Abstract
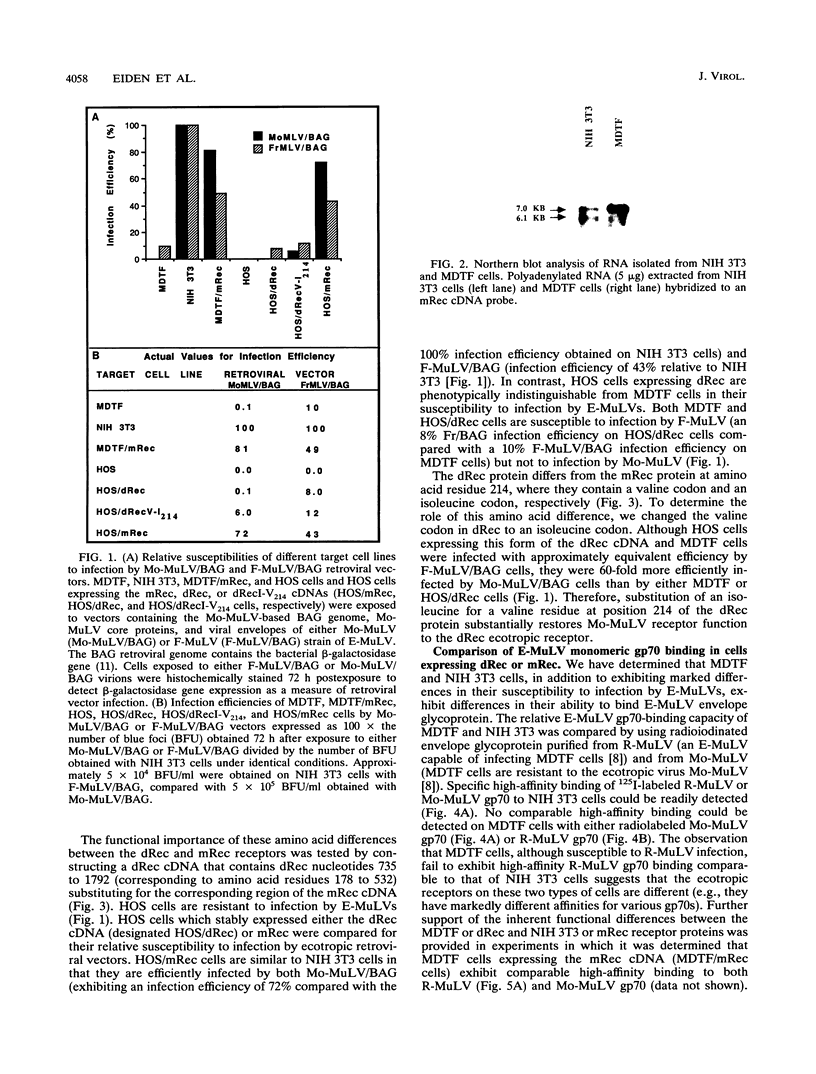
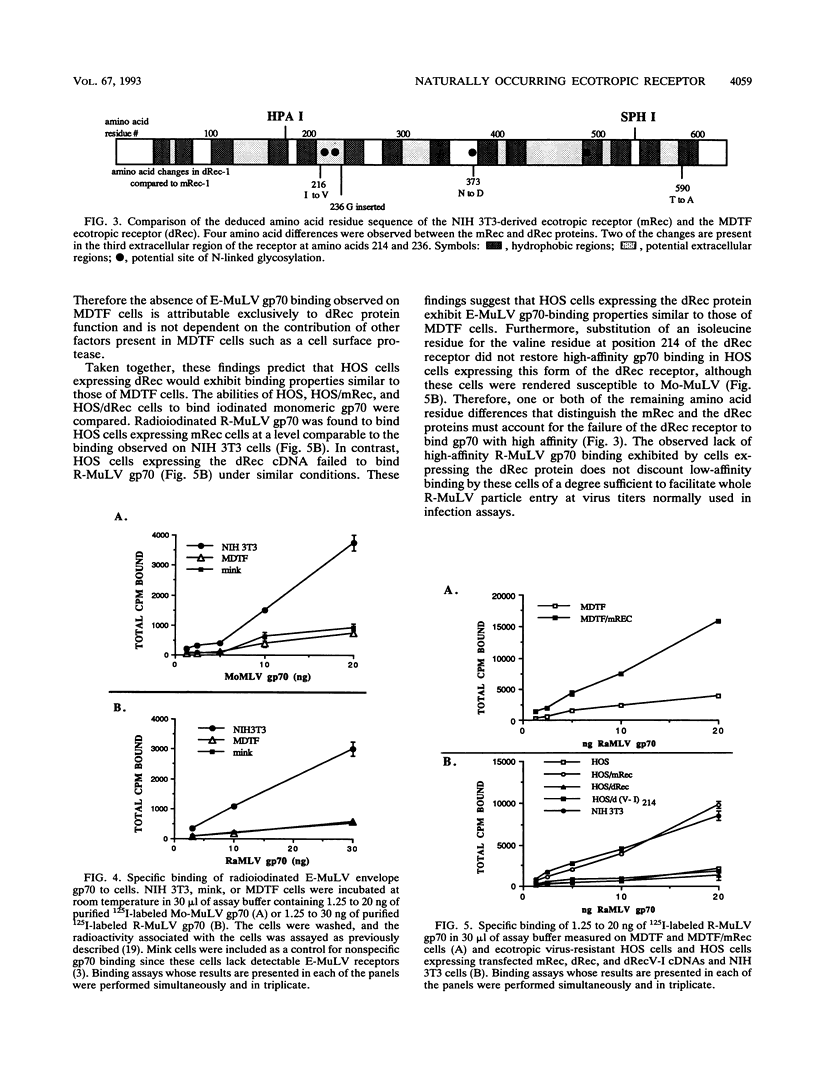
A fibroblast cell line (MDTF) derived from the feral mouse Mus dunni is resistant to infection by Moloney murine leukemia virus (Mo-MuLV), an ecotropic murine leukemia virus (E-MuLV) (M. R. Lander and S. K. Chattopadadhyay, J. Virol. 52:695-698, 1984). MDTF cells can be infected by other E-MuLVs such as Friend MuLV and Rauscher MuLV, which have been demonstrated to use the same receptor as Mo-MuLV in NIH 3T3 cells (A. Rein and A. Schultz, Virology 136:144-152, 1984). We have now shown that the block to Mo-MuLV infection of MDTF cells occurs at the level of the envelope-receptor interaction. We have cloned the ecotropic receptor cDNA from MDTF cells (dRec) and compared its sequence with that of the NIH 3T3 cell receptor (mRec). Although the deduced dRec and mRec proteins differ at only four amino acid residues, we demonstrate that these changes account for the resistance of MDTF cells to Mo-MuLV infection. Our findings suggest that retroviruses in the same receptor class can exhibit different host ranges due to single amino acid differences in their cellular receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Bishayee S., Strand M., August J. T. Cellular membrane receptors for oncovirus envelope glycoprotein: properties of the binding reaction and influence of different reagents on the substrate and the receptors. Arch Biochem Biophys. 1978 Jul;189(1):161–171. doi: 10.1016/0003-9861(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Different murine cell lines manifest unique patterns of interference to superinfection by murine leukemia viruses. Virology. 1985 Feb;141(1):119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Ellerbrok H., Pozo F., Nishio J., Hayes S. F., Evans L. H., Chesebro B. Sequences in the U5-gag-pol region influence early and late pathogenic effects of Friend and Moloney murine leukemia viruses. J Virol. 1990 May;64(5):2135–2140. doi: 10.1128/jvi.64.5.2135-2140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., Eiden M. V. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J Virol. 1991 Nov;65(11):5975–5982. doi: 10.1128/jvi.65.11.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. A., Marsh J. W., Eiden M. V. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J Virol. 1992 Dec;66(12):7262–7269. doi: 10.1128/jvi.66.12.7262-7269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Reitz M. S., Okayama H., Eiden M. V. Formation of infectious hybrid virions with gibbon ape leukemia virus and human T-cell leukemia virus retroviral envelope glycoproteins and the gag and pol proteins of Moloney murine leukemia virus. J Virol. 1989 May;63(5):2374–2378. doi: 10.1128/jvi.63.5.2374-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Yoshimoto E., Meruelo D. Identification of amino acid residues critical for infection with ecotropic murine leukemia retrovirus. J Virol. 1993 Mar;67(3):1310–1314. doi: 10.1128/jvi.67.3.1310-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Yoshimoto E., Meruelo D. Molecular cloning and characterization of a novel human gene homologous to the murine ecotropic retroviral receptor. Virology. 1991 Nov;185(1):10–17. doi: 10.1016/0042-6822(91)90748-z. [DOI] [PubMed] [Google Scholar]




