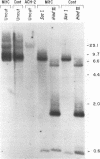
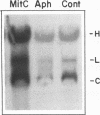
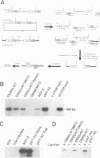
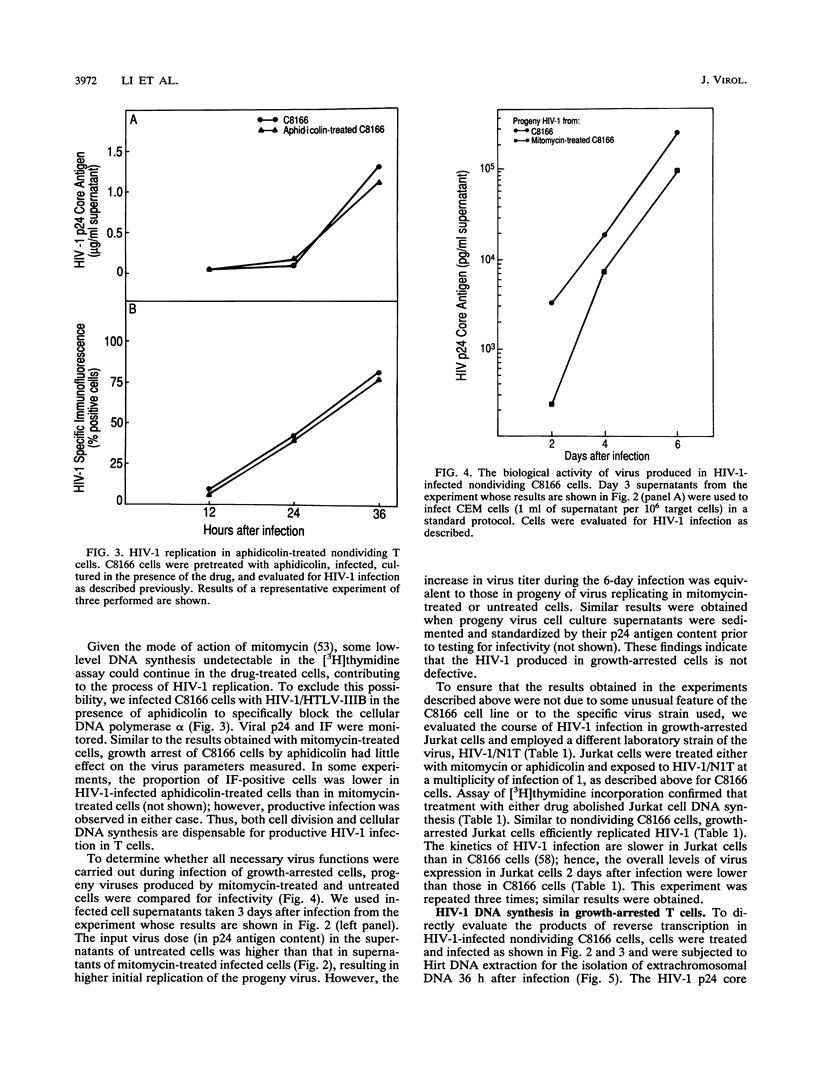
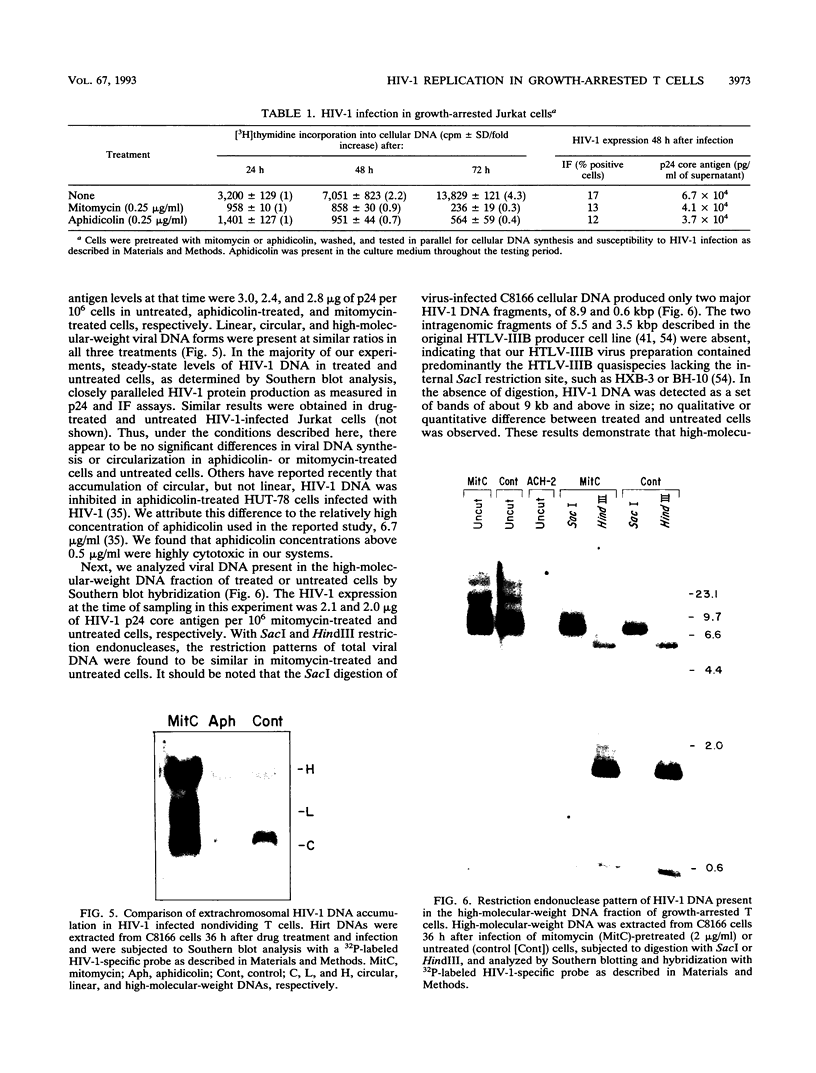
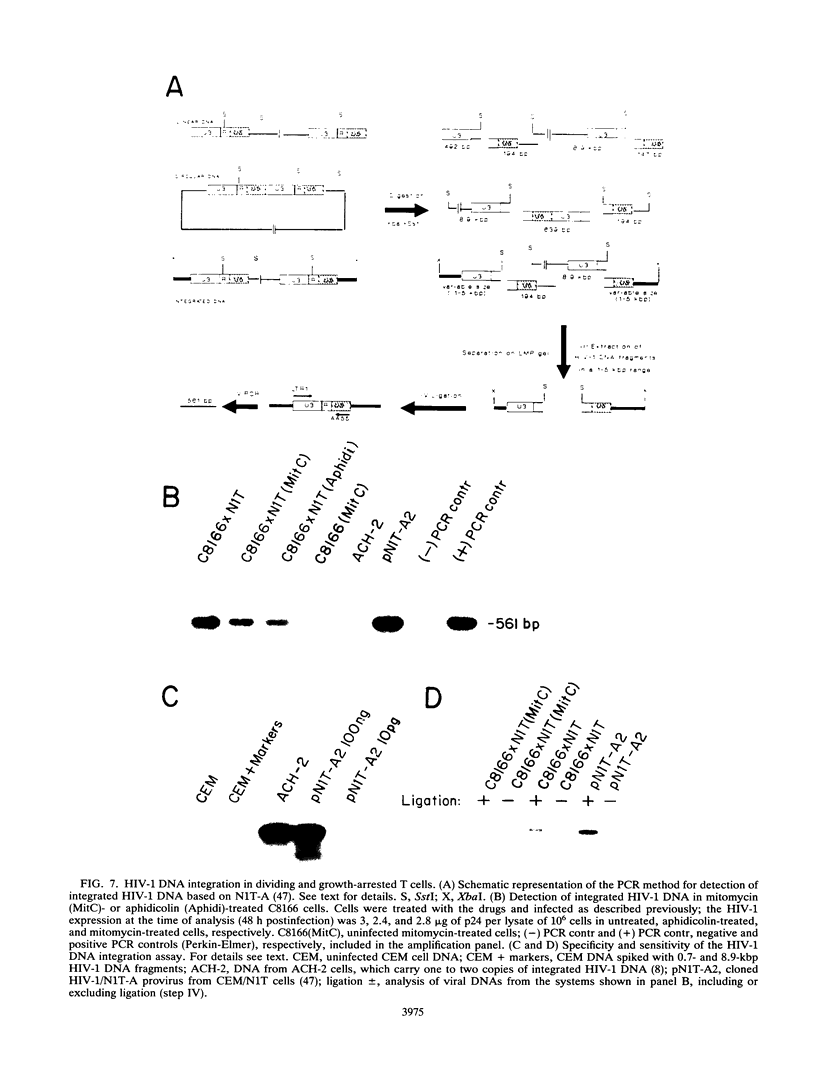
Abstract
Human immunodeficiency virus type 1 (HIV-1) replicates efficiently in nonproliferating monocytes and macrophages but not in resting primary T lymphocytes. To determine the contribution of cell division to the HIV-1 replicative cycle in T cells, we evaluated HIV-1 expression, integration of proviral DNA, and production of infectious progeny virus in C8166 T-lymphoid cells blocked in cell division by treatment with either mitomycin, a DNA cross-linker, or aphidicolin, a DNA polymerase alpha inhibitor. The arrest of cell division was confirmed by assay of [3H]thymidine uptake; the nondividing cells remained viable for at least 3 days after treatment. HIV-1 was expressed and replicated equally well in nondividing and dividing C8166 cells, as judged by the comparison of the levels of p24 core antigens in culture supernatants, the proportion of cells expressing HIV-1 specific antigens, the pattern and quantity of HIV-1 DNA present in the extrachromosomal and total cellular DNA fractions, and the biological activity of progeny viruses. A polymerase chain reaction-based viral DNA integration assay indicated that HIV-1 provirus was integrated in C8166 cells treated with either of the two inhibitors of cell division. Similar results were obtained by using growth-arrested Jurkat T-lymphoid cells. We conclude that cell division and cellular DNA synthesis are not required for efficient HIV-1 expression in T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukrinsky M. I., Sharova N., Dempsey M. P., Stanwick T. L., Bukrinskaya A. G., Haggerty S., Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casareale D., Dewhurst S., Sonnabend J., Sinangil F., Purtilo D. T., Volsky D. J. Prevalence of AIDS-associated retrovirus and antibodies among male homosexuals at risk for AIDS in Greenwich Village. AIDS Res. 1984;1(6):407–421. doi: 10.1089/aid.1.1983.1.407. [DOI] [PubMed] [Google Scholar]
- Casareale D., Stevenson M., Sakai K., Volsky D. J. A human T-cell line resistant to cytopathic effects of the human immunodeficiency virus (HIV). Virology. 1987 Jan;156(1):40–49. doi: 10.1016/0042-6822(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Establishment of infection by spleen necrosis virus: inhibition in stationary cells and the role of secondary infection. J Virol. 1982 Jan;41(1):183–191. doi: 10.1128/jvi.41.1.183-191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Fv-1 host restriction of Friend leukemia virus: analysis of unintegrated proviral DNA. J Virol. 1981 Oct;40(1):45–55. doi: 10.1128/jvi.40.1.45-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Griffin D. E., Johnson R. T. The synthesis and structure of visna virus DNA. Virology. 1979 Mar;93(2):377–386. doi: 10.1016/0042-6822(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Dewhurst S., Sakai K., Bresser J., Stevenson M., Evinger-Hodges M. J., Volsky D. J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987 Dec;61(12):3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Li G. G., Volsky D. J. Differences in the basal activity of the long terminal repeat determine different replicative capacities of two closely related human immunodeficiency virus type 1 isolates. J Virol. 1990 Aug;64(8):3654–3660. doi: 10.1128/jvi.64.8.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Gorrell M. D., Brandon M. R., Sheffer D., Adams R. J., Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992 May;66(5):2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Mohagheghpour N., Benike C. J., Engleman E. G. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989 Feb 1;142(3):773–780. [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Harel J., Rassart E., Jolicoeur P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology. 1981 Apr 15;110(1):202–207. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettman J., Lefkovits I. An attempt to assess the overall diversity of murine T cells using two-dimensional gel electrophoresis. Eur J Immunol. 1984 Sep;14(9):769–777. doi: 10.1002/eji.1830140902. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Lee Y. M., Coffin J. M. Nonrandom integration of retroviral DNA in vitro: effect of CpG methylation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5532–5536. doi: 10.1073/pnas.89.12.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., O'Brien W. A., Zhao J. Q., Golde D. W., Gasson J. C., Chen I. S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep 23;241(4873):1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- Kulkosky J., Skalka A. M. HIV DNA integration: observations and interferences. J Acquir Immune Defic Syndr. 1990;3(9):839–851. [PubMed] [Google Scholar]
- LaFemina R. L., Schneider C. L., Robbins H. L., Callahan P. L., LeGrow K., Roth E., Schleif W. A., Emini E. A. Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol. 1992 Dec;66(12):7414–7419. doi: 10.1128/jvi.66.12.7414-7419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Burrell C. J. Synthesis of human immunodeficiency virus DNA in a cell-to-cell transmission model. AIDS Res Hum Retroviruses. 1992 Feb;8(2):253–259. doi: 10.1089/aid.1992.8.253. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Immunofluorescent localization of equine infectious anemia virus in tissue. Am J Pathol. 1971 Feb;62(2):283–294. [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosborg-Petersen P., Toth F. D., Zachar V., Villadsen J. A., Nørskov-Lauritsen N., Aboagye-Mathiesen G., Chermann J. C., Ebbesen P. Differential HIV replication and HIV-induced interferon production in mononuclear phagocytes: relationship to cell maturation. Res Virol. 1991 Sep-Oct;142(5):353–361. doi: 10.1016/0923-2516(91)90002-k. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989 Jul;70(Pt 7):1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Chen I. S., Zack J. A., Leonard M. L., O'Brien W. A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992 Jan;89(1):176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. M., Krivine A., Pinkston P., Gillis J. M., Huang A., Hammer S. M. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1991 Apr;143(4 Pt 1):850–854. doi: 10.1164/ajrccm/143.4_Pt_1.850. [DOI] [PubMed] [Google Scholar]
- Sakai H., Kawamura M., Sakuragi J., Sakuragi S., Shibata R., Ishimoto A., Ono N., Ueda S., Adachi A. Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J Virol. 1993 Mar;67(3):1169–1174. doi: 10.1128/jvi.67.3.1169-1174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Dewhurst S., Ma X. Y., Volsky D. J. Differences in cytopathogenicity and host cell range among infectious molecular clones of human immunodeficiency virus type 1 simultaneously isolated from an individual. J Virol. 1988 Nov;62(11):4078–4085. doi: 10.1128/jvi.62.11.4078-4085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Schuitemaker H., Kootstra N. A., Koppelman M. H., Bruisten S. M., Huisman H. G., Tersmette M., Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Invest. 1992 Apr;89(4):1154–1160. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Tartar A., Portetelle D., Burny A., Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986 Mar 28;231(4745):1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. Unexpectedly high levels of HIV-1 RNA and protein synthesis in a cytocidal infection. Science. 1988 Dec 16;242(4885):1554–1557. doi: 10.1126/science.3201245. [DOI] [PubMed] [Google Scholar]
- Srivastava K. K., Fernandez-Larsson R., Zinkus D. M., Robinson H. L. Human immunodeficiency virus type 1 NL4-3 replication in four T-cell lines: rate and efficiency of entry, a major determinant of permissiveness. J Virol. 1991 Jul;65(7):3900–3902. doi: 10.1128/jvi.65.7.3900-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Poulin L., Levy J. A. Lack of human immunodeficiency virus type 1 (HIV-1) replication and accumulation of viral DNA in HIV-1-infected T cells blocked in cell replication. J Gen Virol. 1992 Apr;73(Pt 4):933–939. doi: 10.1099/0022-1317-73-4-933. [DOI] [PubMed] [Google Scholar]
- Valentin A., Von Gegerfelt A., Matsuda S., Nilsson K., Asjö B. In vitro maturation of mononuclear phagocytes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4(8):751–759. [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Pellegrino M. G., Li G., Logan K. A., Aswell J. E., Lawrence N. P., Decker S. R. Titration of human immunodeficiency virus type 1 (HIV-1) and quantitative analysis of virus expression in vitro using liquid RNA-RNA hybridization. J Virol Methods. 1990 Jun;28(3):257–271. doi: 10.1016/0166-0934(90)90119-z. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Sakai K., Stevenson M., Dewhurst S. Retroviral etiology of the acquired immune deficiency syndrome (AIDS). AIDS Res. 1986 Dec;2 (Suppl 1):S35–S48. [PubMed] [Google Scholar]
- Weinberg J. B., Matthews T. J., Cullen B. R., Malim M. H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991 Dec 1;174(6):1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M. C., Narayan O., Kennedy P. G., Clements J. E. Pathogenesis of visna/maedi and caprine arthritis-encephalitis: new leads on the mechanism of restricted virus replication and persistent inflammation. Vet Immunol Immunopathol. 1987 May;15(1-2):167–180. doi: 10.1016/0165-2427(87)90110-3. [DOI] [PubMed] [Google Scholar]