Abstract
The functions of the surface glycoproteins (SU) of feline leukemia viruses (FeLVs) are of interest since these proteins mediate virus infection and interference and are critical determinants of disease specificity. In this study, we examined the biochemical and genetic determinants of SU important to virus entry and cell killing. In particular, we developed and used vesicular stomatitis virus (VSV)/FeLV pseudotype virus interference assays to determine interference subgroupings and assess mechanisms of host cell restriction. We also assessed roles of SU in virus growth kinetics and in the inhibition of cell killing caused by superinfection with cytopathic virus. Subgroup classification by VSV/FeLV pseudotype assay was in agreement with that defined previously by focus interference assay and was found to be determined by changes near the N terminus of SU for FeLV subgroups A (FeLV-A) and C. Virus host range restriction was found to be mediated at the level of virus entry in most cases, although postentry events mediated restriction in the failure of a subgroup A-like, T-cell cytopathic and immunodeficiency-inducing clone (FeLV-FAIDS-EECC) to replicate in feline fibroblasts. FeLV-FAIDS-EECC-induced cell killing was also inhibited by prior infection with one of two FeLV-A isolates. This inhibition could be conveyed by as few as four amino acid changes near the N terminus of the FeLV-A SU and also appeared to be mediated at a postentry level. Lastly, the SU-coding sequence was also found to determine differences in growth kinetics of viruses within the same subgroup. These studies demonstrate that subtle alterations in the FeLV SU, particularly in the N-terminal region, impart multiple significant functional differences which distinguish virus variants.
Full text
PDF
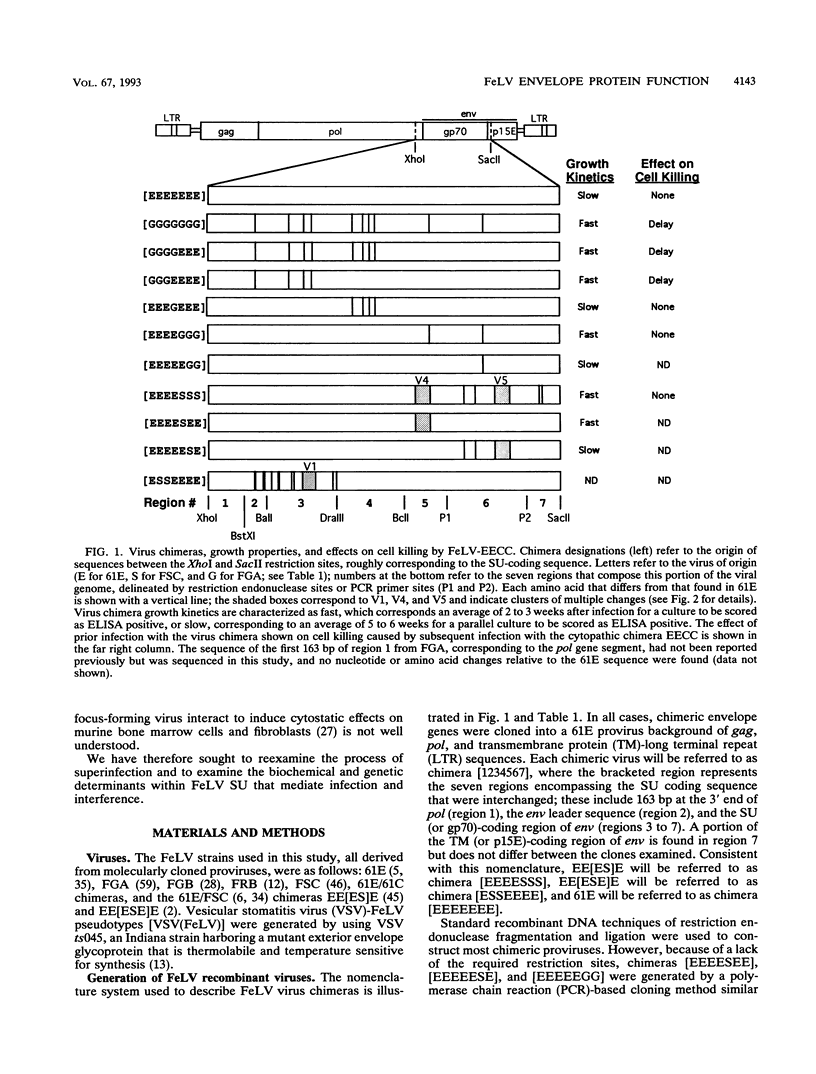



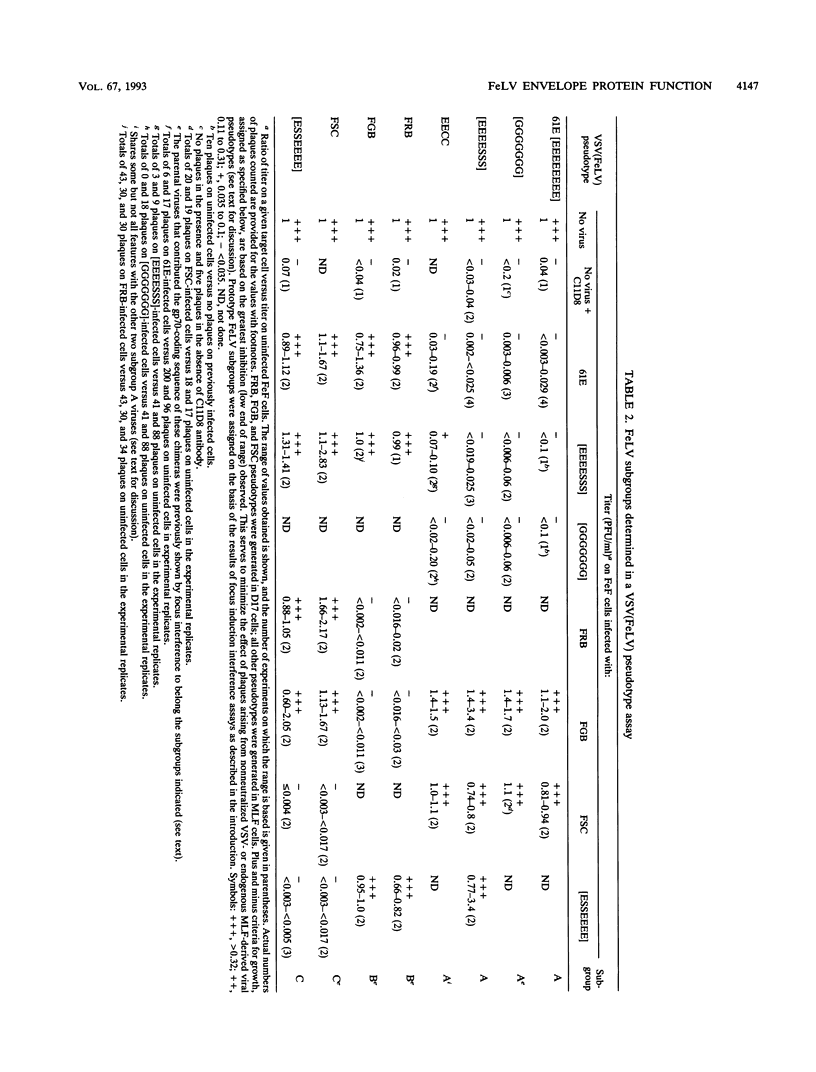
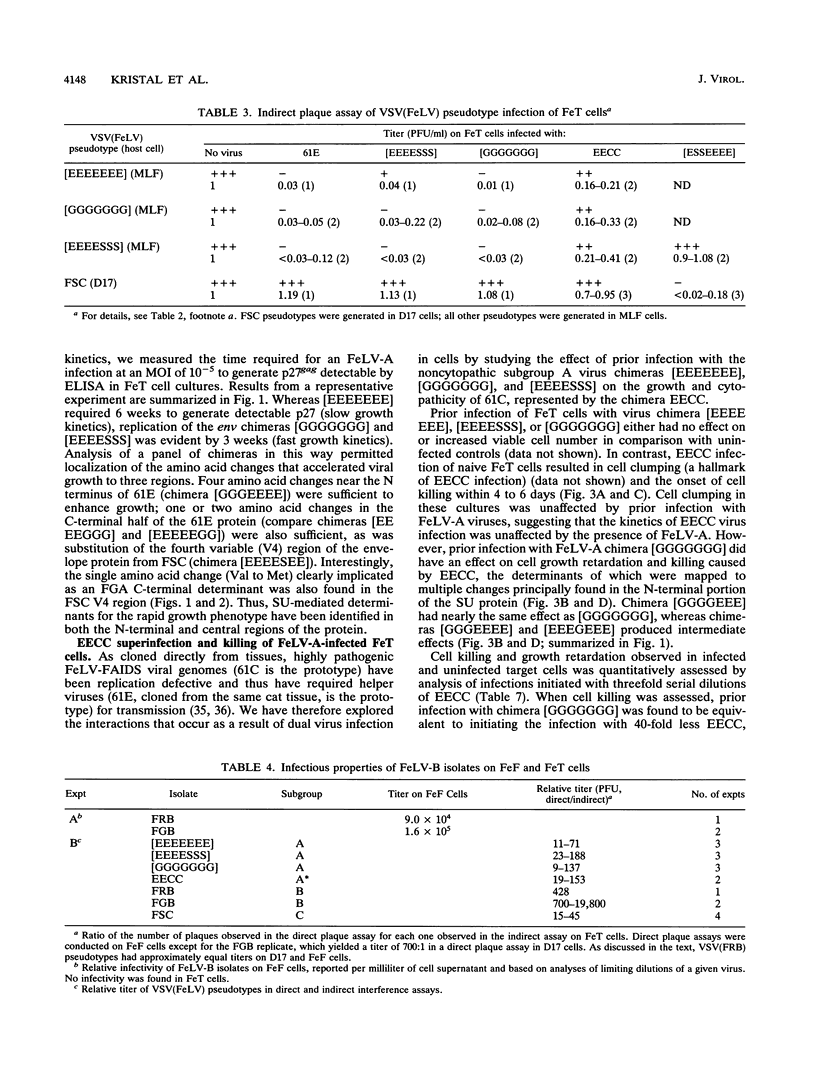
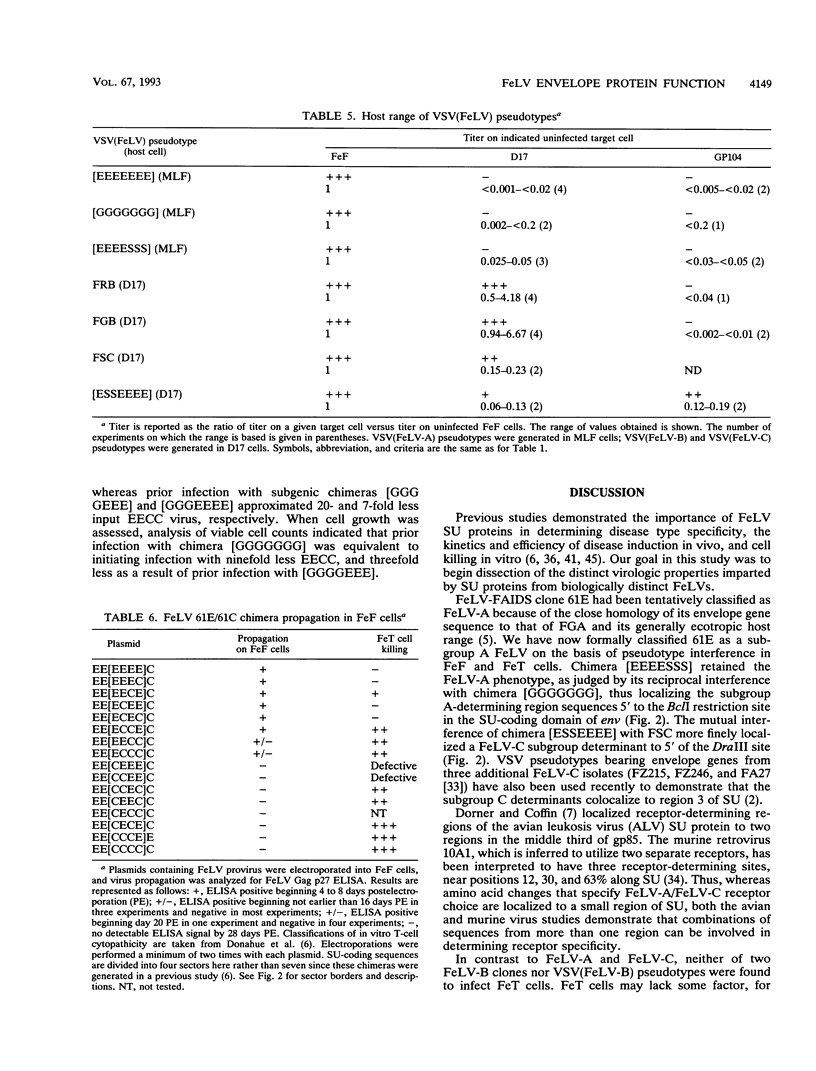


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Tronick S. R., Aaronson S. A. Isolation and characterization of an endogenous type C RNA virus of mink (Mv1Lu) cells. J Virol. 1978 Jan;25(1):129–137. doi: 10.1128/jvi.25.1.129-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brojatsch J., Kristal B. S., Viglianti G. A., Khiroya R., Hoover E. A., Mullins J. I. Feline leukemia virus subgroup C phenotype evolves through distinct alterations near the N terminus of the envelope surface glycoprotein. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8457–8461. doi: 10.1073/pnas.89.18.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E. L., Panganiban A. T. Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J Virol. 1989 Jan;63(1):273–280. doi: 10.1128/jvi.63.1.273-280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Quackenbush S. L., Gallo M. V., deNoronha C. M., Overbaugh J., Hoover E. A., Mullins J. I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1991 Aug;65(8):4461–4469. doi: 10.1128/jvi.65.8.4461-4469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dorsett D. L., Keshet I., Winocour E. Quantitation of a simian virus 40 nonhomologous recombination pathway. J Virol. 1983 Oct;48(1):218–228. doi: 10.1128/jvi.48.1.218-228.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., McGee J. S., Munson M., Houghten R. A., Kloetzer W., Bittle J. L., Grant C. K. Localization of neutralizing regions of the envelope gene of feline leukemia virus by using anti-synthetic peptide antibodies. J Virol. 1987 Jan;61(1):8–15. doi: 10.1128/jvi.61.1.8-15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Bachmann M. H., Hoover E. A., Mullins J. I. Identification of a putative receptor for subgroup A feline leukemia virus on feline T cells. J Virol. 1992 Jun;66(6):3707–3714. doi: 10.1128/jvi.66.6.3707-3714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. K., Ernisse B. J., Jarrett O., Jones F. R. Feline leukemia virus envelope gp70 of subgroups B and C defined by monoclonal antibodies with cytotoxic and neutralizing functions. J Immunol. 1983 Dec;131(6):3042–3048. [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Jarrett O., Golder M. C., Toth S., Onions D. E., Stewart M. F. Interaction between feline leukaemia virus subgroups in the pathogenesis of erythroid hypoplasia. Int J Cancer. 1984 Aug 15;34(2):283–288. doi: 10.1002/ijc.2910340222. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Russell P. H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int J Cancer. 1978 Apr 15;21(4):466–472. doi: 10.1002/ijc.2910210411. [DOI] [PubMed] [Google Scholar]
- Klement V., Dougherty M. F., Roy-Burman P., Pal B. K., Shimizu C. S., Rongey R. W., Nelson-Rees W., Huebner R. J. Endogenous type C RNA virus of mink (Mustela vison). Virology. 1978 Mar;85(1):296–306. doi: 10.1016/0042-6822(78)90433-6. [DOI] [PubMed] [Google Scholar]
- Kumar D. V., Berry B. T., Roy-Burman P. Nucleotide sequence and distinctive characteristics of the env gene of endogenous feline leukemia provirus. J Virol. 1989 May;63(5):2379–2384. doi: 10.1128/jvi.63.5.2379-2384.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. X., Fan H. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J Virol. 1990 Aug;64(8):3701–3711. doi: 10.1128/jvi.64.8.3701-3711.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Chen C. S., Hoover E. A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986 Jan 23;319(6051):333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Hoover E. A., Quackenbush S. L., Donahue P. R. Disease progression and viral genome variants in experimental feline leukemia virus-induced immunodeficiency syndrome. J Acquir Immune Defic Syndr. 1991;4(6):547–557. [PubMed] [Google Scholar]
- Nicolaisen-Strouss K., Kumar H. P., Fitting T., Grant C. K., Elder J. H. Natural feline leukemia virus variant escapes neutralization by a monoclonal antibody via an amino acid change outside the antibody-binding epitope. J Virol. 1987 Nov;61(11):3410–3415. doi: 10.1128/jvi.61.11.3410-3415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onions D., Jarrett O., Testa N., Frassoni F., Toth S. Selective effect of feline leukaemia virus on early erythroid precursors. Nature. 1982 Mar 11;296(5853):156–158. doi: 10.1038/296156a0. [DOI] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Hoover E. A., Mullins J. I., Burns D. P., Rudensey L., Quackenbush S. L., Stallard V., Donahue P. R. Structure and pathogenicity of individual variants within an immunodeficiency disease-inducing isolate of FeLV. Virology. 1992 Jun;188(2):558–569. doi: 10.1016/0042-6822(92)90510-v. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Pandey R., Ghosh A. K., Kumar D. V., Bachman B. A., Shibata D., Roy-Burman P. Recombination between feline leukemia virus subgroup B or C and endogenous env elements alters the in vitro biological activities of the viruses. J Virol. 1991 Dec;65(12):6495–6508. doi: 10.1128/jvi.65.12.6495-6508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M. L., Quackenbush S. L., Mullins J. I., Hoover E. A. Characterization and significance of delayed processing of the feline leukemia virus FeLV-FAIDS envelope glycoprotein. J Virol. 1990 Sep;64(9):4338–4345. doi: 10.1128/jvi.64.9.4338-4345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush S. L., Donahue P. R., Dean G. A., Myles M. H., Ackley C. D., Cooper M. D., Mullins J. I., Hoover E. A. Lymphocyte subset alterations and viral determinants of immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1990 Nov;64(11):5465–5474. doi: 10.1128/jvi.64.11.5465-5474.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Dornsife R. E., Mullins J. I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko J. L., Kociba G. J., Abkowitz J. L., Hamilton K. L., Hardy W. D., Jr, Ihle J. N., O'Brien S. J. Feline lymphomas: immunological and cytochemical characterization. Cancer Res. 1989 Jan 15;49(2):345–351. [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Jain D., Hill P. R. In vitro host range of feline leukemia virus. Bibl Haematol. 1975;(40):489–492. doi: 10.1159/000397566. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Jain D., Hill P. R., Huebner R. J. Differential host range of viruses of feline leukemia-sarcoma complex. Virology. 1975 Apr;64(2):438–446. doi: 10.1016/0042-6822(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Weiss R. A., Zavada J. Pseudotypes of vesicular stomatitis virus with the envelope properties of mammalian and primate retroviruses. J Virol. 1977 Sep;23(3):449–454. doi: 10.1128/jvi.23.3.449-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Benveniste R. E., Todaro G. J. Endogenous mink (Mustela vison) type C virus isolated from sarcoma virus-transformed mink cells. J Virol. 1978 Mar;25(3):738–749. doi: 10.1128/jvi.25.3.738-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C. D., Levy J. A., White J. M. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J Virol. 1990 Nov;64(11):5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]
- Závada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nat New Biol. 1972 Nov 22;240(99):122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]



