Abstract
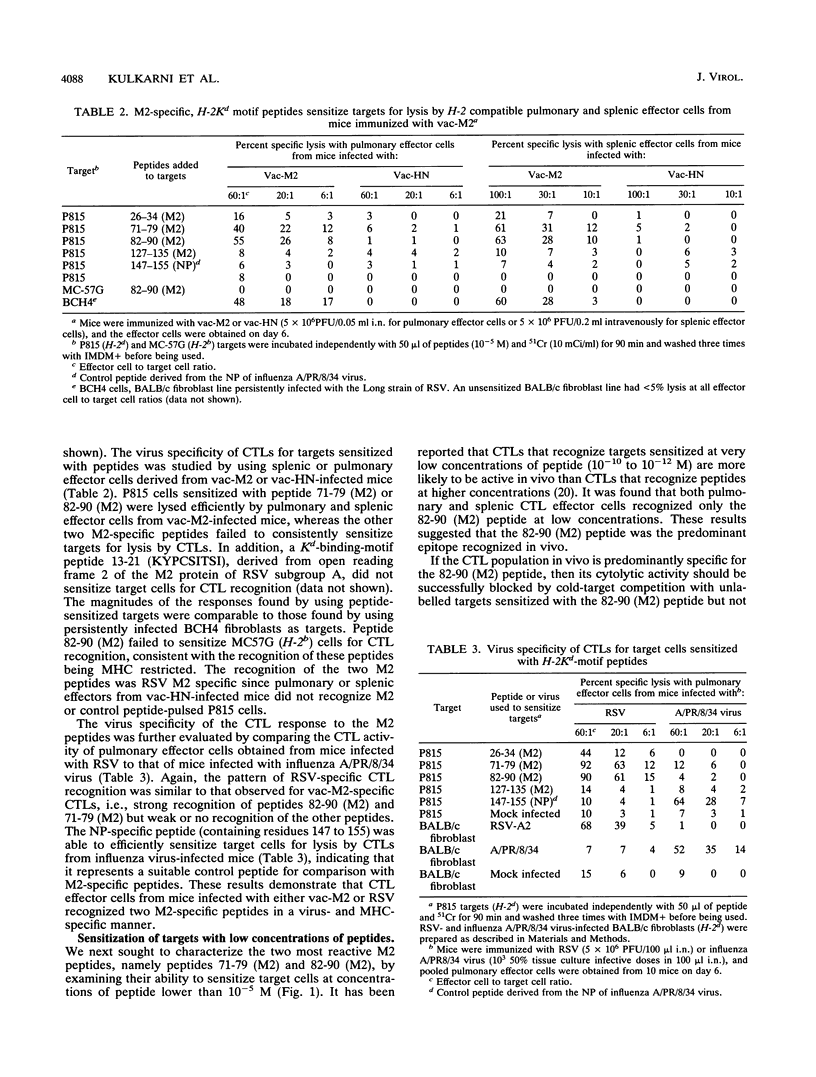
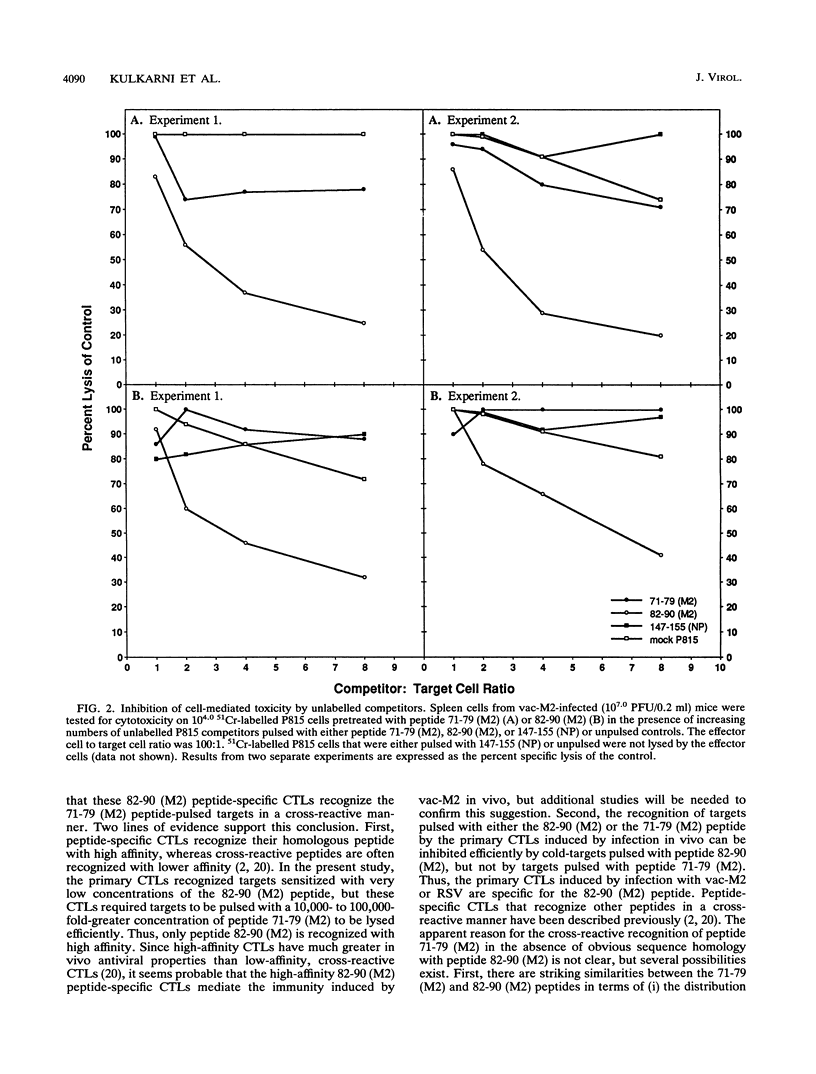
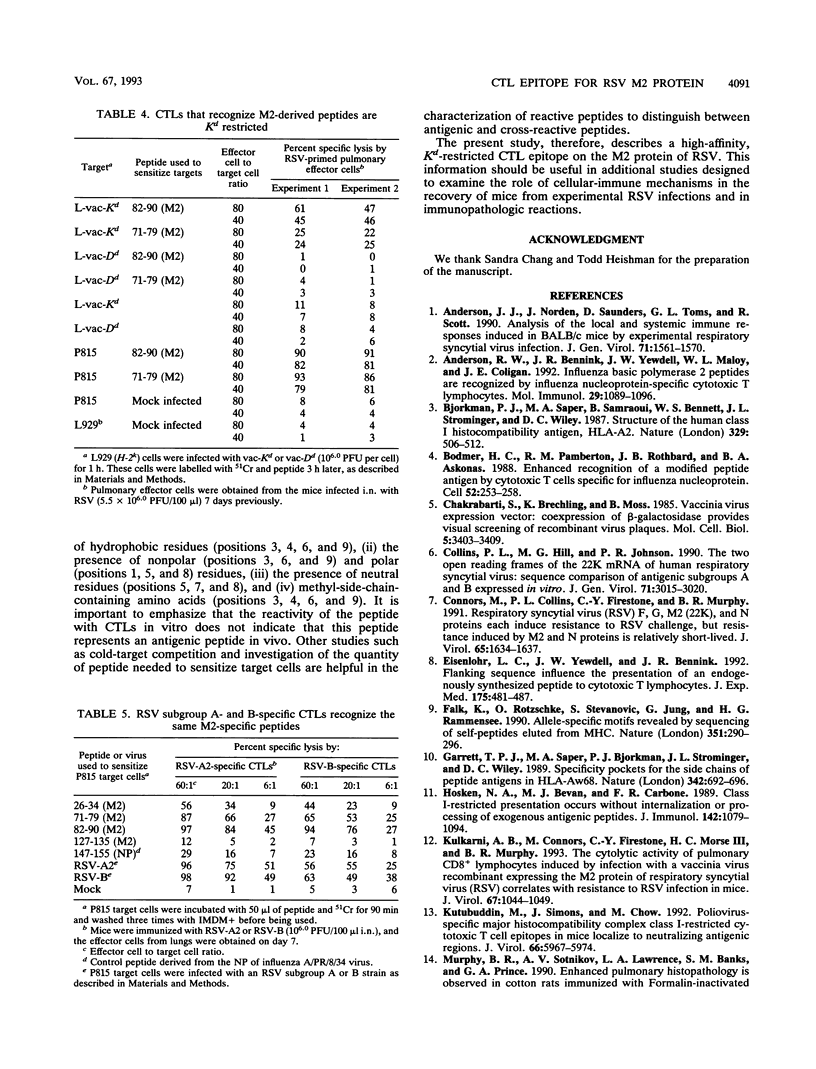
The M2 protein of respiratory syncytial virus (RSV) is a protective antigen in H-2d, but not H-2b or H-2k mice. None of the other RSV proteins, excluding the surface glycoproteins that induce neutralizing antibodies, is protective in mice bearing these haplotypes. Thus, the M2 protein stands alone as a nonglycoprotein-protective antigen of RSV. The M2 protein is a target for murine Kd-restricted cytotoxic T lymphocytes (CTLs), and the resistance induced by infection with a vaccinia virus-RSV M2 (vac-M2) recombinant is mediated by CD8+ CTLs. Since the nonameric consensus sequence for H-2 Kd-restricted T-cell epitopes and the amino acid sequence of the M2 protein of subgroup A and B strains of RSV are known, the present study sought to identify the specific epitope(s) on the M2 protein recognized by CD8+ CTLs. This was done by examining the ability of four predicted Kd-specific motif peptides present in the M2 amino acid sequence of an RSV subgroup A strain to sensitize target cells for lysis by pulmonary or splenic CTLs obtained from mice infected with RSV or vac-M2. The following observations were made. First, two of the four peptides sensitized target cells for lysis by pulmonary or splenic CTLs induced by infection with either vac-M2 or RSV. Second, one of the two peptides, namely the 82-90 (M2) peptide, sensitized targets at a very low peptide concentration (10(-10) to 10(-12) M). Third, cold-target competition experiments revealed that the predominant CTL population induced by infection with vac-M2 or RSV recognized the 82-90 (M2) peptide, and this CTL population appeared to recognize the 71-79 (M2) peptide in a cross-reactive manner. Fourth, CTL recognition of targets sensitized with either the 71-79 (M2) or the 82-90 (M2) peptide was Kd restricted. Fifth, CTLs induced by infection with RSV subgroup A or B strains recognized the two M2 peptides. The findings suggest that the M2 protein of RSV contains an immunodominant Kd-restricted CTL epitope consisting of amino acid residues 82 to 90 (SYIGSINNI), which are shared by subgroup A and B RSVs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Norden J., Saunders D., Toms G. L., Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990 Jul;71(Pt 7):1561–1570. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- Anderson R. W., Bennink J. R., Yewdell J. W., Maloy W. L., Coligan J. E. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol Immunol. 1992 Sep;29(9):1089–1096. doi: 10.1016/0161-5890(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bodmer H. C., Pemberton R. M., Rothbard J. B., Askonas B. A. Enhanced recognition of a modified peptide antigen by cytotoxic T cells specific for influenza nucleoprotein. Cell. 1988 Jan 29;52(2):253–258. doi: 10.1016/0092-8674(88)90514-4. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hill M. G., Johnson P. R. The two open reading frames of the 22K mRNA of human respiratory syncytial virus: sequence comparison of antigenic subgroups A and B and expression in vitro. J Gen Virol. 1990 Dec;71(Pt 12):3015–3020. doi: 10.1099/0022-1317-71-12-3015. [DOI] [PubMed] [Google Scholar]
- Connors M., Collins P. L., Firestone C. Y., Murphy B. R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991 Mar;65(3):1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992 Feb 1;175(2):481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J., Carbone F. R. Class I-restricted presentation occurs without internalization or processing of exogenous antigenic peptides. J Immunol. 1989 Feb 15;142(4):1079–1083. [PubMed] [Google Scholar]
- Kulkarni A. B., Connors M., Firestone C. Y., Morse H. C., 3rd, Murphy B. R. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J Virol. 1993 Feb;67(2):1044–1049. doi: 10.1128/jvi.67.2.1044-1049.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutubuddin M., Simons J., Chow M. Poliovirus-specific major histocompatibility complex class I-restricted cytolytic T-cell epitopes in mice localize to neutralizing antigenic regions. J Virol. 1992 Oct;66(10):5967–5974. doi: 10.1128/jvi.66.10.5967-5974.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. G., Harty J. T., Bevan M. J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991 Oct 31;353(6347):852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Speiser D. E., Kyburz D., Stübi U., Hengartner H., Zinkernagel R. M. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992 Aug 1;149(3):972–980. [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]



