Abstract
Direct inoculation of a cloned bovine leukemia virus (BLV) provirus into sheep has allowed study of the viral infectivity of genetic mutants in vivo. Three BLV variants cloned from BLV-induced tumors and 12 in vitro-modified proviruses were isolated and analyzed for viral expression in cell culture. The proviruses were then inoculated into sheep in order to assess viral infectivity in vivo. Of three variants cloned from BLV-induced tumors (344, 395, and 1345), one (344) was found infectious in vivo. This particular provirus was used to engineer 12 BLV mutants. A hybrid between the 5' region of the complete but noninfectious provirus 395 and the 3' end of mutant 344 was infectious in vivo, suggesting that the tax/rex sequences were altered in virus 395. As expected, several regions of the BLV genome appeared to be essential for viral infection: the protease, pol, and env genes. Even discrete modifications in the fusion peptide located at the NH2 end of the transmembrane gp30 glycoprotein destroyed the infectious potential. In contrast, mutations and deletions in the X3 region present between the env gene and the 3' tax/rex region did not interfere with viral infection in vivo. This region of unknown function could thus be used to introduce foreign sequences. A BLV recombinant carrying a ribozyme directed against the tax/rex sequences was still infectious in vivo. Cotransfection of two noninfectious mutants carrying deletions led to infection in two of four independent injections, the infectious virus being then a recombinant between the two deletants. The experimental approach described here should help to gain insight into essential mechanisms such as in vivo viral replication, cooperation between deletants for viral infectivity, and viral superinfections. The gene products in the X3 and X4 region which are dispensable for in vivo infection could be involved in leukemogenesis, and thus proviruses deleted in these sequences could constitute the basis for a live attenuated vaccine.
Full text
PDF

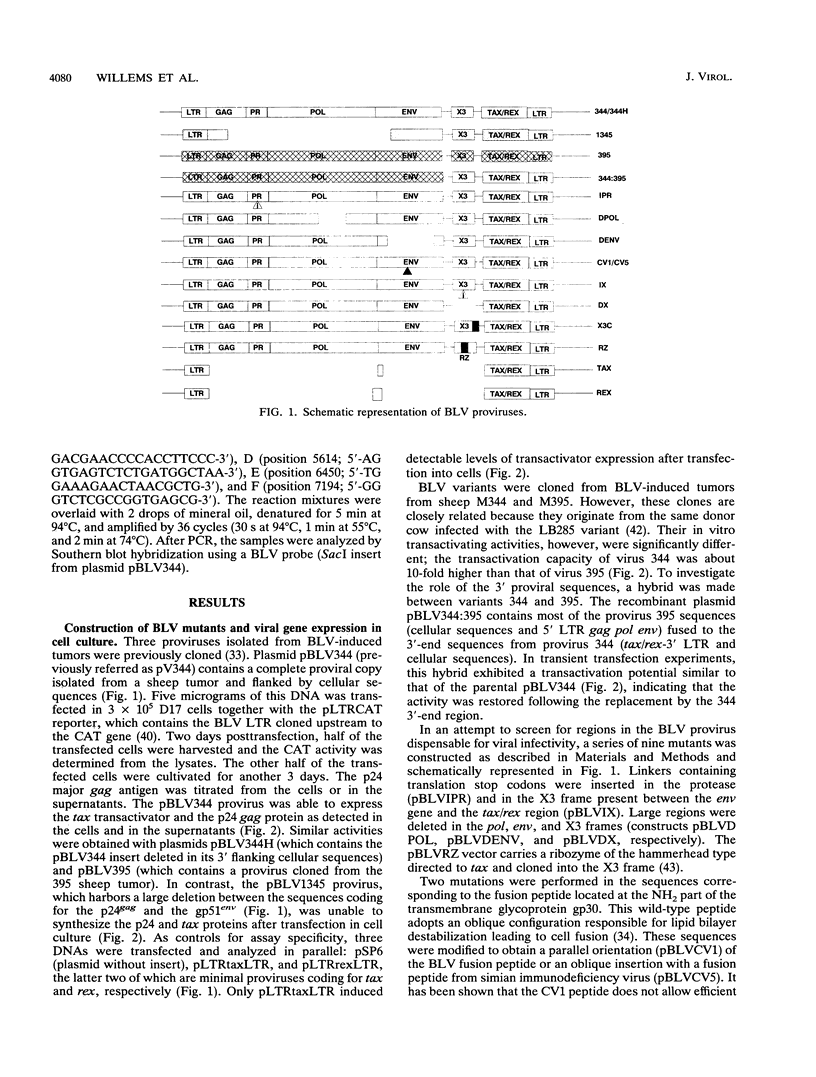



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Carpenter S., Christensen J., Storgaard T., Viuff B., Wannemuehler Y., Belousov J., Roth J. A. Identification of alternatively spliced mRNAs encoding potential new regulatory proteins in cattle infected with bovine leukemia virus. J Virol. 1993 Jan;67(1):39–52. doi: 10.1128/jvi.67.1.39-52.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneman Z. N., Gartenhaus R. B., Reitz M. S., Jr, Blattner W. A., Manns A., Hanchard B., Ikehara O., Gallo R. C., Klotman M. E. Expression of alternatively spliced human T-lymphotropic virus type I pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burny A., Cleuter Y., Kettmann R., Mammerickx M., Marbaix G., Portetelle D., Van den Broeke A., Willems L., Thomas R. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Cancer Surv. 1987;6(1):139–159. [PubMed] [Google Scholar]
- Ciminale V., Pavlakis G. N., Derse D., Cunningham C. P., Felber B. K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992 Mar;66(3):1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Martarano L. Construction of a recombinant bovine leukemia virus vector for analysis of virus infectivity. J Virol. 1990 Jan;64(1):401–405. doi: 10.1128/jvi.64.1.401-405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D. trans-acting regulation of bovine leukemia virus mRNA processing. J Virol. 1988 Apr;62(4):1115–1119. doi: 10.1128/jvi.62.4.1115-1119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res Hum Retroviruses. 1992 Mar;8(3):411–421. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
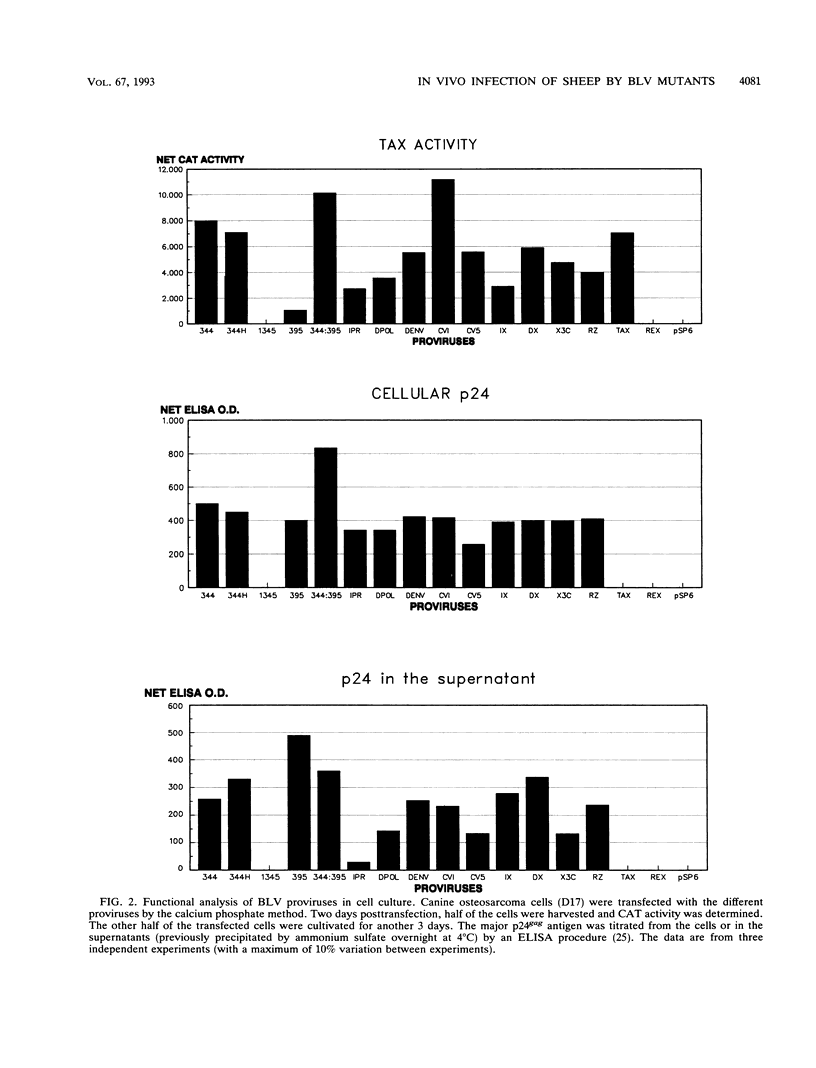
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. A., Rovnak J., Cockerell G. L. In vivo transcription of the bovine leukemia virus tax/rex region in normal and neoplastic lymphocytes of cattle and sheep. J Virol. 1991 May;65(5):2484–2490. doi: 10.1128/jvi.65.5.2484-2490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. A., Wicks-Beard B. J., Cockerell G. L. Inhibition of protein kinase C results in decreased expression of bovine leukemia virus. J Virol. 1992 Jul;66(7):4427–4433. doi: 10.1128/jvi.66.7.4427-4433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Cleuter Y., Gregoire D., Burny A. Role of the 3' long open reading frame region of bovine leukemia virus in the maintenance of cell transformation. J Virol. 1985 Jun;54(3):899–901. doi: 10.1128/jvi.54.3.899-901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I. J., Gessain A., Klotman M. E., Lo Monico A., Berneman Z. N., Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarias D. M., Radke K. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J Virol. 1989 May;63(5):2099–2107. doi: 10.1128/jvi.63.5.2099-2107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Trinh D., Brady J. N. Activation of interleukin-2 receptor alpha expression by extracellular HTLV-I Tax1 protein: a potential role in HTLV-I pathogenesis. Oncogene. 1992 Sep;7(9):1749–1755. [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Milan D., Nicolas J. F. Activator-dependent and activator-independent defective recombinant retroviruses from bovine leukemia virus. J Virol. 1991 Apr;65(4):1938–1945. doi: 10.1128/jvi.65.4.1938-1945.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portetelle D., Limbach K., Burny A., Mammerickx M., Desmettre P., Riviere M., Zavada J., Paoletti E. Recombinant vaccinia virus expression of the bovine leukaemia virus envelope gene and protection of immunized sheep against infection. Vaccine. 1991 Mar;9(3):194–200. doi: 10.1016/0264-410x(91)90153-w. [DOI] [PubMed] [Google Scholar]
- Portetelle D., Mammerickx M., Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukaemia virus envelope protein gp51. J Virol Methods. 1989 Feb;23(2):211–222. doi: 10.1016/0166-0934(89)90135-3. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Elkins D., Zaia J. A., Sullivan S. Ribozymes as anti-HIV-1 therapeutic agents: principles, applications, and problems. AIDS Res Hum Retroviruses. 1992 Feb;8(2):183–189. doi: 10.1089/aid.1992.8.183. [DOI] [PubMed] [Google Scholar]
- Sadaie M. R., Kalyanaraman V. S., Mukopadhayaya R., Tschachler E., Gallo R. C., Wong-Staal F. Biological characterization of noninfectious HIV-1 particles lacking the envelope protein. Virology. 1992 Apr;187(2):604–611. doi: 10.1016/0042-6822(92)90462-x. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Benko D. M., Fenyö E. M., Pavlakis G. N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Van den Broeke A., Cleuter Y., Chen G., Portetelle D., Mammerickx M., Zagury D., Fouchard M., Coulombel L., Kettmann R., Burny A. Even transcriptionally competent proviruses are silent in bovine leukemia virus-induced sheep tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9263–9267. doi: 10.1073/pnas.85.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonèche V., Portetelle D., Kettmann R., Willems L., Limbach K., Paoletti E., Ruysschaert J. M., Burny A., Brasseur R. Fusogenic segments of bovine leukemia virus and simian immunodeficiency virus are interchangeable and mediate fusion by means of oblique insertion in the lipid bilayer of their target cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3810–3814. doi: 10.1073/pnas.89.9.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Nishi M., Ikawa Y., Amanuma H. Conversion of Friend mink cell focus-forming virus to Friend spleen focus-forming virus by modification of the 3' half of the env gene. J Virol. 1991 Jan;65(1):132–137. doi: 10.1128/jvi.65.1.132-137.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Gegonne A., Chen G., Burny A., Kettmann R., Ghysdael J. The bovine leukemia virus p34 is a transactivator protein. EMBO J. 1987 Nov;6(11):3385–3389. doi: 10.1002/j.1460-2075.1987.tb02661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Grimonpont C., Heremans H., Rebeyrotte N., Chen G., Portetelle D., Burny A., Kettmann R. Mutations in the bovine leukemia virus Tax protein can abrogate the long terminal repeat-directed transactivating activity without concomitant loss of transforming potential. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3957–3961. doi: 10.1073/pnas.89.9.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Heremans H., Chen G., Portetelle D., Billiau A., Burny A., Kettmann R. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 1990 May;9(5):1577–1581. doi: 10.1002/j.1460-2075.1990.tb08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Kettmann R., Burny A. The amino acid (157-197) peptide segment of bovine leukemia virus p34tax encompass a leucine-rich globally neutral activation domain. Oncogene. 1991 Jan;6(1):159–163. [PubMed] [Google Scholar]
- Willems L., Kettmann R., Chen G., Portetelle D., Burny A., Derse D. A cyclic AMP-responsive DNA-binding protein (CREB2) is a cellular transactivator of the bovine leukemia virus long terminal repeat. J Virol. 1992 Feb;66(2):766–772. doi: 10.1128/jvi.66.2.766-772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Portetelle D., Kerkhofs P., Chen G., Burny A., Mammerickx M., Kettmann R. In vivo transfection of bovine leukemia provirus into sheep. Virology. 1992 Aug;189(2):775–777. doi: 10.1016/0042-6822(92)90604-n. [DOI] [PubMed] [Google Scholar]
- Willems L., Thienpont E., Kerkhofs P., Burny A., Mammerickx M., Kettmann R. Bovine leukemia virus, an animal model for the study of intrastrain variability. J Virol. 1993 Feb;67(2):1086–1089. doi: 10.1128/jvi.67.2.1086-1089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt C. R., Wingett D., White J. S., Buck C. D., Knowles D., Reeves R., Magnuson N. S. Persistent infection of rabbits with bovine leukemia virus associated with development of immune dysfunction. J Virol. 1989 Nov;63(11):4498–4506. doi: 10.1128/jvi.63.11.4498-4506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]