavior at any time points may be important for clinical studies. Since AEDs are used to prevent seizures during the early post-traumatic period. Many AEDs can worsen post-injury recovery, and consequently, their use in the treatment of acute post-traumatic seizures has been questioned (Hernandez, 1997). As the present data shows, RWJ-333369-A did not negatively affect motor or cognitive performance of brain-injured rats, suggesting that it has no deleterious effects on post-traumatic recovery. Future studies with this compound should employ increased/more chronic dosing paradigms and neurobehavioral evaluation for at least 8−10weeks post-injury to reveal potentially significant behavioral and cognitive effects. Further work with these compounds in TBI models is warranted.
Acknowledgements
The authors acknowledge the technical assistance of Dr. Christian Schütz and Asenia T. McMillan and the administrative support of Robin Armstrong. This research was supported, in part, by grants from R.W. Johnson, LLC, National Institutes of Health (P50-08803, R01-NS40978, and T32-NS 043126, T32-NR 07106), and a Merit Review Grant from the Veterans Administration.
References
- Bareyre F, Wahl F, McIntosh TK, Stutzmann J-M. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. J Neurotrauma. 1997;14:839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Liu Y, et al. Talampanel, a novel noncompetitive AMPA antagonist, is neuroprotective after traumatic brain injury in rats. J Neurotrauma. 2001;18:1031–1038. doi: 10.1089/08977150152693728. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Traumatic Brain Injury: Incidence and Distribution. Centers for Disease Control and Prevention. DHHS. 10−11−2004. http://www.cdc.gov/node.do/id/0900f3ec8000dbdc/aspectId/AS_A0400020.
- Centers for Disease Control and Prevention Traumatic Brain Injury: Outcomes and Consequences. Centers for Disease Control and Prevention. DHHS. 6−3−2004. http://www.cdc.gov/node.do/id/0900f3ec8000dbdc/aspectId/AS_A0400027.
- Cheney JA, Brown AL, Bareyre FM, et al. The novel compound LOE 908 MS attenuates acute neuromotor dysfunction but not cognitive impairment or cortical tissue loss following traumatic brain injury in rats. J Neurotrauma. 2000;17:83–91. doi: 10.1089/neu.2000.17.83. [DOI] [PubMed] [Google Scholar]
- Cheney JA, Weisser JD, Bareyre FM, et al. The Maxi-K potassium channel opener BMS-204352 attenuates regional cerebral edema and neurological motor impairment following experimental brain injury. J Cereb Blood Flow and Metab. 2001;21:403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Current Topics in Medicinal Chemistry. 2005;5:3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sombati S, Coulter DA. Epilepsia. Suppl 1. Vol. 41. S40-S44: 2000. Effects of topiramate on sustained repetitive firing and spontaneous recurrent seizure discharges in cultured hippocampal neurons. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat: Neurological, physiological, and histopathological characteristics. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Ma X, Kline AE, et al. Acute etomidate treatment reduces cognitive deficits and histopathology in rats with traumatic brain injury. Crit Care Med. 2003;31:2222–2227. doi: 10.1097/01.CCM.0000080493.04978.73. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:789–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Faden AI, O'Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA. Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improves outcome after brain trauma. Exp Neurol. 2001;167:435–444. doi: 10.1006/exnr.2000.7577. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces expression of interleukin-l βmRNA in the rat brain. Mol Brain Res. 1995;30:125–130. doi: 10.1016/0169-328x(94)00287-o. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Nehlig A. A new drug RWJ333369 protects hippocampal layer CA1, amygdala, thalamus and ventral cortices in the lithium-pilocarpine model (li-pilo) of epilepsy and delays or prevents the occurrence of spontaneous recurrent seizures; Proceedings of the American Epilepsy Society; Washington, DC. December 2−6.2005. [Google Scholar]
- Graham DI, Gennarelli TA, McIntosh TK. Cellular and molecular consequences of TBI. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathology. 7th ed Arnold; London: 2002. pp. 830–835. [Google Scholar]
- Graham DI, Raghupathi R, Saatman KE, Meaney DF, McIntosh TK. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 2000;99:117–124. doi: 10.1007/pl00007414. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Jenkins LW, Lyeth BG. Neurotransmitter -mediated mechanisms of traumatic brain injury - acetylcholine and excitatory amino acids. J Neurotrauma. 1992:S173–S187. [PubMed] [Google Scholar]
- Hayes RL, Yang K, Whitson JS, et al. Rescue of injury-induced neurofilament loss by BDNF gene transfection in primary septo-hippocampal cell cultures. Neurosci Lett. 1995;121:121–125. doi: 10.1016/0304-3940(95)11561-x. [DOI] [PubMed] [Google Scholar]
- Hernandez TD. Preventing post-traumatic epilepsy after bran injury: Weighing the costs and benefits of anticonvulsant prophylaxis. TIPS. 1997;18:59–62. [PubMed] [Google Scholar]
- Hicks R, Soares H, Smith D, McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol (Berl) 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- Hoover RC, Motta M, Davis J, et al. Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J Neurotrauma. 2004;21:501–512. doi: 10.1089/089771504774129847. [DOI] [PubMed] [Google Scholar]
- Kaplanski J, Pruneau D, Asa I, et al. LF 16−0687 Ms, a bradykinin B-2 receptor antagonist, reduces brain edema and improves long-term neurological function recovery after closed head trauma in rats. J Neurotrauma. 2002;19:953–964. doi: 10.1089/089771502320317104. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Ikezaki K. Early cellular swelling in experimental traumatic brain injury: a phenomenon mediated by excitatory amino acids. Acta Neurochir (Suppl) (Wien) 1990;51:271–273. doi: 10.1007/978-3-7091-9115-6_92. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hendrich KS, Dixon CE, Schiding JK, Williams DS, Ho C. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J Neurotrauma. 2002;19:1029–1037. doi: 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 1995;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Friberg H, Neumar RW, et al. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J Cereb Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Clausen F, Lewander T, Hillered L. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J Neurotrauma. 2001;18:1217–1227. doi: 10.1089/089771501317095250. [DOI] [PubMed] [Google Scholar]
- Marklund N, Clausen F, McIntosh TK, Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat. J Neurotrauma. 2001;18:821–832. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- Mattiasson GJ, Philips MF, Tomasevic G, Johansson BB, Wieloch T, McIntosh TK. The rotating pole test: evaluation of its effectiveness in assessing functional motor deficits following experimental brain injury in the rat. J Neurosci Methods. 2000;95:75–82. doi: 10.1016/s0165-0270(99)00162-4. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Donnelly S, Sun X, Fenton T, Puri N, Graham DI. Axonal cytoskeletal responses to nondisruptive axonal injury and the short-term effects of posttraumatic hypothermia. J Neurotrauma. 1999;16:1225–1234. doi: 10.1089/neu.1999.16.1225. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Didion SP. Glutamate-induced disruption of the blood-brain barrier in rats. Role of nitric oxide, Stroke. 1996;27:965–969. doi: 10.1161/01.str.27.5.965. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Voddi M, Perri BR, Stutzmann J-M. Riluzole, a novel neuroprotective agent, attenuates both neurologic motor and cognitive dysfunction following experimental brain injury in the rat. J Neurotrauma. 1996;13:767–780. doi: 10.1089/neu.1996.13.767. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Faden AI. Traumatic brain injury in the rat: Characterization of a lateral fluid percussion model. Neurosci. 1989;238:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Nehlig A, Rigoulot M, Boehrer A. A new drug, RWJ 333369 displays potent antiepileptic properties in genetic models of absence and audiogenic epilepsy; Proceedings of the American Epilepsy Society; Washington, DC. December 2−6.2005. [Google Scholar]
- O'Dell DM, Gibson CJ, Wilson MS, DeFord SM, Hamm RJ. Positive and negative modulation of the GABA(A) receptor and outcome after traumatic brain injury in rats. Brain Res. 2000;861:325–332. doi: 10.1016/s0006-8993(00)02055-2. [DOI] [PubMed] [Google Scholar]
- Okiyama K, Smith DH, Thomas MJ, McIntosh TK. Evaluation of a novel calcium channel blocker (S)-emopamil on regional cerebral edema and neurobehavioral function after experimental brain injury. J Neurosurg. 1992;77:607–615. doi: 10.3171/jns.1992.77.4.0607. [DOI] [PubMed] [Google Scholar]
- Okiyama K, Smith DH, White WF, McIntosh TK. Effects of the NMDA antagonist CP-98,113 on regional cerebral edema and cardiovascular, cognitive, and neurobehavioral function following experimental brain injury in the rat. Brain Res. 1998;792:291–298. doi: 10.1016/s0006-8993(98)00158-9. [DOI] [PubMed] [Google Scholar]
- Okiyama K, Smith DH, White WF, Richter K, McIntosh TK. Effects of the novel NMDA antagonists CP-98,113, CP-101,581, and CP-101,606 on cognitive function and regional cerebral edema following experimental brain injury in the rat. J Neurotrauma. 1997;14:211–222. doi: 10.1089/neu.1997.14.211. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson S. A Stereotactic Atlas of the Rat Brain. Academic Press; New York: 1990. [Google Scholar]
- Pierce JES, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral, and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neurosci. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Kontos HA. The role of oxygen radicals in the pathobiology of traumatic brain injury. Human Cell. 1992;5:345–353. [PubMed] [Google Scholar]
- Povlishock JT, Pettus E,H. Traumatically induced axonal damage: Evidence for enduring changes in axolemma permeability with associated cytoskeletal change. Acta Neurochir. 1996;66:81–86. doi: 10.1007/978-3-7091-9465-2_15. [DOI] [PubMed] [Google Scholar]
- Royo NC, Schouten JW, Fulp CT, et al. From cell death to neuronal regeneration: building a new brain after traumatic brain injury. J Neuropathol Exp Neurol. 2003;62:801–811. doi: 10.1093/jnen/62.8.801. [DOI] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Martin D, Millerm G, McIntosh TK. Systemic administration of interleukin-1 receptor antagonist (IL-1ra) attenuates neuronal death and cognitive dysfunction but not neurological motor deficits following lateral fluid-percussion brain injury in the rat. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–S9. [PubMed] [Google Scholar]
- Smith DH, Perri BR, Raghupathi R, Saatman KE, McIntosh TK. Remacemide hydrochloride reduces cortical lesion volume following brain trauma in the rat. Neurosci Lett. 1997;231:135–138. doi: 10.1016/s0304-3940(97)00551-x. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen X-H, Pierce JES, et al. Progressive atrophy and neuronal death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy VR, Siman VR, McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for imparied mitochondrial and transport function. J Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Toulmond S, Rothwell NJ. Interleukin-l receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Soares HD, Thomas MJ, Cloherty K, McIntosh TK. Development of prolonged focal cerebral edema and regional cation change following experimental brain injury in the rat. J Neurochem. 1992;58:1845–1852. doi: 10.1111/j.1471-4159.1992.tb10061.x. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Urbanska EM, Czuczwar SJ, Kleinrok Z, Turski WA. Excitatory amino acids in epilepsy. Restorat Neurol Neurosci. 1998;13:25–39. [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, et al. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–S20. [PubMed] [Google Scholar]
- Yeh GC, Bonhaus DW, Nadler JV, McNamara JO. N-methyl-D-aspartate receptor plasticity in kindling: quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proc Natl Acad Sci USA. 1989;86:8157–8160. doi: 10.1073/pnas.86.20.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neurosci Letts. 1997;236:123–126. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Raghupathi R, Saatman KE, Smith DH, Stutzmann JM. Riluzole attenuates cortical size, but not hippocampal neuronal loss, following traumatic brain injury in the rat. J Neurosci Res. 1998;52:342–349. doi: 10.1002/(SICI)1097-4547(19980501)52:3<342::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]