Abstract
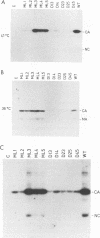
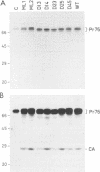
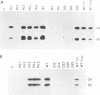
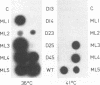
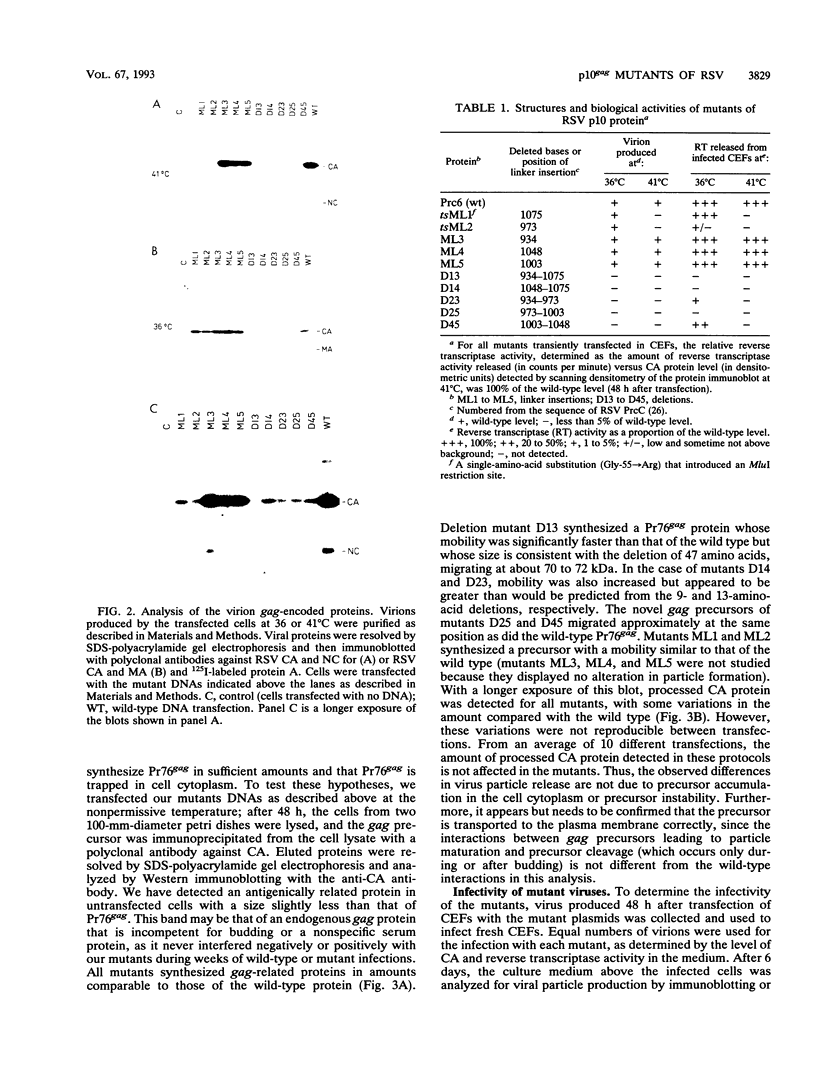
Rous sarcoma virus protein p10 is a gag component of the virion present in stoichiometric amount but of unknown function. To characterize this protein, a series of mutants of p10 with linker insertions or deletions was generated by site-directed mutagenesis of a cloned proviral DNA. The deletions and two of the linkers insertions, which disrupted proline pairs, reduced the yield of virus particles upon transfection. These two linker insertion mutants were moreover thermosensitive for this phenotype, producing fewer virus particles at 41 degrees C than at 36 degrees C. Examination of the intracellular viral proteins demonstrated that for all mutants, the amount of gag precursor was similar to the wild-type level. Moreover, the amount of mature gag CA that could be detected by this analysis was similar between each of the mutants and the wild type. This finding suggests that the transport of gag to the membrane and the initial stages of maturation were not affected by the mutations. The virus particles contained normal amounts of active reverse transcriptase, showing that the gag-pol polyprotein was incorporated and cleaved properly. Viral RNA was quantitatively and qualitatively similar in mutant and wild-type virions. However, the infectivity of the mutants virions differed; one of the thermosensitive linker insertions that had no effect on particle production at 36 degrees C was nevertheless noninfectious at that temperature. Together, these data suggest that the p10 protein is involved in a late steps of virus maturation, possibly budding, and perhaps also in an early event of viral infection.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988 Nov;8(11):4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K., Ryden T. A., Beemon K. Localization and footprinting of an enhancer within the avian sarcoma virus gag gene. J Virol. 1988 May;62(5):1617–1624. doi: 10.1128/jvi.62.5.1617-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984 Mar;49(3):909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz P., Oertle S., Meric C., Damay P., Spahr P. F. Point mutations in the proximal Cys-His box of Rous sarcoma virus nucleocapsid protein. J Virol. 1990 Oct;64(10):4978–4987. doi: 10.1128/jvi.64.10.4978-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz P., Spahr P. F. Specificity of Rous sarcoma virus nucleocapsid protein in genomic RNA packaging. J Virol. 1992 Aug;66(8):4662–4670. doi: 10.1128/jvi.66.8.4662-4670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Bennett J. C., Bhown A., Pepinsky R. B., Vogt V. M. Amino-terminal amino acid sequence of p10, the fifth major gag polypeptide of avian sarcoma and leukemia viruses. J Virol. 1983 Feb;45(2):885–888. doi: 10.1128/jvi.45.2.885-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskólski M., Miller M., Rao J. K., Leis J., Wlodawer A. Structure of the aspartic protease from Rous sarcoma retrovirus refined at 2-A resolution. Biochemistry. 1990 Jun 26;29(25):5889–5898. doi: 10.1021/bi00477a002. [DOI] [PubMed] [Google Scholar]
- Katoh I., Ikawa Y., Yoshinaka Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J Virol. 1989 May;63(5):2226–2232. doi: 10.1128/jvi.63.5.2226-2232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkait T., Strebel K., Hoggan M. D., Martin M. A., Orenstein J. M. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990 Feb;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur M. W., Thornton J. M. Influence of proline residues on protein conformation. J Mol Biol. 1991 Mar 20;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- McNally M. T., Gontarek R. R., Beemon K. Characterization of Rous sarcoma virus intronic sequences that negatively regulate splicing. Virology. 1991 Nov;185(1):99–108. doi: 10.1016/0042-6822(91)90758-4. [DOI] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Méric C., Gouilloud E., Spahr P. F. Mutations in Rous sarcoma virus nucleocapsid protein p12 (NC): deletions of Cys-His boxes. J Virol. 1988 Sep;62(9):3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle S., Bowles N., Spahr P. F. Complementation studies with Rous sarcoma virus gag and gag-pol polyprotein mutants. J Virol. 1992 Jun;66(6):3873–3878. doi: 10.1128/jvi.66.6.3873-3878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle S., Spahr P. F. Role of the gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990 Dec;64(12):5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Mattaliano R. J., Vogt V. M. Structure and processing of the p2 region of avian sarcoma and leukemia virus gag precursor polyproteins. J Virol. 1986 Apr;58(1):50–58. doi: 10.1128/jvi.58.1.50-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Purification and properties of a fifth major viral gag protein from avian sarcoma and leukemia viruses. J Virol. 1983 Feb;45(2):648–658. doi: 10.1128/jvi.45.2.648-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Endogenous feline (RD-114) and baboon type C viruses have related specific RNA-binding proteins and genome binding sites. Virology. 1978 Jan;84(1):99–107. doi: 10.1016/0042-6822(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. Specificity of in vitro binding of primate Type C viral RNA and the homologous viral p12 core protein. Science. 1976 Jul 23;193(4250):326–328. doi: 10.1126/science.180601. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Stewart L., Vogt V. M. trans-acting viral protease is necessary and sufficient for activation of avian leukosis virus reverse transcriptase. J Virol. 1991 Nov;65(11):6218–6231. doi: 10.1128/jvi.65.11.6218-6231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Martin M. A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988 Sep 2;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Pepinsky R. B., Southard L. E. Primary structure of p19 species of avian sarcoma and leukemia viruses. J Virol. 1985 Oct;56(1):31–39. doi: 10.1128/jvi.56.1.31-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon R. A., Jr, Erdie C. R., Oliver M. G., Wills J. W. Incorporation of chimeric gag protein into retroviral particles. J Virol. 1990 Sep;64(9):4169–4179. doi: 10.1128/jvi.64.9.4169-4179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]