Abstract
Cytotoxic T lymphocytes (CTL) play an essential role in recovery from viral infections, but induction of CTL responses with nonreplicating antigens is difficult to achieve. Exogenous antigens, such as viral proteins and peptides, normally induce CD4+ T-cell responses unless appropriately delivered to the major histocompatibility complex class I antigen presentation pathway. In vitro studies performed to address this issue revealed a similar scenario, and primary CTL induction with nonreplicating antigens has rarely been reported. This study demonstrated primary antiviral CTL induction in vitro with exogenous antigens delivered in vivo to dendritic cells. This study also evaluated the efficacy of glycoprotein B peptide (free or encapsulated in liposomes), peptide-tripalmitoyl-S-glyceryl cysteinyl conjugate (acylpeptide), and glycoprotein B protein encapsulated in pH-sensitive liposomes as antigen delivery vehicles. Our results show that higher levels of cytotoxicity against herpes simplex virus type 1 resulted from exposure of dendritic cells to peptide-tripalmitoyl-S-glyceryl cysteinyl in liposomes. Macrophages treated in a similar manner were not effective stimulators for primary CTL induction. Our data have relevance to the understanding of mechanisms of antigen processing and presentation and the design of antiviral vaccines.
Full text
PDF



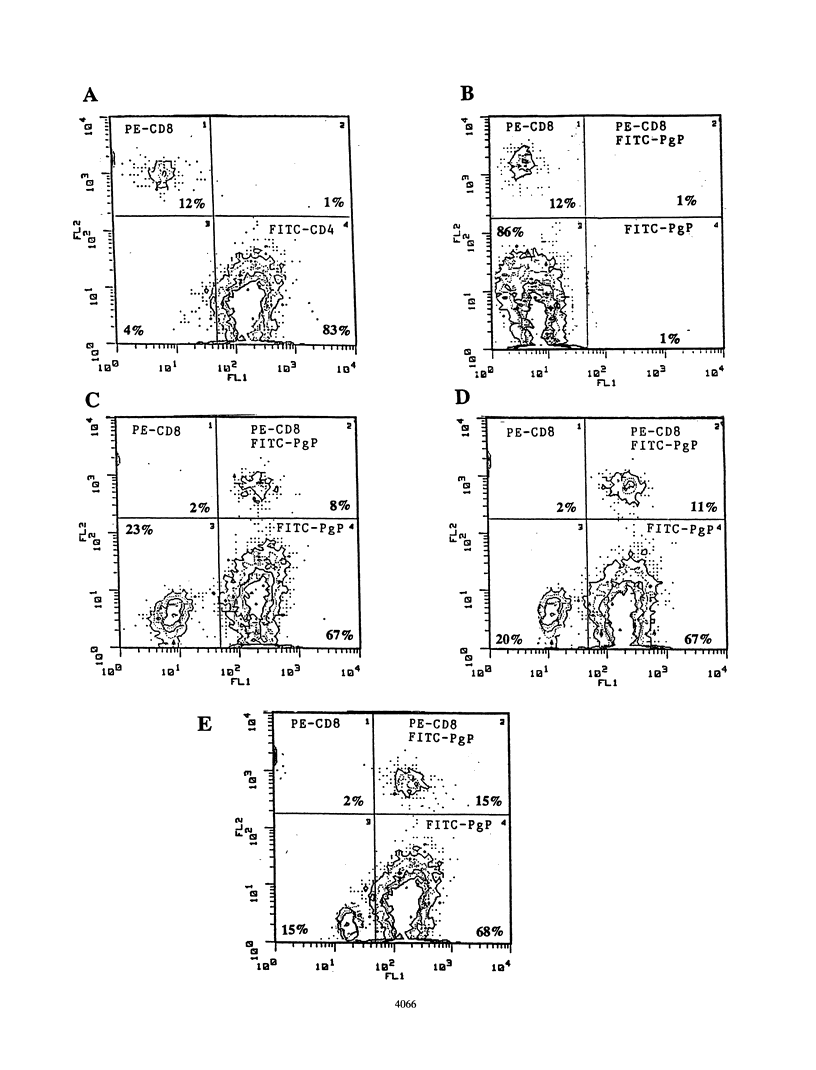
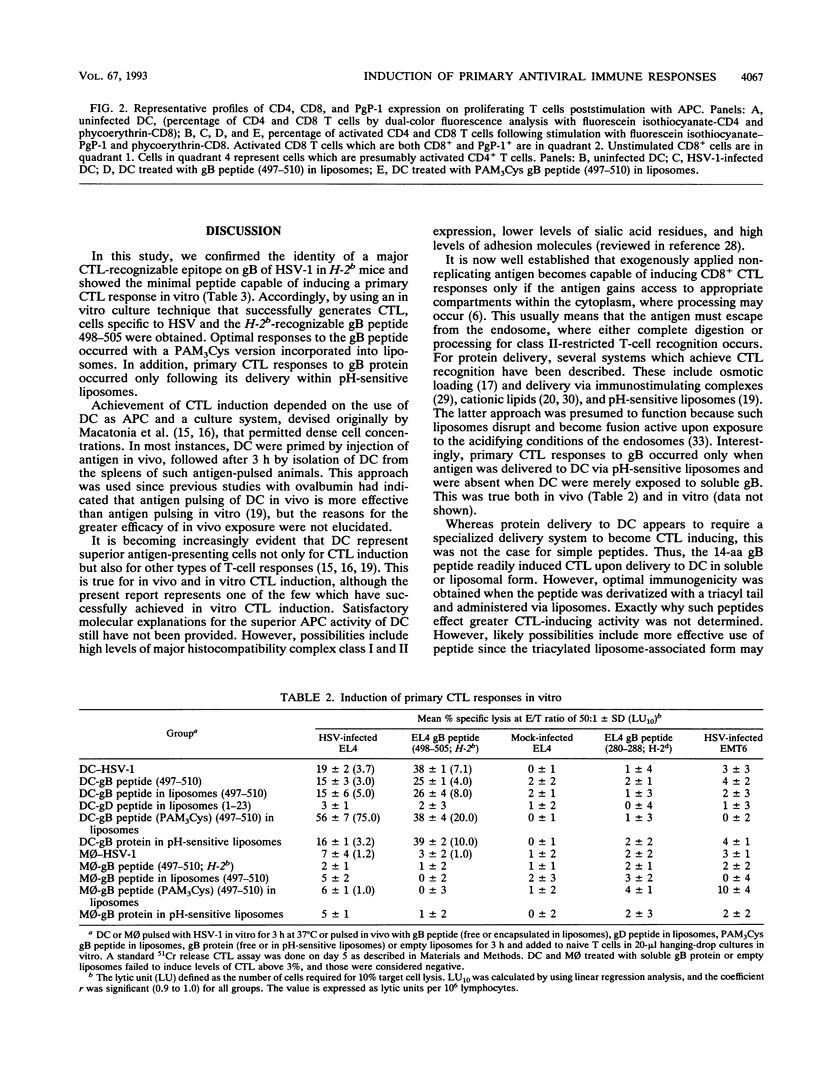


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichele P., Hengartner H., Zinkernagel R. M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990 May 1;171(5):1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Immunodominance in T lymphocyte recognition. Immunol Lett. 1988 Jun;18(2):83–92. doi: 10.1016/0165-2478(88)90046-6. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987 Jan 15;325(6101):192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Guagliardi L. E. The cell biology of antigen processing and presentation. Annu Rev Immunol. 1991;9:707–744. doi: 10.1146/annurev.iy.09.040191.003423. [DOI] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., Horvath C., Bron C., Pedrazzini T., Howe R. C., MacDonald H. R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987 May 15;138(10):3120–3129. [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Cantin E. M., Eberle R., Baldick J. L., Moss B., Willey D. E., Notkins A. L., Openshaw H. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gao X. M., Zheng B., Liew F. Y., Brett S., Tite J. Priming of influenza virus-specific cytotoxic T lymphocytes vivo by short synthetic peptides. J Immunol. 1991 Nov 15;147(10):3268–3273. [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Rosenthal K. L., Johnson D. C. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991 Mar;65(3):1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Patterson S., Knight S. C. Primary proliferative and cytotoxic T-cell responses to HIV induced in vitro by human dendritic cells. Immunology. 1991 Nov;74(3):399–406. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Zhou F., Reddy R., Huang L., Rouse B. T. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992 Feb 1;175(2):609–612. doi: 10.1084/jem.175.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Zhou X., Huang L., Rouse B. T. Class I restricted CTL recognition of a soluble protein delivered by liposomes containing lipophilic polylysines. J Immunol Methods. 1992 Aug 10;152(2):237–243. doi: 10.1016/0022-1759(92)90145-j. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Witmer M. D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Zhou F., Nair S., Huang L., Rouse B. T. In vivo cytotoxic T lymphocyte induction with soluble proteins administered in liposomes. J Immunol. 1992 Mar 1;148(5):1585–1589. [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Stevanović S., Jung G., Walden P., Rammensee H. G. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991 Nov;21(11):2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- Schild H., Deres K., Wiesmüller K. H., Jung G., Rammensee H. G. Efficiency of peptides and lipopeptides for in vivo priming of virus-specific cytotoxic T cells. Eur J Immunol. 1991 Nov;21(11):2649–2654. doi: 10.1002/eji.1830211102. [DOI] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Walker C., Selby M., Erickson A., Cataldo D., Valensi J. P., Van Nest G. V. Cationic lipids direct a viral glycoprotein into the class I major histocompatibility complex antigen-presentation pathway. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7915–7918. doi: 10.1073/pnas.89.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmüller K. H., Bessler W., Jung G. Synthesis of the mitogenic S-[2,3-bis(palmitoyloxy)propyl]-N-palmitoylpentapeptide from Escherichia coli lipoprotein. Hoppe Seylers Z Physiol Chem. 1983 May;364(5):593–606. doi: 10.1515/bchm2.1983.364.1.593. [DOI] [PubMed] [Google Scholar]
- Witmer L. A., Rosenthal K. L., Graham F. L., Friedman H. M., Yee A., Johnson D. C. Cytotoxic T lymphocytes specific for herpes simplex virus (HSV) studied using adenovirus vectors expressing HSV glycoproteins. J Gen Virol. 1990 Feb;71(Pt 2):387–396. doi: 10.1099/0022-1317-71-2-387. [DOI] [PubMed] [Google Scholar]
- Zhou F., Rouse B. T., Huang L. An improved method of loading pH-sensitive liposomes with soluble proteins for class I restricted antigen presentation. J Immunol Methods. 1991 Dec 15;145(1-2):143–152. doi: 10.1016/0022-1759(91)90320-f. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



